Introduction
Scorpion venom contains various groups of compounds
that exhibit a wide range of biological properties and actions in
cells. The general composition and expression level of scorpion
venom depends on genetic variation and geographical environment
(1,2). The scorpion Buthus martensii
Karsch (BmK) and its products have been used as a traditional
Chinese medicine for thousands of years. Traditional healers use
scorpions to treat various types of condition, such as epilepsy,
rheumatism and cancer. It has previously been reported that crude
scorpion venom or isolated peptides from scorpion venom may inhibit
the proliferation of cancer cells and induce cell apoptosis
(3,4). However, the antitumor molecular
mechanisms are poorly understood.
Nuclear factor κB (NF-κB) is an important
transcription factor, which plays a part in many cellular
activities such as proliferation and activation of immunocytes,
development of T and B lymphocytes and cell apoptosis (5). However, substantial evidence also
indicates that NF-κB plays a pivotal role in the onset and
development of malignancies. Recent observations have shown that
there is a close relationship between NF-κB and hematopoietic
malignancies such as leukemia, lymphoma and multiple myeloma
(6,7), as aberrant activation of the NF-κB
pathway is involved in the pathogenesis of these diseases.
Moreover, some studies have suggested that blocking the NF-κB
signaling pathway can cause tumor cells to cease proliferation,
die, or become more sensitive to the action of antitumor agents
(7). The NF-κB signaling pathway
has therefore become a promising target for cancer therapy. In the
present study, we attempted to elucidate the antiproliferation and
cell cycle arresting properties of scorpion venom component III
(SVCIII) from BmK venom and its effects on the NF-κB signaling
pathway in human leukemic cell line Jurkat and THP-1 cells.
Materials and methods
Chemicals
RPMI-1640 medium and fetal bovine serum (FBS) were
purchased from Gibco-BRL (Carlsbad, CA, USA). TransFast™
Transfection Reagent was obtained from Promega Corporation
(Madison, WI, USA). NF-κB luciferase reporter plasmid was a gift
from Dr Luan Haojiang (US National Institutes of Health).
Antibodies to cyclin D1, IκBα and p-IκBα were purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Nuclear extract kit
was purchased from Active Motif (Carlsbad, CA, USA).
Chemiluminescent electrophoretic mobility shift assay (EMSA) kit
was purchased from Beyotime Institute of Biotechnology (Nantong,
China). All other reagents used in the study were of analytical
grade and purchased locally.
Scorpion venom
BmK venom was extracted by mild electrical
stimulation of the telsons and dissolved in 0.02 M phosphate
buffer, pH 7.2, and centrifuged at 10,000 x g for 15 min at 4°C.
Gel chromatography was utilized to isolate partial peptide
fractions from crude scorpion venom. Seven fractions were obtained
and named scorpion venom components (SVC)I, II, III, IV, V, VI,
VII, respectively. The molecular weight of SVCIII was calculated to
be approximately 70–80 kDa through comparison with protein markers
of known molecular weights run in a 12% SDS-PAGE.
Cell culture and treatments
The THP-1 (human acute monocytic leukemia) cell line
was provided by the Southern Medical University, and the Jurkat
(human T lymphoma) cell line was obtained from the American Type
Culture Collection (Manassas, VA, USA). Cells were grown in 50-ml
plastic flasks in RPMI-1640 medium containing 10% heat-inactivated
fetal bovine serum (FBS), 100 μg/ml streptomycin, and 100
U/ml penicillin and incubated in a 5% CO2 humidity
incubator at 37°C. The medium was refreshed three times a week.
Cells in log phase were seeded in sterile 6-, 24- or 96-well plates
with a fixed number in each well and then treated with varying
amounts of SVCIII for 48 h.
Cell viability assay by MTT
Cell viability was determined by MTT assay. Cells
were seeded in a 96-well plate at a density of 1x105
cells per well and treated with various concentrations (0, 1, 5,
10, 20, 30, 40 and 50 μg/ml) of SVCIII for 48 h. MTT dye was
added to each well for the last 4 h of treatment. When purple
precipitates were visible, the medium was carefully discarded. The
formazan crystals were dissolved by adding 200 μl of
dimethyl sulfoxide to each well. The cell viability index was
calculated by measuring the absorbance value at 570 nm.
Flow cytometry for cell cycle
analysis
A cell cycle assay was performed using propidium
iodide (PI) staining of the nuclei. Following treatment for 48 h
with SVCIII, cells were fixed in 70% cold alcohol overnight and
then centrifuged. The pellet was re-suspended in 500 μl PI
staining buffer (250 μg/ml PI, 10 μg/ml RNase in PBS)
in a dark room for 30 min at room temperature and analyzed with a
flow cytometer. For each measurement, at least 10,000 cells were
counted.
NF-κB luciferase reporter luciferase
assay
To determine the effect of SVCIII on NF-κB
activation, cells were transiently transfected with a NF-κB
luciferase reporter plasmid. Cells were seeded in 24-well plates
(105/well) and transfected with 0.5 μg of a NF-κB
luciferase reporter plasmid or pGL3 basic as a negative control
using TransFast™ Transfection Reagent according to the
manufacturer’s instructions and co-transfected with 40 ng of pRL-TK
Renilla luciferase vector to control transfection
efficiency. Transfected cells were exposed to SVCIII for 6 h. Cells
were then harvested and lysed according to the manufacturer’s
instructions. Supernatants were analyzed for firefly and
Renilla luciferase activity using the dual-luciferase
reporter assay system.
EMSA
To assess NF-κB activation, EMSA was performed
according to the manufacturer’s instructions for the
Chemiluminescent EMSA Kit. Biotin-labeled double-stranded
oligonucleotides were used which included commercially available
consensus NF-κB gel shift oligonucleotide
5′-biotin-AGTTGAGGGGACTTTCCCAGG-3′. Specific binding was confirmed
by competition experiments with a 100-fold excess of unlabeled or
mutated oligonucleotides. The bands were detected by enhanced
chemiluminescent (ECL) assay kit.
Cell extracts and western blotting
Nuclear extracts were isolated using a nuclear
extract kit. Cells were briefly washed twice with ice-cold
PBS/phosphatase inhibitors and incubated in 500 μl of
hypotonic buffer for 15 min on ice. Subsequently, 25 μl
detergent was added and the cells were vortexed at the highest
setting and centrifuge suspended for 30 sec at 14,000 x g at 4°C.
Nuclei were washed with 50 μl complete lysis buffer and
vortexed for 10 sec at the highest setting. Thereafter the lysate
was incubated for 30 min on ice and centrifuged for 10 min at
14,000 x g. Protein concentrations were determined using the
Bradford assay. Proteins were resolved by 12% SDS-PAGE gels,
transferred onto a PVDF membrane and subjected to western blot
analysis using anti-cyclin D1, IκBα and p-IκBα antibody. Proteins
were visualized with an ECL assay kit according to the
manufacturer’s instructions.
Statistical analysis
Data are presented as mean ± S.D. and one-way
analysis of variance was used to identify significant differences
among the results. Statistical significance was defined as
P<0.05.
Results
Effect of SVCIII on cell viability
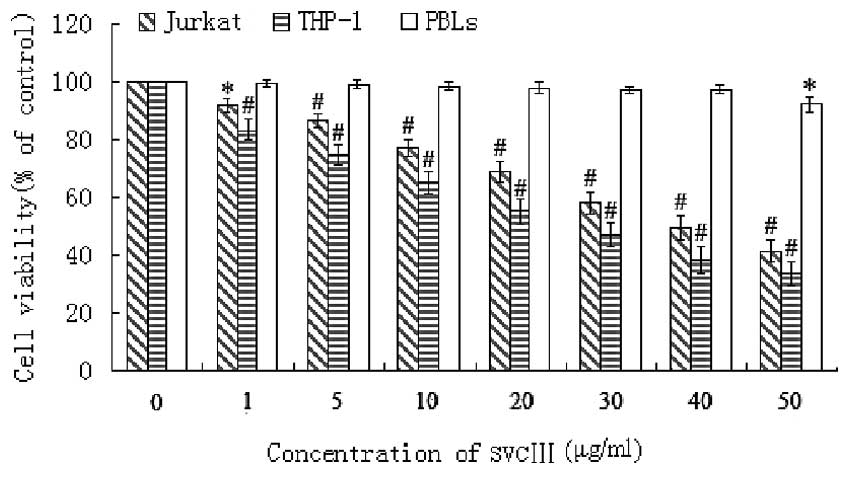
MTT assay was used to determine the effect of SVCIII
on cell viability. As shown in Fig.
1, the cell viability of THP-1 and Jurkat cells was decreased
by SVCIII in a dose-dependent manner. The percentage of viable
THP-1 cells following treatment with 1, 5, 10, 20, 30, 40 and 50
μg/ml of SVCIII decreased to 83.4, 74.7, 65.3, 55.0, 46.9,
38.2 and 33.4% respectively. Viability of Jurkat cells after
exposure to increasing concentrations of SVCIII was reduced to
91.9, 86.7, 77.3, 68.8, 58.2, 49.4 and 41.3% respectively (Fig. 1). However, there were no
significant differences in the human peripheral blood lymphocytes
(PBLs) between SVCIII-treated cells and controls. The
IC50 value was calculated to be 29 μg/ml for
THP-1 and 39.6 μg/ml for Jurkat. The concentrations 1/2
IC50 and IC50 were used to investigate the
effects of venom in subsequent experiments.
Effect of SVCIII on cell cycle
distribution
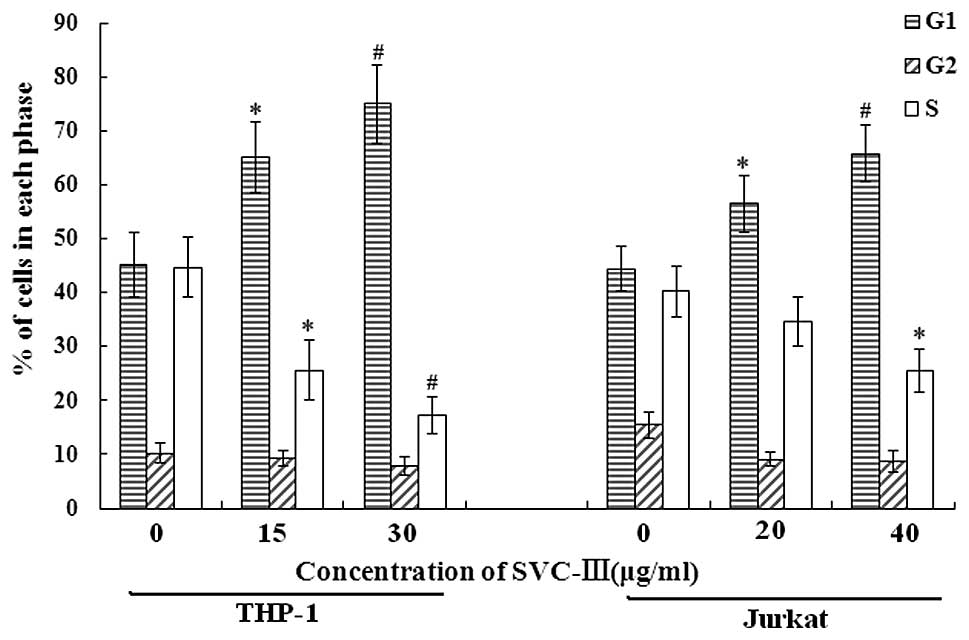
We then tested the effect of SVCIII on cell cycle
distribution using flow cytometry. A dose-dependent increase in the
G1 phase cell population was observed from 45.1% in controls to
65.1 and 74.9%, respectively, due to SVCIII treatment at 1/2
IC50 (15 μg/ml) and IC50 (30
μg/ml) concentration for THP-1, and from 44.4% in controls
to 56.4 and 65.7%, respectively, due to SVCIII treatment at 1/2
IC50 (20 μg/ml) and IC50 (40
μg/ml) concentration for Jurkat. A decrease in the S phase
cell population was observed from 44.7% in controls to 25.6 and
17.3%, respectively, due to SVCIII treatment at 1/2 IC50
(15 μg/ml) and IC50 (30 μg/ml)
concentration for THP-1, and from 40.2% in controls to 34.6 and
25.6%, respectively, due to SVCIII treatment at 1/2 IC50
(20 μg/ml) and IC50 (40 μg/ml)
concentration for Jurkat (Fig. 2).
These results indicate that SVCIII inhibits cell growth through
arrest at G1 phase and reduces transition to the S and G2/M phases
of the cell cycle in both THP-1 and Jurkat cells.
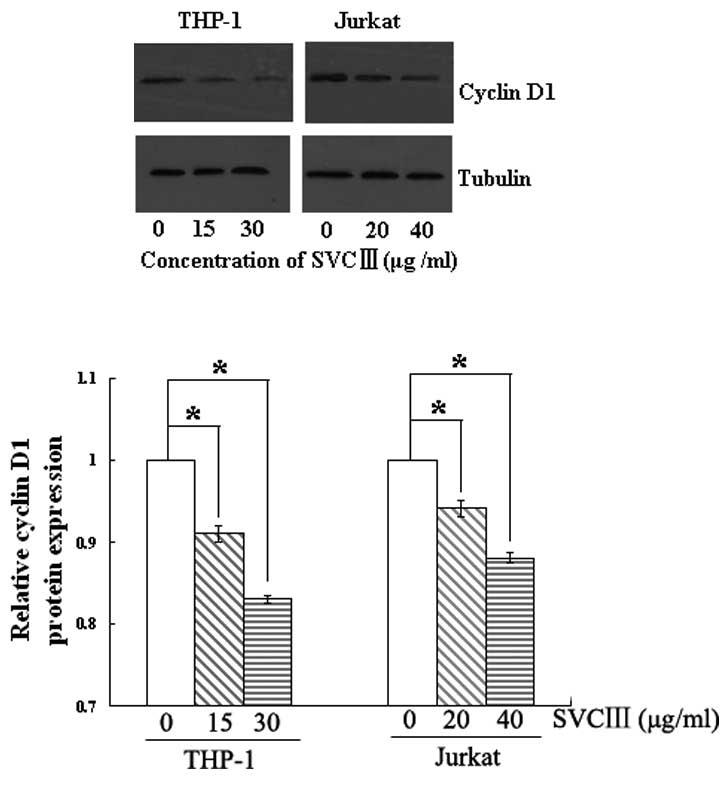
Effect of SVCIII on cyclin D1
protein
Cyclin D1, an NF-κB-regulated gene, is required for
transition from G1 to S phase and plays a vital role in cell
proliferation. We, therefore, examined whether SVCIII suppresses
the expression of cyclin D1 protein. As shown in Fig. 3, SVCIII significantly inhibited the
expression of cyclin D1 in a dose-dependent manner in both cell
types. This result suggests a potential mechanism for how SVCIII
suppresses tumor cell proliferation.
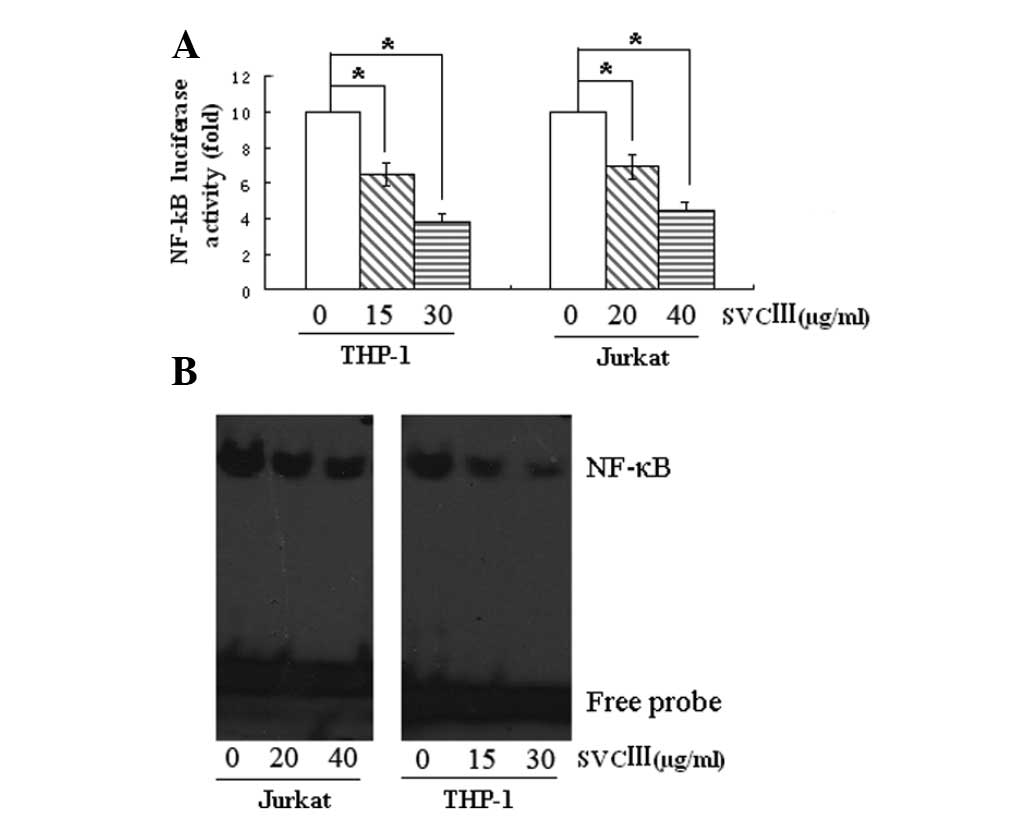
Effect of SVCIII on NF-κB activation
In order to determine whether NF-κB is involved in
cell growth suppression induced by SVCIII, we first measured
NF-κB-luciferase activity by using a luciferase plasmid containing
six tandem NF-κB sites as a minimal promoter. Fig. 4A showed that treatment with SVCIII
resulted in a significant decrease in NF-κB-luciferase
activity.
We then examined the NF-κB activation using EMSA.
Exposure of cells to SVCIII led to a decrease in NF-κB-DNA binding
in a dose-dependent manner (Fig.
4B). The suppression of NF-κB-DNA binding activity was
consistent with luciferase reporter activity. These results suggest
that SVCIII inhibits NF-κB activation.
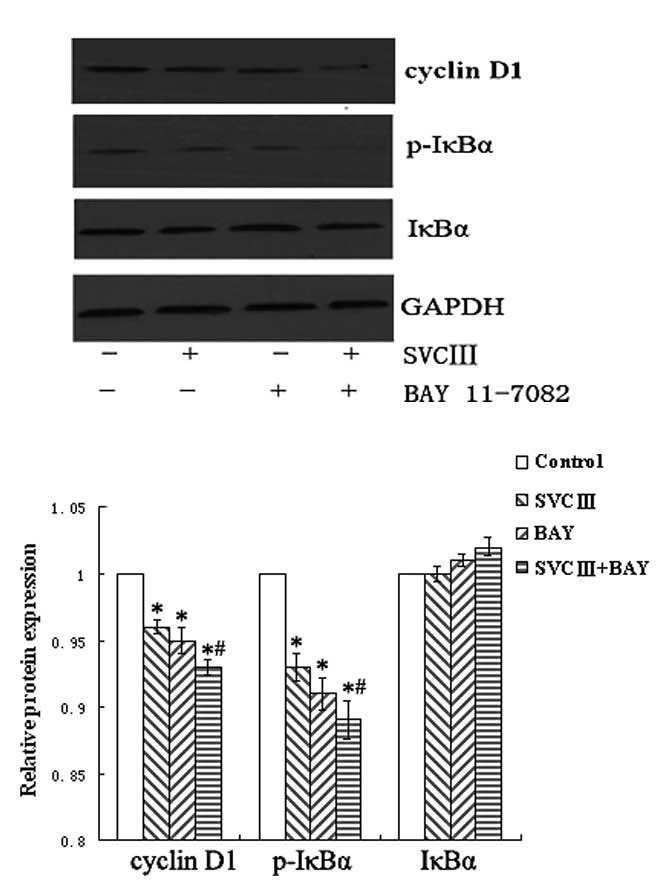
Effect of SVCIII on IκBα
The translocation of NF-κB to the nucleus is
preceded by the phosphorylation and proteolytic degradation of
IκBα. To elucidate the signaling pathways involved in the
suppression of NF-κB activation, we pretreated THP-1 cells with
NF-κB inhibitor BAY11-7082 for 1 h and then subjected the cells to
SVCIII. Although the expression of p-IκBα was markedly inhibited by
SVCIII treatment, it was further decreased by the pre-treatment
with the NF-κB inhibitor (Fig. 5).
Next, we investigated whether the NF-κB inhibitor would also
suppress the expression of cyclin D1. As expected, NF-κB inhibitor
led to a decrease in the expression of cyclin D1, whereas it was
further decreased by the NF-κB inhibitor combined with SVCIII
(Fig. 5).
Discussion
In the present study, we investigated the effects of
SVCIII on cell growth in the THP-1 and Jurkat cell lines, as well
as on the NF-κB signaling pathway. We found that SVCIII inhibited
the cell proliferation and cell cycle arrest at G1 phase in a
dose-dependent manner, and suppressed NF-κB activation through
inhibition of IκBα phosphorylation, degradation and p65 nuclear
translocation.
It is well established that normal cells divide and
create new cells only when needed. One of the hallmark
characteristics of cancer cells is their uncontrolled proliferation
(8). Our results demonstrated that
SVCIII inhibited cell proliferation in human THP-1 and Jurkat cells
in a dose-dependent manner. These results agree with previous
reports that scorpion venom inhibited the growth of lymphoma
(3,9), leukemia (10), neuroblastoma (4), gliomas (11–13),
breast cancer (14) and prostate
cancer (15,16).
It is well known that cell proliferation is closely
related to cell cycle distribution. Under normal conditions, cells
are believed to be in the G0 phase in most mammals. Cells progress
through the cell cycle phase from G0/G1 to S after stimulation from
extracellular signals. It was demonstrated that scorpion venom
induced cell cycle arrest mainly in the G0/G1 phase and decreased
in the S phase (3). The analysis
of cell cycle distribution in the present study also showed that
SVCIII inhibited cell proliferation with cell cycle arrest at the
G1 phase and reduced transition to the S phase and G2/M phases of
the cell cycle in a dose-dependent manner. This reinforces the
evidence that suppression of cell cycle transition is involved in
the SVCIII-induced antitumor action in human leukemia cells.
NF-κB plays a pivotal role in physiological immune
reactions, as well as in the onset and maintenance of malignancies
(17–20). It targets many genes that promote
tumor progression, cell survival, proliferation, angiogenesis and
metastasis (21–23). Aberrant or persistent activation of
NF-κB is believed to be an important mechanism in the generation of
various tumor types (24,25). In this study, we investigated the
activity of NF-κB using the luciferase reporter gene and EMSA.
Results showed that SVCIII inhibited the activity of NF-κB in a
dose-dependent manner as well. This suggests that SVCIII prevents
the binding of NF-κB to its target gene, and thus downregulates the
expression of NF-κB-regulated gene products.
NF-κB is expressed in the cytoplasm of virtually all
cell types. NF-κB activation is initiated by the signal-induced
degradation of IκB proteins (26,27).
In the classical NF-κB signaling pathway, IκB proteins are
phosphorylated by an activated IκB kinase (IKK) complex and then
degraded by the proteasome. The degradation of IκB allows NF-κB
protein to translocate to the nucleus and bind to their cognate DNA
binding sites to regulate the transcription of many genes (28,29).
We found that the suppression of NF-κB activation was accompanied
by inhibition of IκBα phosphorylation and degradation. Moreover,
SVCIII also inhibited p65 nuclear translocation. Therefore, the
inhibition of cell proliferation by SVCIII may be associated with
downregulation of constitutive NF-κB activation.
It is clear that NF-κB transcription factor
regulates expression of various genes, including cyclin D1 which
has been linked with proliferation of tumor cells. Cyclin D1
modulates the cell cycle transition from G1 to S phase and is
over-expressed in a variety of human malignancies (30,31).
To reveal the inhibitory mechanism of SVCIII on cell proliferation,
we investigated the effect of this compound on the cell cycle. It
was found that treatment with SVCIII significantly inhibited the
expression of cyclin D1 in a dose-dependent manner. These results
suggest a molecular mechanism for the manner in which SVCIII
suppresses tumor cell proliferation. Further studies are required
to clarify the effects of SVCIII on other signaling pathways.
In conclusion, this study has demonstrated that
SVCIII suppresses cell proliferation and cell cycle arrest at the
G1 phase by targeting the NF-κB signal pathway in THP-1 and Jurkat
cells. This suggests that SVCIII may have a potential and/or
adjuvant therapeutic application in the treatment of human
leukemia.
Acknowledgements
This research was funded by the
Education Department of Henan Province, P.R. China (No.
2010A310005). We thank Cang-bao Xu for linguistic advice. We also
thank Dr Luan Haojiang of the US National Institutes of Health for
his generous gift of the NF-κB luciferase reporter plasmid.
References
|
1
|
Batista CVF, Pozo LD, Zamudio FZ,
Contreras S, Becerril B, Wanke E and Possani LD: Proteomics of the
venom from the Amazonian scorpion Tityus cambridgei and the
role of prolines on mass spectrometry analysis of toxins. J
Chromatogr B Analyt Technol Biomed Life Sci. 803:55–66. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zargan J, Sajad M, Umar S, Naime M, Ali S
and Khan HA: Scorpion (Androctonus crassicauda) venom limits
growth of transformed cells (SH-SY5Y and MCF-7) by cytotoxicity and
cell cycle arrest. Exp Mol Pathol. 91:447–454. 2011.
|
|
3
|
Gao F, Li H, Chen YD, Yu XN, Wang R and
Chen XL: Upregulation of PTEN involved in scorpion venom-induced
apoptosis in a lymphoma cell line. Leuk Lymphoma. 50:633–641. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zargan J, Sajad M, Umar S, Naime M, Ali S
and Khan HA: Scorpion (Odontobuthus doriae) venom induces
apoptosis and inhibits DNA synthesis in human neuroblastoma cells.
Mol Cell Biochem. 348:173–181. 2011.
|
|
5
|
Hayden MS, West AP and Ghosh S: NF-κB and
the immune response. Oncogene. 25:6758–6780. 2006.
|
|
6
|
Garg A and Aggarwal BB: Nuclear
transcription factor-kappaB as a target for cancer drug
development. Leukemia. 16:1053–1068. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Escárcega RO, Fuentes-Alexandro S,
García-Carrasco M, Gatica A and Zamora A: The transcription factor
nuclear factor-κB and cancer. Clin Oncol (R Coll Radiol).
19:154–161. 2007.
|
|
8
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar
|
|
9
|
Gupta SD and Gomes A, Debnath A, Saha A
and Gomes A: Apoptosis induction in human leukemic cells by a novel
protein Bengalin, isolated from Indian black scorpion venom:
through mitochondrial pathway and inhibition of heat shock
proteins. Chem Biol Interact. 183:293–303. 2010. View Article : Google Scholar
|
|
10
|
Das Gupta S, Debnath A, Saha A, Giri B,
Tripathi G, Vedasiromoni JR and Gomes A: Indian black scorpion
(Heterometrus bengalensis Koch) venom induced
antiproliferative and apoptogenic activity against human leukemic
cell lines U937 and K562. Leukemia Res. 31:817–825. 2007.
|
|
11
|
Fan S, Sun Z, Jiang D, Dai C, Ma Y, Zhao
Z, Liu H, Wu Y, Cao Z and Li W: BmKCT toxin inhibits glioma
proliferation and tumor metastasis. Cancer Lett. 291:158–166. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu YJ, Yin LT, Liang AH, Zhang CF, Wang W,
Chai BF, Yang JY and Fan XJ: Therapeutic potential of
chlorotoxin-like neurotoxin from the Chinese scorpion for human
gliomas. Neurosci Lett. 412:62–67. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang WX and Ji YH: Scorpion venom induces
glioma cell apoptosis in vivo and inhibits glioma tumor growth in
vitro. J Neurooncol. 73:1–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
D’Suze G, Rosales A, Salazar V and Sevcik
C: Apoptogenic peptides from Tityus discrepans scorpion
venom acting against the SKBR3 breast cancer cell line. Toxicon.
56:1497–1505. 2010.
|
|
15
|
Zhang YY, Wu LC, Wang ZP, Wang ZX, Jia Q,
Jiang GS and Zhang WD: Anti-proliferation effect of polypeptide
extracted from scorpion venom on human prostate cancer cells in
vitro. J Clin Med Res. 1:24–31. 2009.PubMed/NCBI
|
|
16
|
Omran MAA: In vitro anticancer effect of
scorpion Leiurus quinquestriatus and Egyptian Cobra venom on
human breast and prostate cancer cell lines. J Med Sci. 3:66–86.
2003.
|
|
17
|
Bonizzi G and Karin M: The two NF-kappaB
activation pathways and their role in innate and adaptive immunity.
Trends Immunol. 25:280–288. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamamoto M and Takeda K: Role of nuclear
IkappaB proteins in the regulation of host immune responses. J
Infect Chemother. 14:265–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Y and Zhou BP:
TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and
invasion. Br J Cancer. 102:639–644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sarkar FH, Li Y, Wang Z and Kong D:
NF-kappaB signaling pathway and its therapeutic implications in
human diseases. Int Rev Immunol. 27:293–319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishikori M: Classical and alternative
NF-κB activation pathways and their roles in lymphoid malignancies.
J Clin Exp Hematopathol. 45:15–24. 2005.
|
|
22
|
Wong JH, Lui VW, Umezawa K, Ho Y, Wong EY,
Ng MH, Cheng SH, Tsang CM, Tsao SW and Chan AT: A small molecule
inhibitor of NF-kappaB, dehydroxymethylepoxyquinomicin (DHMEQ),
suppresses growth and invasion of nasopharyngeal carcinoma (NPC)
cells. Cancer Lett. 287:23–32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Warfel JM and D’Agnillo F: Anthrax lethal
toxin enhances TNF-induced endothelial VCAM-1 expression via an IFN
regulatory factor-1-dependent mechanism. J Immunol. 180:7516–7524.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiao Q, Nozaki Y, Sakoe K, Komatsu N and
Kirito K: NF-κB mediates aberrant activation of HIF-1 in malignant
lymphoma. Exp Hematol. 38:1199–1208. 2010.
|
|
25
|
Huber AV, Saleh L, Prast J, Haslinger P
and Knöfler M: Human chorionic gonadotrophin attenuates NF-kappaB
activation and cytokine expression of endometriotic stromal cells.
Mol Hum Reprod. 13:595–604. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karin M and Ben-Neriah Y: Phosphorylation
meets ubiquitination: the control of NF-kB activity. Annu Rev
Immunol. 18:621–663. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Korn SH, Wouters EF, Vos N and
Janssen-Heininger YM: Cytokine-induced activation of nuclear
factor-kappa B is inhibited by hydrogen peroxide through oxidative
inactivation of IkappaB kinase. J Biol Chem. 276:35693–35700. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumar A, Takada Y, Boriek AM and Aggarwal
BB: Nuclear factor-κB: its role in health and disease. J Mol Med.
82:434–448. 2004.
|
|
29
|
Sethi G, Sung B and Aggarwal BB: Nuclear
factor-kappaB activation: from bench to bedside. Exp Biol Med
(Maywood). 233:21–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Biliran H Jr, Wang Y, Banerjee S, Xu H,
Heng H, Thakur A, Bollig A, Sarkar FH and Liao JD: Overexpression
of cyclin D1 promotes tumor cell growth and confers resistance to
cisplatin-mediated apoptosis in an elastase-myc
transgene-expressing pancreatic tumor cell line. Clin Cancer Res.
11:6075–6086. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sung B, Ahn KS and Aggarwal BB: Noscapine,
a benzylisoquinoline alkaloid, sensitizes leukemic cells to
chemotherapeutic agents and cytokines by modulating the NF-kappaB
signaling pathway. Cancer Res. 70:3259–3268. 2010. View Article : Google Scholar : PubMed/NCBI
|