Introduction
Probiotics are the live cultures of beneficial
organisms that selectively stimulate the growth of native bacteria
in the gut, thereby benefiting the host (1). Probiotics from the genera
Bifidobacteria, Lactobacillus and yeast are the most
beneficial for the host intestine (2). Probioticos have been reported to be
beneficial in controlling inflammation, lactose intolerance and
cholesterol and have been found to exhibit antitumor activity and
stimulation of the immune response, and also prevent allergies and
reduce the incidence of respiratory diseases (3). It has also been reported that
probiotics aid in the prevention of all types of diarrhea,
inflammatory bowel diseases, ulcerative colitis, Crohn's disease,
pouchitis, irritable bowel syndrome, constipation, liver diseases,
lung cancer, mammary cancer and colorectal cancer (4).
Colorectal cancer (CRC) is one of the most common
types of cancer worldwide and the second leading cause of
cancer-related death in developing and developed countries
(5). L. bulgaricus (LAB)
has been found to inhibit colon cancer by altering the metabolic
activities of intestinal microflora, by altering physiochemical
conditions, by binding, degradation, and inhibiting promoters to
carcinogenesis, by producing certain metabolites, such as bile
acid, by producing antimutagenic metabolites, by enhancing the host
immune compounds, or by enhancing the host immune response
(6). Modulation of enzymes, such
as β glucosidase and β glucopyranose by probiotics results in a
reduction of ras p-21 oncoprotein activity which aids in cancer
prevention (7).
It has been found that the consumption of probiotics
resulted in an increase in the daily weight gain, the egg
production, shell weight, shell thickness and concentration of
cholesterol in the serum and yolk of leghorn chickens (8). It has also been reported that the
consumption of Lactobacillus sp. by newborn ducks (Anas
platyrhynchos domestica) and chicks (Gallus gallus
domesticus) resulted in weight gain. Furthermore, the positive
effect of probiotics in bringing health benefits also depends on
the doses. A significant increase in body weight and liver mass was
noted after the second dose of Lactobacillus administration
in newborn chicks (9). In IL-10
knockout mice, probiotic Lactobacilli prevented and reduced
the prevalence of colon cancer and mucosal inflammatory activity
(10). Antitumorigenic activity of
L. rhamnosus and Bifidobacterium lactis
administration on azoxymethane-induced colon cancer was found to
decrease carcinogenesis in rats (11). In laboratory investigations,
certain strains of LAB have demonstrated antimutagenic effects due
to their ability to bind to heterocyclic amines, which are
carcinogenic substances formed in cooked meat (12). Animal studies have demonstrated
that several LAB strains protect against colon cancer in rodents,
although human data are limited and conflicting. Most human trials
have found that the strains tested may exert anticarcinogenic
effects by decreasing the activity of the β glucuronidase enzyme
(7). Possible mechanisms by which
probiotics prevent colon cancer include the lowering of the pH of
the intestine, the modulation of enzymes, such as β glucosidase,
which convert pro-carcinogens to proximate carcinogens, the
reduction in the expression of ras-P21 oncoprotein, production of
sodium butyrate by fermenting lactose, which is a powerful
inhibitor of growth and an inducer of phenotype differentiation and
apoptosis (13).
The plant Catharanthus roseus has been used
from ancient times in treating various types of cancer, and this
use has been described in Ayurveda (14). Its leaf, flower and root extracts
contains more than 400 alkaloids approved as antineoplastic agents
to cure leukemia, Hodgkin's disease, malignant lymphoma,
neuroblastoma, rhabdomyosarcoma and Wilm's tumor (15). One ton of its leaves yields 50 g of
vincristine sulfate in pure form. Vincristine and vinblastin are
two important alkaloids used in the treatment of several types of
cancer. Junior et al (16)
evaluated the effects of vincristine on the gastrointestinal
motility of awake rats and found an increase in the colon weight of
these rats.
The antitumor effect as a result of the synergistic
cytotoxic effect of a combination of betulinic acid and vincristine
on malignant melanoma cells has also been reported (17). Furthermore, administration of
topotecan and vincristine for pediatric solid tumor xenografts
significantly resulted in a synergistic effect in controlling nine
different types of cell lines: four neuroblastomas, three brain
tumors and two rhabdomyosarcomas (18). Although Lactobacillus and
vincristine are antitumorigenic in nature, reports on their
combination in controlling CRC are scarce. Therefore, in the
present study we investigated the activity of vincristine either
alone and/or in combination with L. fermentum and L.
plantarum in 1,2-dimethylhydrazine (DMH)-induced CRC in female
Swiss mice.
Materials and methods
Mice
Experiments were performed on female Swiss albino
mice weighing 25–35 g. All procedures and animal treatments were
conducted in accordance with the Guide for the Care and Use of
Laboratory Animals (S.S. Medical College Hospital, Davangere,
India; Institutional Ethics committee) and were approved by the
Animal Ethics Committee.
The mice were anesthetized with ether and treated
with vincristine, which was slowly injected intraperitoneously i.p.
for 21 days. Experimental animals received an equivalent amount of
saline according to their weight.
Vincristine sulphate
Vincristine sulfate (MP Bioneutricals, Solon, OH,
USA) was diluted with 1 mg/ml of saline, and 1 μl of this solution
was administered to each experimental mouse regularly up to 21
days. Every experimental group was injected with a single dose.
Group 10 mice (VCR + DMH + normal diet) received only a vincristine
dose, one week prior to carcinogen induction. Group 11 mice (DMH +
VCR + normal diet) received a vincristine injection after 1 week of
carcinogen induction. Other groups received a vincristine injection
along with L. fermentum and L. plantarum feeding pre-
or post-carcinogen induction (Table
I).
 | Table I.Percentage of the weight gain of the
mice in the experimental groups. |
Table I.
Percentage of the weight gain of the
mice in the experimental groups.
| Groups | Feeding schedule | Change in body
weight | Percentage of weight
gain |
|---|
| Group 1 | Normal diet (Control
1) | +2 | 6.00% |
| Group 2 | DMH + normal diet
(Control 2) | −2 | −6.25% |
| Group 3 | Lf3 + DMH + normal
diet | +6 | 21.00% |
| Group 4 | DMH + Lf3 + normal
diet | +2 | 7.14% |
| Group 5 | Lp1 + DMH + normal
diet | +3 | 10.34% |
| Group 6 | DMH + Lp1 + normal
diet | +2 | 6.89% |
| Group 7 | Lf3 + Lp1 + DMH +
normal diet | +3 | 13.33% |
| Group 8 | DMH + Lf3 + Lp1 +
normal diet | +1 | 6.66% |
| Group 9 | VCR + normal diet
(Control 3) | +3 | 10.71% |
| Group 10 | VCR + DMH + normal
diet (Control 4) | +5 | 17.85% |
| Group 11 | DMH + VCR + normal
diet (Control 5) | +3 | 10.71% |
| Group 12 | Lf3 + VCR + DMH +
normal diet | +6.5 | 23.21% |
| Group 13 | DMH + Lf3 + VCR +
normal diet | +3 | 10.71% |
| Group 14 | Lp1 + VCR + DMH +
normal diet | +4 | 13.79% |
| Group 15 | DMH + Lp1 + VCR +
normal diet | +3 | 10.34% |
| Group 16 | Lf3 + Lp1 + VCR +
DMH + normal diet | +2 | 10.00% |
| Group 17 | DMH + Lf3 + Lp1 +
VCR + normal diet | +1 | 3.33% |
Carcinogen
DMH was purchased from Sigma Aldrich (C.3050). The
carcinogen (0.1 mg/kg) was dissolved in physiological saline and
0.1 μl was injected i.p., twice a week except for Group 1 (normal
diet Control 1) and Group 9 (normal diet + VCR; Control 3). The
injection was administered as a single dose and the animals were
held carefully and injected without causing injury or
discomfort.
Animals and treatments
We used 2- to 4-week-old female Swiss albino mice.
The animals were housed in plastic cages with steel tops, and
maintained in a 12-h light/12-h dark cycle at 28±2°C and humidity
62±2%. After their arrival, the animals (n=17 per group) were
acclimatized for 1 week, during which they were fed with standard
ground nut cake. All the experimental animals were fed an equal
amount of the diet (100 g/kg w/w). The pre-carcinogen-induced
groups were fed with the probiotic diet or vincristine 1 week prior
to carcinogen induction. Post-carcinogen-induced groups were fed
with the probiotic diet or vincristine 1 week after the induction
of the carcinogen. All the dietary groups were fed with a normal
diet, apart from the pre- and post-carcinogen groups for which
vincristine was also administered, which served as Control Group
3.
Bacterial strains
L. fermentum and L. plantarum were
isolated from curd sample from remote regions of Karnataka, India.
The sample was serially diluted and plated on MRS media and
incubation was carried out at 37°C for 24 h. The colonies obtained
on MRS media were identified by physiological, fermentation test
and were further subjected to PCR and identified as L.
fermentum and L. plantarum (19). Overnight cultures of L.
fermentum and L. plantarum were centrifuged at 8,000
g/10 min/twice. Cells (2×108) were suspended in 1 ml of
skimmed milk and 0.1 ml of this suspension was fed to the
experimental mice regularly, apart from Control 2 (Group 2; DMH +
normal diet).
Diets
Control diet, designed as a normal diet, consisted
of starch 320 g/kg, casein 150 g/kg, ground nut malt 200 g/kg, a
vitamin mix 15 g/kg and a mineral mix 23 g/kg. The compositions of
the normal diet have been obtained from the Ministry of Animal
Husbandry, Government of Karnataka, India.
Lactobacillus fermentum diet. The culture in
skim milk was added to the normal diet to provide 2×108
cfu/g diet.
Lactobacillus plantarum diet. The culture in
skim milk was added to the normal diet to provide 2×108
cfu/g diet.
Lactobacillus fermentum/L. plantarum diet.
This diet contained L. fermentum in skim milk (50%) and
L. plantarum in skim milk (50%) added to the normal diet.
The diets were prepared each day before feeding.
Experimental design
A total of 17 female 2- to 4-week-old Swiss albino
mice were obtained, and after optimizing the physiological
conditions, the mice were divided into 17 groups and fed the
experimental diets as described above. Pre-carcinogen-induced mice
were administered the DMH dose i.p. twice a week prior to 1 week of
the probiotic/vincristine diet. Post-carcinogen-induced mice were
administered DMH i.p. after 1 week of probiotic/vincristine dose.
Food intake and body weight were measured each day. The rats were
sacrificed by ether anesthetic 21 days after the start of the
experimental diets. The cecum was removed and frozen at −20°C for
subsequent enzyme assay. The colon was removed and fixed for
histopathological examination.
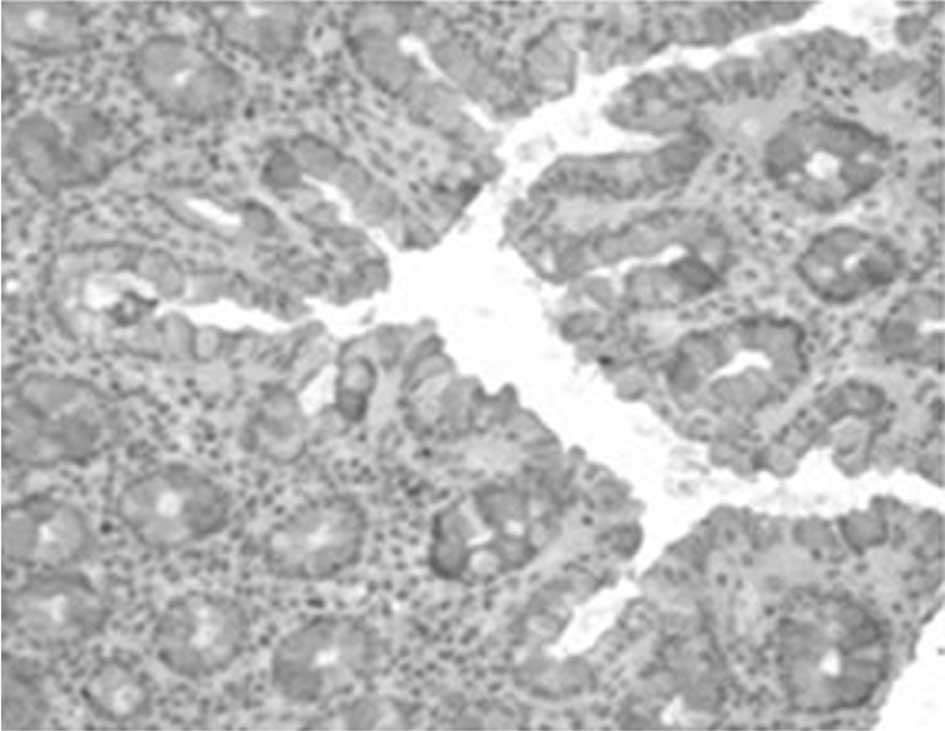
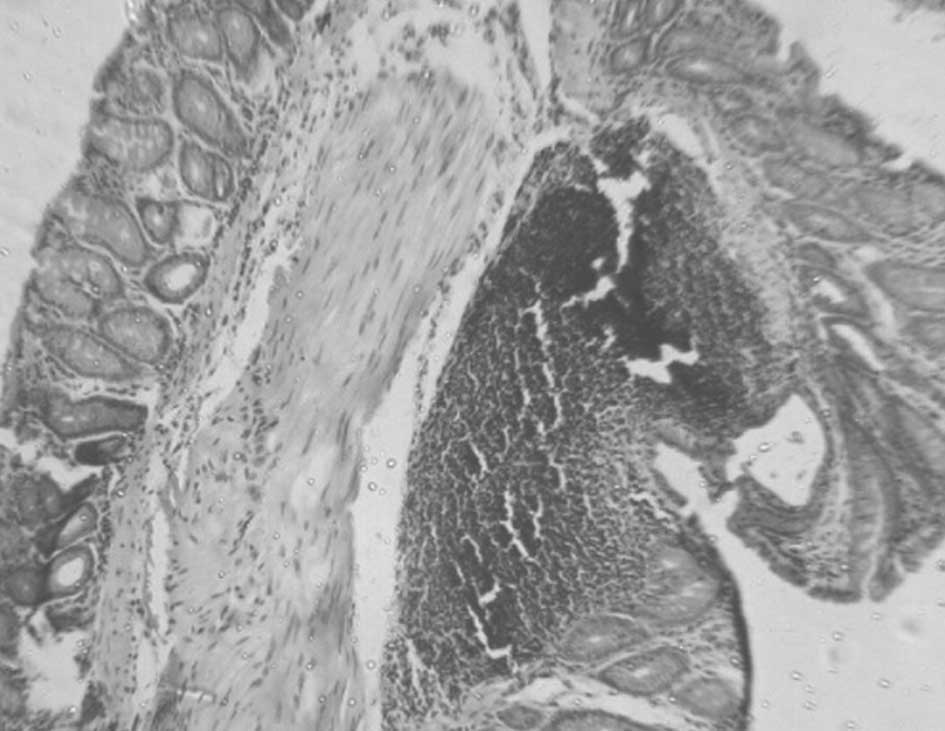
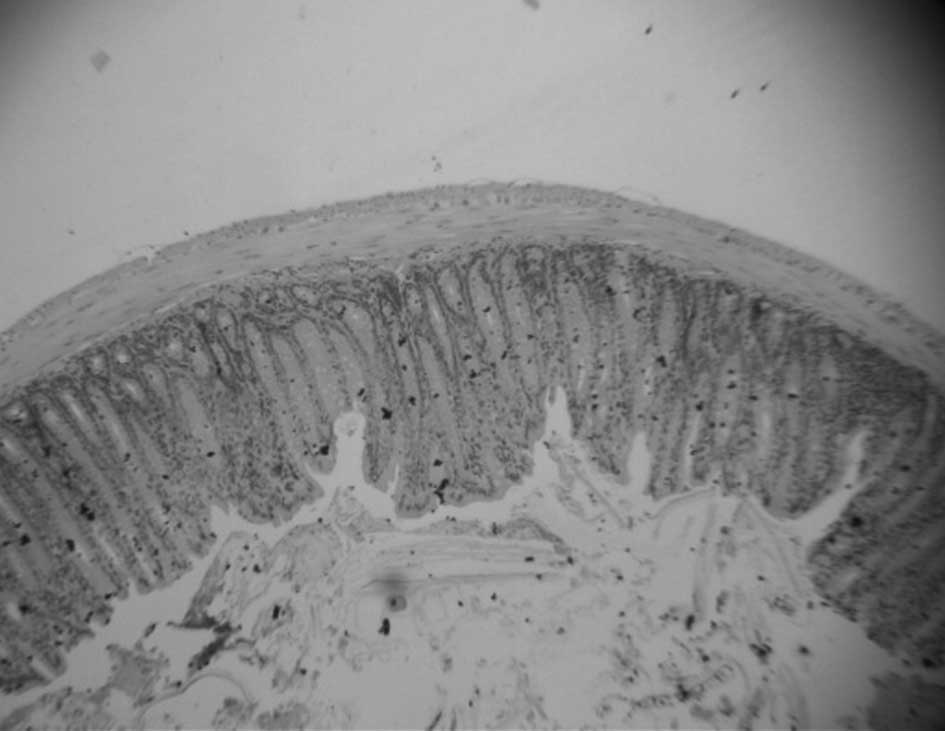
Assessment of aberrant crypt foci
(ACF)
Colon (distal cecum to rectum) was removed from the
anesthetized (sacrificed) experimental mice and slit
longitudinally. Each colon was placed on a piece of white card,
mucosal surface uppermost and the surface was cleaned gently with
0.9% saline. The back of the card was sprayed with absolute ethanol
and the card and its attached colon was placed in 10% buffered
formalin for at least 24 h for fixation of the tissue. The tissues
were processed, sectioned using standard tissue processing
procedure (0.2 μm) and stained with H&E. The number of ACF was
determined using a low-power stereo microscope (MS-224; Magnus,
Olympus, India).
Measurement of β glucosidase and β
glucuronidase activity in cecal content
Samples of cecal contents from mice were homogenized
(anerobic condition) in 0.1 M potassium phosphate buffer/pH 7.2 to
provide 10% (w/w) suspensions. The suspensions, in screw-cap
bottles, were incubated anerobically with
P-nitrophenyl-β-D-glucopyranoside (ONDP) (3 mM) or
P-nitrophenyl-β-D-glucuronide to determine the concentration of the
enzyme β glucosidase and β glucuronidase, respectively. Released
product P-nitrophenol was measured spectrophotometrically at 420 nm
(20).
Measurement of cecal ammonia
concentration
Samples of the 10% cecal homogenates were assayed
for ammonia content using phenol nitroprusside and alkaline
hypochloride (19). Blue color was
developed and was measured spectrophotometrically (570 nm). The
ammonia concentration was calculated with reference to a standard
curve of ammonium chloride.
Results
Food and probiotic intake and body weight
gain
The food intake of mice was similar for all of the
experimental diets, and ranged between 2.8 and 3.3 g/mice/day. The
daily probiotic dose was 2×108 cfu/kg body weight per
day for 30-g mice. There was a significant difference between the
dietary mouse groups in final body weight or in body weight gain
during the experiment. A significant increase in body weight of
∼6.5 g was noted for the mice fed with a combination of vincristine
and L. fermentum (Lf3 + VCR + DMH + normal diet; Group 12),
whereas an increase in weight by ∼6 g was observed in the Lf3 + DMH
+ normal diet group (Group 4). There was a significant weight gain
observed in other groups ranging between 1 and 6 g. However, the
pre-carcinogenic group showed a marked increase in body weight,
while the post-carcinogenic group showed a relatively lower
increase in body weight (Table
I).
Effect of L. fermentum, L. plantarum and
vincristine on ammonia and activity of β glucosidase and β
glucuronidase in cecal content
The concentration of ammonia in the cecal contents
of the mice was significantly decreased upon the administration of
L. fermentum (250 mg, 19.36%) or L. plantarum (270
mg, 12.19%) or vincristine and combination of L. fermentum
(240 mg, 22.59%), L. plantarum (260 mg, 16.13%) and
vincristine (250 mg, 19.36%) (Fig.
1). Similarly, the activity of the enzyme β glucuronidase was
significantly decreased in all of the experimental groups in
comparison to the mice fed the control diet (normal diet + DMH;
Control 2) (Fig. 2).
Concentrations of ammonia and β glucuronidase activity (0.0027 IU,
56.46%) were lowest in the mice fed vincristine and L.
fermentum (Lf3 + VCR + DMH + normal diet; Group 12) (240 mg,
22.59%) (Fig. 2). A maximum
decrease in β glucosidase enzyme activity (0.0023 IU, 73.6%) was
recorded in mice fed the combination of L. plantarum (Lp1 +
VCR + DMH + normal diet; Group 14) (Fig. 2). Animals fed with L.
fermentum alone exhibited slightly smaller (250 mg, 19.36%)
reduction in ammonia concentration (0.0035 IU, 43.55%), a
relatively less decrease in β glucuronidase activity and a smaller
decrease (0.0025 IU, 70.4%) in β glucosidase enzyme activity. A
significant reduction in ammonia concentration, β glucuronidase
activity and β glucosidase enzyme activity was observed only in the
pre-carcinogen-induced probiotic groups, while a smaller decrease
was observed in the post-carcinogen-induced probiotic groups.
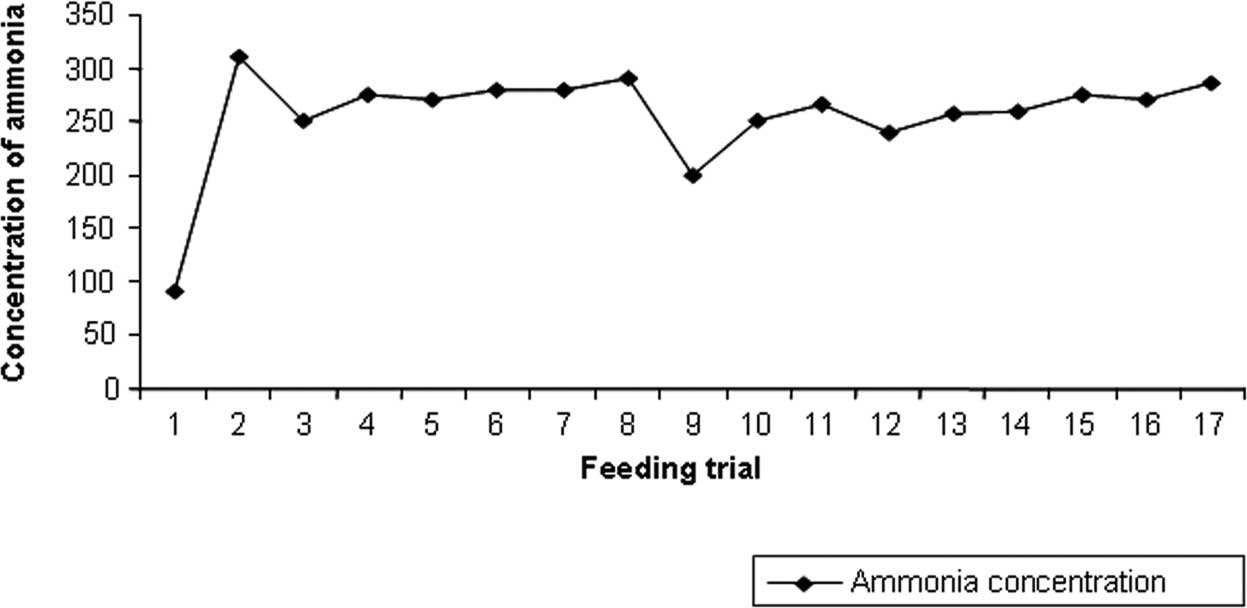 | Figure 1.Concentration of ammonia. 1, normal
diet (Control 1); 2, normal diet + DMH (Control 2); 3, Lf3 + DMH +
normal diet; 4, DMH + Lf3 + normal diet; 5, Lp1 + DMH + normal
diet; 6, DMH + Lp1 + normal diet; 7, Lf3 + Lp1 + VCR + DMH + normal
diet; 8, DMH + Lf3 + Lp1 + normal diet; 9, VCR + normal diet
(Control 3); 10, VCR + DMH + normal diet (Control 4); 11, DMH + VCR
+ normal diet (Control 5); 12, Lf3 + VCR + DMH + normal diet; 13,
DMH + Lf3 + VCR + normal diet; 14, Lp1 + VCR + DMH + normal diet;
15, DMH + Lp1 + VCR + normal diet; 16, Lf3 + Lp1 + DMH + normal
diet; 17, DMH + Lf3 + Lp1 + VCR + normal diet. |
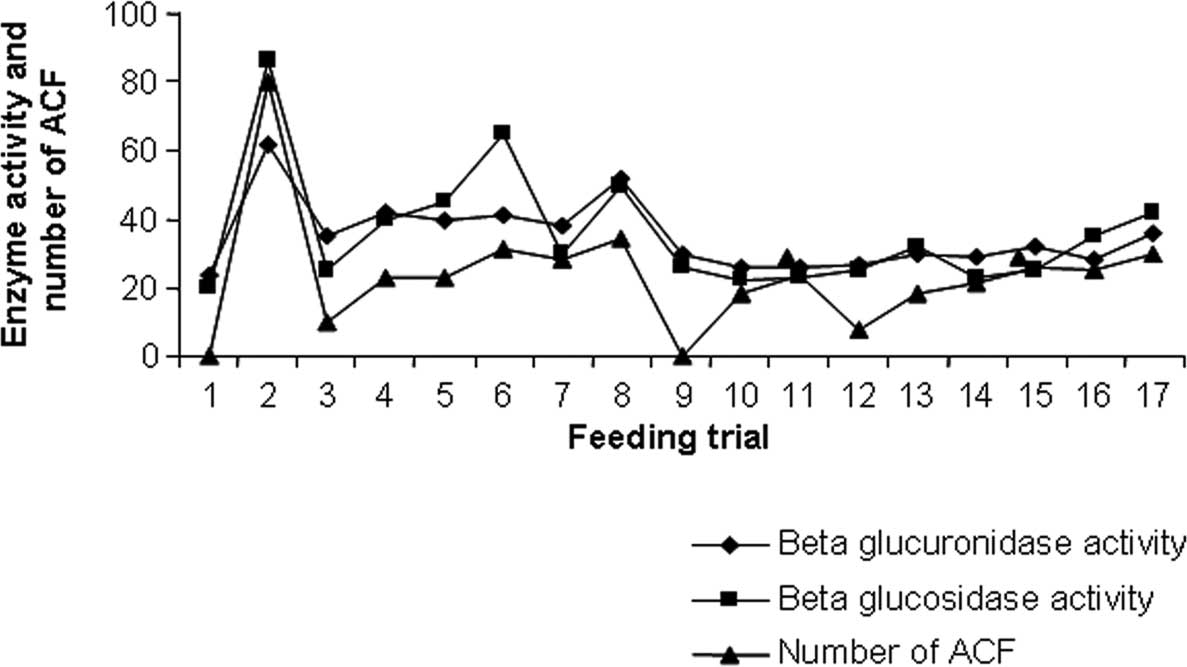 | Figure 2.Enzyme activity of β glucuronidase, β
glucosidase and number of ACF in experimental mice. 1, normal diet
(Control 1); 2, normal diet + DMH (Control 2); 3, Lf3 + DMH +
normal diet; 4, DMH + Lf3 + normal diet; 5, Lp1 + DMH + normal
diet; 6, DMH + Lp1 + normal diet; 7, Lf3 + Lp1 + VCR + DMH + normal
diet; 8, DMH + Lf3 + Lp1 + normal diet; 9, VCR + normal diet
(Control 3); 10, VCR + DMH + normal diet (Control 4); 11, DMH + VCR
+ normal diet (Control 5); 12, Lf3 + VCR + DMH + normal diet; 13,
DMH + Lf3 + VCR + normal diet; 14, Lp1 + VCR + DMH + normal diet;
15, DMH + Lp1 + VCR + normal diet; 16, Lf3 + Lp1 + DMH + normal
diet; 17, DMH + Lf3 + Lp1 + VCR + normal diet. |
Effect of L. fermentum, L. plantarum and
vincristine on ACF induction by 1,2-dimethylhydrazine
dihydrochloride
In the DMH-treated mice, induced ACF were mainly
distributed at the distal part of the colon. The number of aberrant
crypts per focus was significantly decreased by 10 (87.5%), 23
(71.25%) and 18 (77.5%) in the Lf3 + DMH + normal diet, Lp1 + DMH +
normal diet and VCR + DMH + normal diet subgroups, respectively, in
the pre-carcinogen-induced groups. However, the decrease in ACF was
not as significant in the post-carcinogen-induced groups (Figs. 3–5). No ACF were detected in mice fed a
normal diet in the control group that was not treated with a
carcinogen.
The most observable inhibitory effect on DMH-induced
ACF was found in the mice treated with the combination of L.
fermentum and vincristine (Lf3 + VCR + DMH + normal diet; Group
12), which decreased the total ACF number by (8 ACF) 90% in the
pre-carcinogen-induced group (Fig.
4). However, there was no significant reduction in the numbers
of ACF in the animals treated with L. fermentum, L.
plantarum and vincristine in the post-carcinogen-induced
groups.
Discussion
The present study was designed to assess the
potential of L. fermentum and L. plantarum in
preventing colorectal cancer and to study their synergistic impact
in combination with a conventional chemotherapeutic drug
vincristine. The Lactobacillus strains were isolated from
remote regions of Karnataka, India, where individuals are regular
consumers of curd, exhibiting longevity and disease endurance to
gastrointestinal disorders.
Swiss albino mice (2- to 4-weeks of age) were
administered DMH to produce CRC. The food intake was uniform,
although there was a significant difference in the different
experimental conditions. There was a marked weight gain in the
experimental animals fed Lf3 in the pre-cancer induction group.
Lactobacillus fermentum alone increased the body weight to
approximately 6 g (21%), while an increase to 6.5 g (23.21%) was
observed in group 12 fed a combination of Lf3 + VCR + DMH + normal
diet. A 14% weight gain was previously noted when L.
fermentum AD1 was administered for 4 days to 2-day-old Japanese
quill Coturnix coturnix japonica (21), and the weight gain was 0.39 g more
than that of the control group. A 23% increase upon administration
of Lf3 + VCR in the pre-cancerous group perhaps indicates the
colonization of Lf3 in the intestine. A significant increase in IgG
concentration and body weight was previously noted in 3- to
4-day-old male calves fed with L. acidophilus and L.
plantarum containing a basal diet during the 5th week (22).
Although a more rapid increase in body weight in Lactobacillus
sp.-fed female broiler chicks (Gallus gallus domesticus)
and ducks (Platyrhychos domestica) has been observed after
the second dose (9), in the
present study, a marked increase in body weight was recorded after
the first dose. Intravenous vincristine treatment delayed gastric
emptying and gastrointestinal motility transit of liquid in awake
rats and an increase in colonic weight was also reported (20). Therefore, in the present study the
synergistic effect of vincristine and the Lactobacillus
isolates was evident; particularly Lf3 was potent in increasing
body weight.
Ammonia concentration was significantly decreased in
the cecal contents of the mice fed L. fermentum/vincristine
(250 mg, 19.36%) and L. plantarum (270 mg, 12.19%). Mice fed
a combination of L. fermentum and vincristine (Lf3 + VCR +
DMH + normal diet; Group 12) showed a maximum reduction in ammonia
concentration (240 mg, 22.59%). The other experimental groups of
mice did not show a significant reduction in ammonia concentration,
while in the cecal contents of rats a significant decrease by
25–30% was previously reported after administration of inulin or
B. longum or both (20).
Although β glucuronidase activity was found to be decreased in
L. fermentum (0.0035 IU, 43.55%), L. plantarum
(0.0040 IU, 35.49%) and vincristine (0.0030 IU, 54%)-fed groups, a
maximum decrease in β glucuronidase activity was found in the mice
fed a combination of L. fermentum and vincristine [Lf3 + VCR
+ DMH + normal diet (0.0027 IU, 56.46%)]. However, β glucuronidase
activity was previously found to decrease to 55% in rats fed inulin
+ B. longum (20), which
perhaps indicates the potential of Lp1 in addition to Lf3
isolate.
β glucosidase activity was found to decrease in the
L. fermentum (0.0025 IU, 70.4%), L. plantarum (0.0045
IU, 46.68%) and vincristine (0.0025 IU, 70.94%)-fed group. A
maximum decrease in β glucosidase enzyme activity was found in mice
fed a combination of L. plantarum and vincristine [Lp1 + VCR
+ DMH + normal diet; Group 14 (0.0023 IU, 73.26%)]. By contrast, a
7-fold increase in rats fet inulin + B. longumcompared to
control rats was documented (20).
Therefore, the fact that Lp1 and vincristine combination decreased
the β glucosidase activity perhaps indicates the novelty of this
finding.
In the present study, L. fermentum (10 number
of ACF, 87.5%), L. plantarum (23 number of ACF, 71.25%) and
vincristine (18 number of ACF, 77.5%)-fed mice showed a decreased
ACF in DMH-induced carcinogenesis. A maximum reduction in ACF (8
number of crypts, 90%) was found in the mice fed a combination of
L. fermentum and vincristine [Lf3 + VCR + DMH + normal diet]
and with DMH-induced carcinogenesis. Bifidobacterium longum
and inulin were found to decrease the number of ACF in rats
injected with AOM by 29 and 21%, respectively, while the
combination of inulin with B. longum decreased the total ACF
by 74% (20). Although the
combination of L. rhamnosus and B. lactis along with
oligofructose inulin decreased the number of tumors in AOM-induced
4-to 5-week-old male rats (11),
the extent of decrease was not reported. Furthermore,
Lactobacillus salivarius was shown to be associated with a
reduced prevalence of colon cancer and mucosal inflammatory
activity (10). Vincristine in
combination with betulinic acid was also found to inhibit the
metastasis of lung tumor cells in C57BL/6 mice (17). Therefore, Lf3 isolate in
combination with vincristine may be a potential molecular complex
for combating CRC showing a 90% reduction in the number ACF.
Activity of β glucuronidase and the cecal ammonia concentration
appeared to follow a similar pattern as the inhibition of ACF.
These results suggest that the potential effects of
Lp1/Lf3/vincristine feeding would be beneficial only when it is fed
prior to induction of CRC carcinogen. However, the combination
treatment strongly inhibited the number of ACF and the effect
appeared to be more than additive. Administration of L.
fermentum, L. plantarum or vincristine decreased the
incidence in the colon of ACF, and combined treatment achieved a
level of inhibition of 90%, providing convincing evidence for a
symbiotic effect.
Acknowledgements
The authors thank the S.S. Institute
of Medical College Hospital, Davanagere, India, for providing the
animal laboratory facilities to conduct the experiments.
References
|
1.
|
Jacela JY, DeRouchey JM, Tokach MD, et al:
Feed additives for Swine: fact sheets – prebiotics and probiotics,
and phytogenetics. J Swine Health Prod. 18:132–136. 2010.
|
|
2.
|
Tomasik PJ and Tomasik P: Probiotics and
prebiotics. Cereal Chem. 80:113–117. 2003. View Article : Google Scholar
|
|
3.
|
Vanderhoof JA: Probiotics: future
directions. Am J Clin Nutr. 73:1152–1155. 2001.PubMed/NCBI
|
|
4.
|
Harish K and Varghese T: Probiotics in
humans – evidence based review. Calicut Med J. 4:211–223. 2006.
|
|
5.
|
Jiang T and Savaiano DA: In vitro lactose
fermentation by human colonic bacteria is modified by
Lactobacillus acidophilus supplementation. J Nutr.
127:1489–1495. 1997.PubMed/NCBI
|
|
6.
|
Rafter J: Lactic acid bacteria and cancer:
mechanistic perspective. Br J Nutr. 88:89–94. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Brady LJ, Gallaher DD and Busta FF: The
role of probiotic cultures in the prevention of colon cancer. J
Nutr. 130:410–414. 2000.PubMed/NCBI
|
|
8.
|
Panda AK, Reddy MR, Rao RSV, et al:
Production performance, serum/yolk cholesterol and immune
competence of white leghorn layers as influenced by dietary
supplementation with probiotic. Trop Anim Health Prod. 35:85–94.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Angelakis E and Raoult D: The increase of
Lactobacillus species in the gut flora of new born broiler
chicks and ducks is associated with weight gain. PLoS One. 5:1–5.
2010.
|
|
10.
|
Mahony LO, Feeney M, Halloran SO, et al:
Probiotic impact on microbial flora, inflammation and tumour
development in IL-10 knockout mice. Aliment Pharmacol Ther.
15:1219–1225. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Femia AP, Luceri C, Dolara P, et al:
Antitumorigenic activity of the prebiotic inulin enriched with
oligofructose in combination with the probiotic Lactobacillus
rhamnosus and Bifidobacterium lactis on
azoxymethane-induced colon carcinogenesis in rats. Carcinogenesis.
23:1953–1960. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Wollowski I, Rechkemmer G and Pool-Zobel
BL: Protective role of probiotics and prebiotics in colon cancer.
Am J Clin Nutr. 73:451–455. 2001.PubMed/NCBI
|
|
13.
|
Jung JJ, Jeung HC, Lee JO, et al: Putative
chemosensitive genes in colorectal cancer cell lines for anticancer
agents. Oncol Rep. 18:593–599. 2007.PubMed/NCBI
|
|
14.
|
Garodia P, Ichikawa H, Malani N, et al:
From ancient medicine to modern medicine: ayurvedic concepts of
health and their role in inflammation and cancer. J Soc Integr
Oncol. 5:1–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Nayak BS and Pereira LMP: Catharanthus
roseus flower extract has wound healing activity in Sprague
Dawley rats. BMC Complement Altern Med. 6:1–6. 2006. View Article : Google Scholar
|
|
16.
|
Junior AAP, Teles BCV, Castro EFB, et al:
Vincristine delays gastric emptying and gastro intestinal transit
of liquids in awake rats. Braz J Med Biol Res. 42:567–573. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Sawada N, Kataoka K, Kondo K, et al:
Betulinic acid augments the inhibitory effects of vincristine on
growth and lung metastasis of B16F10 melanoma cells in mice. Br J
Cancer. 90:1672–1678. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Thompson J, George EO, Poquette CA, et al:
Synergy of topotecan in combination with vincristine for treatment
of pediatric solid tumor xenografts. Clin Cancer Res. 5:3617–3631.
1999.PubMed/NCBI
|
|
19.
|
Asha, Gayathri D and Batish V: Molecular
characterization and variation of Lactobacillus sp. of
remote malnad regions of Karnataka, India. Adv Environ Bio.
6:481–486. 2012.
|
|
20.
|
Rowland IR, Rumney CJ, Coutts JT, et al:
Effect of Bifidobacterium longum and inulin on gut bacterial
metabolism and carcinogen-induced aberrant crypt foci in rats.
Carcinogenesis. 19:281–285. 1998.
|
|
21.
|
Strompfova V, Marcinakova M, Gancarcikova
S, et al: New probiotic strain Lactobacillus fermentum AD1
and its effect in Japanese quail. Vet Med Czech. 50:415–420.
2005.
|