Introduction
Asthma is a chronic airway inflammatory disease
characterized by inflammation, airway hyperresponsiveness (AHR),
reversible airway obstruction and elevated production of cytokines
(1). AHR is mainly caused by lung
inflammatory responses in asthma. Eosinophil (Eos) and T-lymphocyte
(CD4+) infiltration and the increased secretion of
inflammatory mediators during asthma adversely affect the large and
small airways (2). In particular,
Th2 cells play a critical role in initiating and sustaining
asthmatic inflammation (3).
Increased numbers of activated T cells are found in the peripheral
blood of patients during acute episodes of asthma (4).
CD44-hyaluronate interactions are able to promote
extravasation and egress of antigen-activated lymphocytes in
inflamed vascular beds (5).
Furthermore, Katoh et al demonstrated that CD44 plays an
important role in the accumulation of T helper type 2 (Th2) cells
in the airways of mice (6). CD44
expressed on CD4+ T cells plays a critical role in the
accumulation of antigen-specific Th2 cells in the development of
AHR induced by antigen challenge in the airways (7).
Adhesion molecule CD44 is a type 1 cell surface
transmembrane glycoprotein which is encoded on the short arm of
chromosome 11 (8). The CD44 family
contains two types of CD44: a standard form (CD44s) and variant
isoforms (CD44v).
The genetic sequence is composed of two groups of
exons. One group comprising exons 1–5 and 16–20 is expressed
together on all cell types as the standard form. The non-variant
exon mRNA encoded isoform has been termed CD44s. The 9 variable
exons v2–v10 (exons 7–15, vl was isolated in rats and is not
present in humans) may be alternatively spliced and included within
the standard exons at an insertion site between exons 5 and 16,
which code for a variety of proteins by selecting certain exons
within the sequence - for example, CD44v can contain one or more
variant regions, such as CD44v3 or CD44v4–7.
Although the specific functions of CD44v remain
unclear in humans, CD44 and its many variant isoforms were
demonstrated to exert some of their functions through docking OPN
and growth factors to their cognate cell surface receptors or
substrates (9–11).
It was found that there is a significant increase in
the expression of anti-apoptotic Bcl-2 protein and a decrease in
the expression of pro-apoptotic protein in asthmatic patients when
compared to normal individuals. Thus, inhibition of apoptosis may
be one of the reasons for the chronic persistent inflammation in
the airways of asthmatic patients (12). This may result in the increase in
memory T cells. It has been demonstrated that CD45RO+
may provide pro-inflammatory signals that contribute to the
persistent airway inflammation of asthma (13).
The CD44v also promote ECM-derived survival signals
mediated through integrin activation through OPN-CD44V interaction
(14). As such, CD44v should play
a vital role in the pathogenesis of asthma and there may even be
some CD44v uniquely expressed on the peripheral blood lymphocyte
cells of asthmatic patients. The aim of this study was to determine
whether there are specific CD44 isoforms expressed on peripheral
blood lymphocytes of asthmatic patients compared with normal
individuals and pneumonia patients.
Patients and methods
Subjects
We collected blood samples from individuals (103
normal, 165 with asthma and 104 with pneumonia) who presented to
The 4th Affiliated Hospital of Harbin Medical University from March
2010 to May 2011. The normal controls had no history of asthma,
other allergic diseases or AHR. They had normal total IgE values
and normal lung function tests.
We enrolled patients with asthma who were diagnosed
on the basis of a history of dyspnea and wheezing during the
previous 12 months and lung function tests >12% FEV1 following
β2-agonist inhalation. Patients with pneumonia were recruited on
the basis of definite clinical diagnosis by chest X-ray and/or
computerized tomography, which revealed bilateral diffused
pulmonary infiltrations.
A routine blood test (including eosinophil,
neutrophil, monocyte and lymphocyte counts) was performed for each
participant. Approval of the ethics committee of our institution
was obtained for this study. Written informed consent was obtained
from all participants.
Lymphocyte isolation
Whole blood samples (10 ml) were drawn from the
antecubital vein for lymphocyte isolation, and mononuclear cells
were isolated by gradient centrifugation on Histopaque-1083 (Sigma,
St. Louis, MO, USA) according to the manufacturer’s instructions.
The unadhered lymphocytes were harvested. Centrifugation was
performed at 600 × g for 15 min at room temperature. Following
centrifugation, the cells were washed twice. The isolated
lymphocytes were confirmed by flow cytometry with fluorescently
labeled antibodies and stained with CD3 and CD19 (BD Pharmigen, San
Diego, CA, USA). The lymphocytes used in our experiments had a
purity of >90%.
Reverse transcriptase and nested PCR for
specific CD44v on lymphocytes
Total RNA was extracted from the isolated
lymphocytes. TRIzol reagent was used according to the
manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA) and
then RNA was reverse transcribed into cDNA using M-MLV Reverse
Transcriptase (Promega Corporation, Madison, WI, USA).
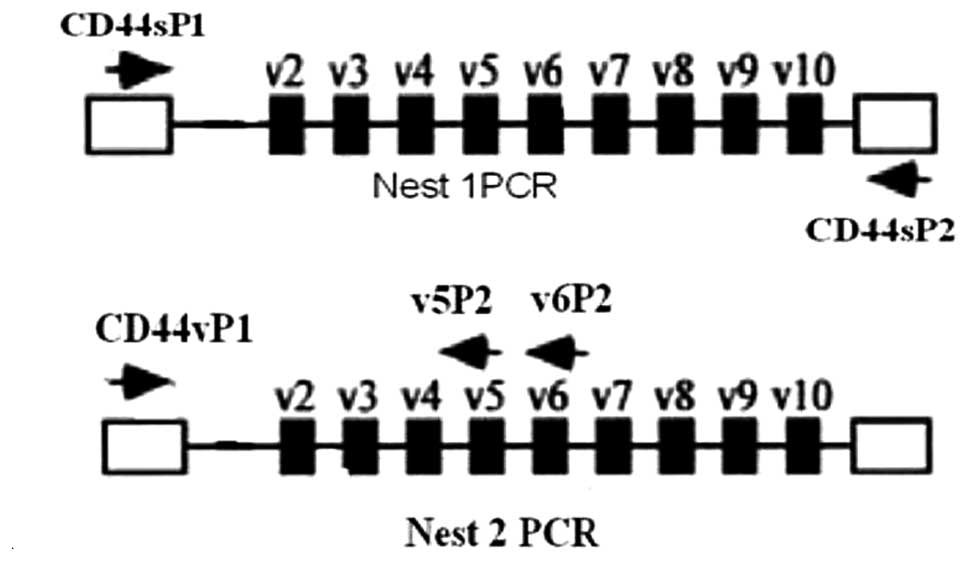
Nest 1
Plasmodium genus-specific PCR amplification was
performed in 25 μl reaction mixtures, 0.25 μM of each primer
(primer for CD44s), 1.25 unit Taq DNA polymerase (Promega), 2 μl of
cDNA template, and the final volume was adjusted to 25 μl with
deionized water. The cycling conditions of nest 1 PCR amplification
of total CD44 isoforms were as follows: initial denaturation at
95°C for 3 min, 10 cycles of denaturation at 95°C for 30 sec,
annealing at 59°C for 30 sec, and extension at 72°C for 40 sec,
followed by final extension at 72°C for 7 min.
Nest 2
Two microliters of the nest 1 diluted amplification
product (diluted to 50 times with deionized water) was used as the
template DNA in the nest 2 PCR amplification. The concentration of
the constituents and nest 2 primers were similar to nest 1 for the
annealing temperature which was 57°C for CD44v specific primers
(CD44v5 and CD44v6) and PCR amplification for specific primers
consisted of 30 cycles (Fig. 1).
The sequences of the specific primers are presented in Table I.
 | Table I.Sequences of the specific primers. |
Table I.
Sequences of the specific primers.
| Gene | Primer sequence | Length (bp) |
|---|
| CD44s (Nest1) | | |
| P1 5′-3′ |
AAGACATCTACCCCAGCAACCC | 329 |
| P2 5′-3′ |
TGCAGTAACTCCAAAGGACCCA | |
| CD44v5 (Nest2) | | |
| P1 5′-3′ |
CTGAAGACATCTACCCCAGCAAC | 206 |
| P2 5′-3′ |
ATAAGCAGTGGTGCCATTTCTG | |
| CD44v6 (Nest2) | | |
| P1 5′-3′ |
CTGAAGACATCTACCCCAGCAAC | 239 |
| P2 5′-3′ |
TTGCCAAACCACTGTTCCTTC | |
| GAPDH (qPCR) | | |
| P1 5′-3′ |
AAGGTCGGAGTCAACGGATTTGG | 239 |
| P2 5′-3′ |
TTGGAGGGATCTCGCTCCTGGAA | |
The nest 2 PCR amplification products were analyzed
by gel electrophoresis on a 2% agarose gel and visualized by
staining with ethidium bromide (4 μg/ml) and ultraviolet
transillumination.
The levels of CD44s and GAPDH mRNA were measured by
quantitative RT-PCR using the SYBR-Green PCR Core reagents kit
(Applied Biosystems, USA) and specific primers on a DA7600 PCR
amplifier (DAAN, China). The CD44 (CD44s, CD44v5 and CD44v6)
primers were designed according to the reported gene sequences of
Homo sapiens CD44 molecule, transcript variant 1, mRNA
(NM_000610.3) and GAPDH (NM_002046.3). The sequences of the
specific primers are presented in Table I.
The PCR reactions (50 ml/tube, in duplicate) were
denatured at 95°C for 2 min and subjected to 30 cycles of 95°C for
30 sec, 57°C for 30 sec and 72°C for 30 sec. The relative levels of
mRNA transcripts were analyzed by normalizing the values of
individual samples to GAPDH.
Data analysis
The clinical parameters for IgE were converted into
log-based values in order to produce a normal distribution for the
statistical analyses, and then the data were analyzed using the
Mann-Whitney U-test. The χ2 test was used to examine the
difference in clinical indices among the three groups. The
demographic variables of the subjects (for example, gender and age)
were analyzed by linear regression in order to determine whether or
not the expression of CD44v was correlated with the clinical
features. Values are expressed as the means ± SD. The expression of
CD44v among the three groups was statistically compared using
χ2 test and Student-Newman-Keuls. Data were analyzed
using the SAS 9.1 statistical program. A p-value <0.05 was
considered to indicate statistical significance.
Results
We divided the subjects into three groups: 113
normal subjects, 165 subjects with asthma and 104 subjects with
pneumonia. We compared the gender, age and blood indices among the
three groups, finding no differences in gender and age. The blood
examination results were different in the three groups. The Eos and
the IgE levels were significantly higher in the asthmatic patients
than the levels in the other groups (p<0.001, compared with the
normal and pneumonia groups). In the normal group, the lymphocyte
count was slightly lower than the count in the other groups
(p<0.05), and the neutrophil count in the pneumonia group was
obviously higher than that in the other groups (p<0.001)
(Table II).
 | Table II.Clinical characteristics of the study
participants. |
Table II.
Clinical characteristics of the study
participants.
| Clinical
parameters | Normal | Asthmatic | Pneumonia |
|---|
| Gender (M/F) | 59/54 | 71/94 | 56/48 |
| Age (years) | 45.4±13.5 | 43.4±12.1 | 51.7±10.6 |
| Eos
(×109) | 0.31±0.22 | 5.21±2.15a | 0.24±0.17 |
| Mono
(×109) | 0.28±0.03 | 0.31±0.03 | 0.56±0.04 |
| Lym
(×109) | 2.4±0.33 | 3.1±0.54b | 3.5±0.61b |
| Neu
(×109) | 3.6±1.42 | 3.4±2.11 | 9.6±2.33a |
| Log IgE
(IU/ml−1) | 0.7±0.09 | 2.1±0.32a | 0.65±0.11 |
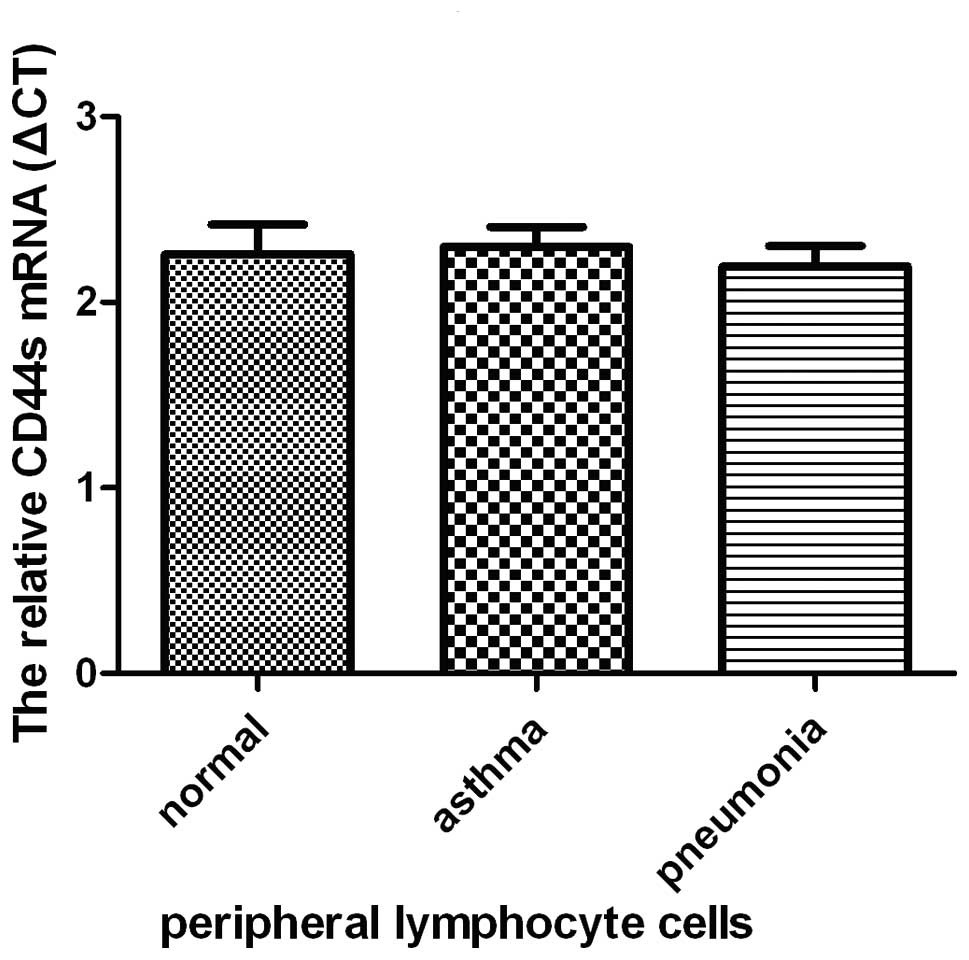
From the results of the nested PCR of the peripheral
lymphocyte cells of the subjects, we demonstrated that expression
levels of CD44s, CD44v5, and CD44v6 differed among the normal,
asthmatic and pneumonia groups. The level of CD44s expression was
not significantly difference among the normal, asthmatic and
pneumonia groups as determined by quantitative real-time PCR
(Fig. 2). However, expression of
CD44v was clearly different (Table
III). CD44v5 was expressed in 55.2% (91/165) of asthma patients
which was significantly higher than the other groups. The
expression level of CD44s was identical in the three groups. CD44v5
expression was found to be independent of the levels of CD44s. We
also investigated the expression of other CD44v (data not shown),
but the results did not reveal any significant difference among the
groups.
 | Table III.Expression of CD44 isoforms on
peripheral blood lymphocyte cells in normal, asthmatic and
pneumonia patients. |
Table III.
Expression of CD44 isoforms on
peripheral blood lymphocyte cells in normal, asthmatic and
pneumonia patients.
| n |
CD44v5+ |
CD44v6+ |
|---|
| Normal | 113 | 24 | 26 |
| Asthmatic | 165 | 91 | 23 |
| Pneumonia | 104 | 27 | 67 |
In contrast, in the normal group only 21.2% (24/113)
of individuals expressed CD44v5, and a low percentage of 23.0%
(26/113) also expressed CD44v6. The data revealed that 26.0%
(27/104) of the pneumonia group weakly expressed CD44v5, while a
high proportion of 64.4% (67/104) patients expressed CD44v6. The
high expression of CD44v6 appears to be independent of CD44s.
The χ2 tests demonstrated that CD44v was
differentially expressed among the three groups
(χ2=117.710, p<0.0001). Student-Newman-Keuls tests
were performed, which indicated that the asthmatic patients had a
significantly higher CD44v5 expression than the other groups
(p<0.001), and CD44v6 expression was highly expressed in the
subjects with pneumonia (p<0.001).
The data were analyzed by multiple logistic
regression, and correlations between age and gender and the
expression of CD44s, CD44v5, and CD44v6 were not found. Regression
analysis revealed that CD44v5 expression was not significantly
correlated with blood indices such as Eos, neutrophil and
lymphocyte counts, while a positive correlation with IgE level
(p=0.032) was noted in the asthma group, indicating that the
expression of CD44v5 is correlated with the extent of the allergic
state. CD44v6 was significantly positively correlated with the
neutrophil count (p<0.05). The reason for this observation is
unclear, but it may be explained by the CD44v6 expression of T
cells inducing Th1 inflammation in pneumonia.
Discussion
According to our data, CD44v5 was found to be
expressed on the T cells of asthmatic patients and CD44v6 was
expressed in patients with pneumonia. The expression of CD44v5 was
positively correlated with the IgE level. These data have not
previously been reported. Although the reason for these
observations are still unknown, it may be suggested that CD44v5
expression correlates with the extent of the allergic state. CD44v5
expressed on T cells may participate in Th2 polarization or may
enhance Th2 cytokine secretion. Certainly there is a need to
confirm our findings with further research.
There are three possible explanations for the
differential expression of CD44 in peripheral lymphocyte cells
between asthmatic and pneumonia patients. First, they may play
varying roles in the T cell activation of the Th1/Th2 condition
respectively. Hegde et al (15) found that mobilization of CD44 in
allogeneic dendritic cell-T cell immunological synapse plays a key
role in T cell activation. Upon TCR engagement, TCR and important
signaling molecules such as Lck, Fyn, PKC, phospholipase C and
linker for activation of T cells were found to be recruited to the
raft aggregates at the T cell-APC contact (16,17).
CD44 has been shown to partition into lipid rafts (18), associated, activated tyrosine
kinases p59fyn and p56lck (19,20),
but there should be different signal transduction pathways among
the various isoforms.
The present controversy among studies may be due to
their omitting the various CD44 isoforms which could play a variety
of roles in the different immunoreactions.
Secondly, CD44 as an adhesion molecule should take
part in the T cell infiltration into the lung and the Th2 memory T
cell resident in the lung. The adhesion between inflammatory cell
and bronchial smooth muscle (BSM) cell types may contribute to
long-term effects leading to hyperresponsiveness and airway
remodeling but the mechanism by which this occurs remains unknown.
Therefore, CD44v5 may be the specific isoform which interacts with
the other adhesion molecules on BSM lung tissue to finally induce T
cell infiltration.
Thirdly, it is well known that subpopulations of
allergen-specific memory Th2 cells are generated during an immune
response to allergens. Following recovery from allergic asthma,
allergen-specific memory Th2 cells reside within the lungs of mice
for their remaining life span rather than going into programmed
cell death. Upon specific allergen exposure, these
allergen-specific Th2-skewed memory T cells rapidly induce the Th2
inflammation (including eosinophilic mucus production, airway
hyperresponsiveness and allergen-specific IgE production) (21). The Th2 cells do not undergo
apoptosis following immunoreaction, which is the main cause for the
elevation of Th2-skewed memory T cells. Fas is a major trigger for
apoptosis, especially in activated immune cells. There is evidence
that the CD44v region may be involved in apoptosis (22).
Mielgo et al (23) demonstrated that CD44v6 and v9
colocalize and interact with Fas in the presence or absence of
FasL. Based on these findings, we propose a model in which CD44v
interacts extracellularly with Fas, preventing FasL binding and
consequently Fas death signaling in the Jurkat cell line. The
CD44v5 expressed on the peripheral lymphocyte cells of asthma
patients may be an anti-apoptosis molecule; by CD44v5 interacting
extracellularly with Fas, this reduced the Th2 cell skewed into
memory T cells.
In this study, we demonstrated that 64.4% of the
pneumonia patients expressed CD44v6. Previous studies demonstrated
that CD44v7 is expressed on T cells and macrophages in T-helper-1
(Th1)-mediated chronic inflammation and autoimmune diseases
(24). CD44v may also have a
ligation with OPN that induces an inside-out signaling transduced
through Src, leading to integrin activation, which in turn
facilitates cell adhesion and enhances matrix survival signaling
(25).
In the present study, the normal subjects also
expressed CD44v5 and CD44v6. This may be due to individual
differences and the normal subjects we enrolled may not have been
in perfect health. Equally we could not guarantee that the asthma
and pneumonia patients did not have co-morbidities. Following this
study, we will proceed to verify our findings in an animal model
and investigate the mechanism of CD44v in asthma.
CD44v5 was expressed by a higher proportion of
asthmatic patients than the other subjects and should also play an
important role in the pathogenesis of asthma, which may provide a
new target for diagnosis of asthma and shed light on the mechanism
of asthma. This finding opens new strategies for therapeutic
intervention in asthma.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (No. 81171657).
References
|
1
|
Bochner BS, Undem BJ and Lichtenstein LM:
Immunological aspects of allergic asthma. Annu Rev Immunol.
12:2951994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fabbri LM, Romagnoli M, Corbetta L, et al:
Differences in airway inflammation in patients with fixed airflow
obstruction due to asthma or chronic obstructive pulmonary disease.
Am J Respir Crit Care Med. 167:418–424. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Robinson DS, Hamid Q, Ying S, Tsicopoulos
A, Barkans J, et al: Predominant TH2-like bronchoalveolar
T-lymphocyte population in atopic asthma. N Engl J Med.
326:2981992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shalaby KH and Martin JG: Overview of
asthma: the place of the T cell. Curr Opin Pharmacol. 10:218–225.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
DeGrendele HC, Estess P and Siegelman MH:
Requirement for CD44 in activated T cell extravasation into an
inflammatory site. Science. 278:672–675. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Katoh S, Ishii N, Nobumoto A, et al:
Galectin-9 inhibits CD44-hyaluronan interaction and suppresses a
murine model of allergic asthma. Am J Respir Crit Care Med.
176:27–35. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Katoh S, Kaminuma O, Hiroi T, et al: CD44
is critical for airway accumulation of antigen-specific Th2, but
not Th1, cells induced by antigen challenge in mice. Eur J Immunol.
41:3198–3207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goodfellow PN, Banting G, Wiles MV, et al:
The gene, MIC4, which controls expression of the antigen defined
bymonoclonal antibody F10.44.2 is on human chromosome 11. Eur J
Immunol. 12:659–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ponta H, Sherman L and Herrlich PA: CD44:
from adhesion molecules to signalling regulators. Nat Rev Mol Cell
Biol. 4:33–45. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Denhardt DT, Noda M, O’Regan AW, et al:
Osteopontin as a means to cope with environmental insults:
regulation of inflammation, tissue remodeling, and cell survival. J
Clin Invest. 107:1055–1061. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bennett KL, Jackson DG, Simon JC, et al:
CD44 isoforms containing exon v3 are responsible for the
presentation of heparin binding growth factor. J Cell Biol.
128:687–698. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xue J, Xu Y and Zhang Z: Lymphocyte
apoptosis in asthmatic patients and its molecular mechanism.
Zhonghua Jie He He Hu Xi Za Zhi. 22:555–557. 1999.(In Chinese).
|
|
13
|
Lamb JP, James A, Carroll N, et al:
Reduced apoptosis of memory T-cells in the inner airway wall of
mild and severe asthma. Eur Respir J. 26:265–270. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JL, Wang MJ, Sudhir PR, et al:
Osteopontin promotes integrin activation through outside-in and
inside-out mechanisms: OPN-CD44V interaction enhances survival in
gastrointestinal cancer cells. 67:2089–2097. 2007.
|
|
15
|
Hegde VL, Singh NP, Prakash S, et al: CD44
mobilization in allogeneic dendritic cell-T cell immunological
synapse plays a key role in T cell activation. J Leukoc Biol.
84:134–142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xavier R, Brennan T, Li Q, et al: Membrane
compartmentation is required for efficient T cell activation.
Immunity. 8:723–732. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Viola A, Schroeder S, Sakakibara Y, et al:
T lymphocyte costimulation mediated by reorganization of membrane
microdomains. Science. 283:680–682. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Neame SJ, Uff CR, Sheikh H, et al: CD44
exhibits a cell type dependent interaction with Triton X-100
insoluble, lipid rich, plasma membrane domains. J Cell Sci.
108:3127–3135. 1995.PubMed/NCBI
|
|
19
|
Ilangumaran S, Briol A and Hoessli DC:
CD44 selectively associates with active Src family protein tyrosine
kinases Lck and Fyn in glycosphingolipid-rich plasma membrane
domains of human peripheral blood lymphocytes. Blood. 91:3901–3908.
1998.
|
|
20
|
Foger N, Marhaba R and Zoller M:
Involvement of CD44 in cytoskeleton rearrangement and raft
reorganization in T cells. J Cell Sci. 114:1169–1178.
2001.PubMed/NCBI
|
|
21
|
Mojtabavi N, Dekan G, Stingl G and Epstein
MM: Long-lived Th2 memory in experimental allergic asthma. J
Immunol. 169:4788–4796. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Günthert U and Johansson B: CD44 – a
protein family involved in autoimmune diseases and apoptosis.
Immunologist. 8:106–109. 2001.
|
|
23
|
Mielgo A, van Driel M, Bloem A, et al: A
novel antiapoptotic mechanism based on interference of Fas
signaling by CD44 variant isoforms. Cell Death Differ. 13:465–477.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wittig BM, Johansson B, Zoller M, et al:
Abrogation of experimental colitis correlates with increased
apoptosis in mice deficient for CD44v7. J Exp Med. 191:2053–2063.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hoffmann U, Heilmann K, Hayford C, et al:
CD44v7 ligation downregulates the inflammatory immune response in
Crohn’s disease patients by apoptosis induction in mononuclear
cells from the lamina propria. Cell Death Differ. 14:1542–1551.
2007.PubMed/NCBI
|