Introduction
Colon cancer is the third most common type of
cancer, with an increasing incidence each year (1). The proliferation and migration of
colon cancer cells are two significant determinants of the
prognosis of colon cancer. Jagged1 is a ligand in the Notch1
signaling pathway. A recent study has demonstrated that Jagged1 is
highly expressed in colon cancer (2). However, the role of Jagged1 in the
occurrence and development of colon cancer remains poorly
understood. Another study revealed that the Jagged1/Notch signaling
pathway was involved in the proliferation and migration of certain
types of cancer (3). This study
aimed to investigate the effect of Jagged1 on the in vitro
proliferation and migration of colon cancer cells.
Materials and methods
Materials
Human colon cancer cells (HT-29) were purchased from
the American Type Culture Collection (ATCC; Manassas, VA, USA), and
DMEM-F12 and fetal bovine serum (FBS) were purchased from Gibco
(Carlsbad, CA, USA). The Jagged1-carrying adenovirus (Ad-Jagged1)
and the blank adenovirus (Ad-CMV) were kindly provided by Professor
Weinmaster from the University of California, Los Angeles, CA, USA.
The adenovirus carrying small interfering RNA (siRNA) targeting the
Jagged1 gene (Ad-si/hJagged1) and the control adenovirus without
siRNA expression were kindly provided by Professor Gabrilovich from
the University of South Florida, FL, USA. Rabbit anti-human
antibodies against Jagged1, phosphorylated Akt (p-Akt), Akt and
GAPDH were purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). The modified Boyden chamber was purchased from
Zhongshan Co., Ltd. (China).
Cell culture and adenovirus
transfection
The HT-29 cells were maintained in DMEM-L containing
10% FBS at 37°C in a 5% CO2 atmosphere in a 100-ml
flask. When the cell confluence reached 60–70%, the
1x106 pfu/ml adenovirus solution (10 μl) was added to
the flask and the cells were incubated for 48 h.
[3H]-thymidine
([3H]-TdR) incorporation
The cells were maintained in serum-free medium for 4
h, then 10 μl of [3H]-TdR was added at a final
concentration of 37 kBq/ml. Following 24-h incubation, the medium
was removed and the cells were washed with PBS of an equal volume.
Then, these cells were treated with 500 μl of 0.125% trypsin and a
cell suspension was prepared which was then transferred onto grade
49 glass-fiber filter paper. Following washing in 0.9% NaCl three
times, the paper containing the cells was rinsed with 2 ml of 5%
trichloroacetic acid for fixation. Then, the cells were washed once
in 1 ml of absolute ethanol and dehydrated. The scintillation
solution was added to the dry paper, which was kept in a dark room
for 3 h. Detection was performed and the data were expressed as
counts per min per well (cpm/well). The amount of incorporated
[3H]-TdR positively correlated with the DNA
synthesis.
Cell migration
Following digestion, cells were collected and
re-suspended in 500 μl of medium for counting. Then, 100 μl of
medium was added to the lower chamber, and the cell suspension
containing 1x105 cells (100 μl) was added to the upper
chamber followed by incubation at 37°C in a 5% CO2
atmosphere for 6 h. The filter was collected and the cells that did
not migrate were removed. The remaining cells were fixed in
absolute ethanol and then underwent haemotoxylin staining. The
filter was kept at 37°C overnight and made transparent with xylene.
Three fields were randomly selected at a magnification of x400 and
the migrated cells were counted.
Western blot analysis
Cells were washed in PBS three times and then lysed
in a lysis buffer containing phenylmethanesulfonylfluoride (PMSF)
for 30 min. The lysate was transferred into an EP tube followed by
centrifugation at 4°C for 20 min at 12000 x g. The supernatant was
collected and stored at −70°C. Protein of equal content was
subjected to SDS-PAGE at a constant 80 V, and then transferred onto
a polyvinylidene fluoride (PVDF) membrane, which was blocked in 5%
non-fat milk in phosphate-buffered saline Tween-20 (PBST) at room
temperature for 1 h. Subsequently, the membrane was treated with
rabbit anti-human Jagged1, p-Akt, Akt or GAPDH (1:200)
independently at 4°C overnight, and then with horseradish
peroxidase (HRP)-conjugated goat anti-rabbit IgG at 37°C for 1 h.
Visualization was conducted according to the manufacturer’s
instructions (ECL kit). Representative images were collected using
a gel imaging system (Bio-Rad, Hercules, CA, USA). The expression
of the target proteins were normalized by that of GAPDH.
Statistical analysis
Statistical analysis was performed using SPSS
version 11.5 and quantitative data were expressed as the means ±
standard deviation. Comparisons between the two groups were
conducted using an independent samples t-test, and between three
groups using one-way analysis of variance. A P-value <0.05 was
considered to indicate a statistically significant difference.
Results
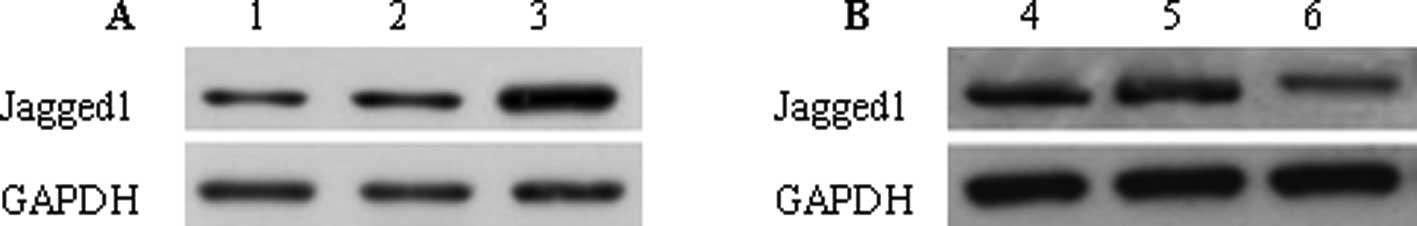
Jagged1 intervention and detection
Ad-Jagged1 adenovirus and blank adenovirus (Ad-CMV)
were used to transfect colon cancer cells. The results demonstrated
that when compared with cells without transfection [mock (control)
group], the Jagged1 expression in the Ad-Jagged1 group was
significantly increased (2.17±0.45 vs. 0.78±0.06, P<0.01), and
there was no significant difference between the Ad-CMV group and
the mock group in the Jagged1 expression (0.81±0.05vs. 0.78±0.06,
P>0.05) (Fig. 1A). Following
transfection with adenovirus carrying siRNA targeting Jagged1
(Ad-si/hJagged1 group) and adenovirus without Jagged1 siRNA (Ad-NSC
group), the results revealed that the Jagged1 expression in the
Ad-si/hJagged1 group was markedly reduced when compared with the
mock group (0.24±0.01 vs. 0.79±0.05, P<0.01), but no significant
difference was observed between the Ad-NS group and the mock group
(0.78±0.04 vs. 0.79±0.05, P>0.05) (Fig. 1B).
Effect of Jagged1 on the proliferation of
colon cancer cells
The proliferation of the colon cancer cells was
determined by [3H]-TdR incorporation. The results
demonstrated that cell proliferation in the Ad-Jagged1 group was
higher than that in the mock group (22048±1235 vs. 14750±867
cpm/well, P<0.01). However, no significant difference was
observed between the Ad-CMV group and the mock group (14946±722 vs.
14750±867 cpm/well±0.06, P>0.05). In addition, when compared
with the mock group, the cell proliferation in the Ad-si/hJagged1
group was significantly reduced (10084±922 vs. 15027±1008 cpm/well,
P<0.01). However, the cell proliferation in the mock group was
comparable with that in the Ad-NSC group (14895±964 vs. 15027±1008
cpm/well, P>0.05).
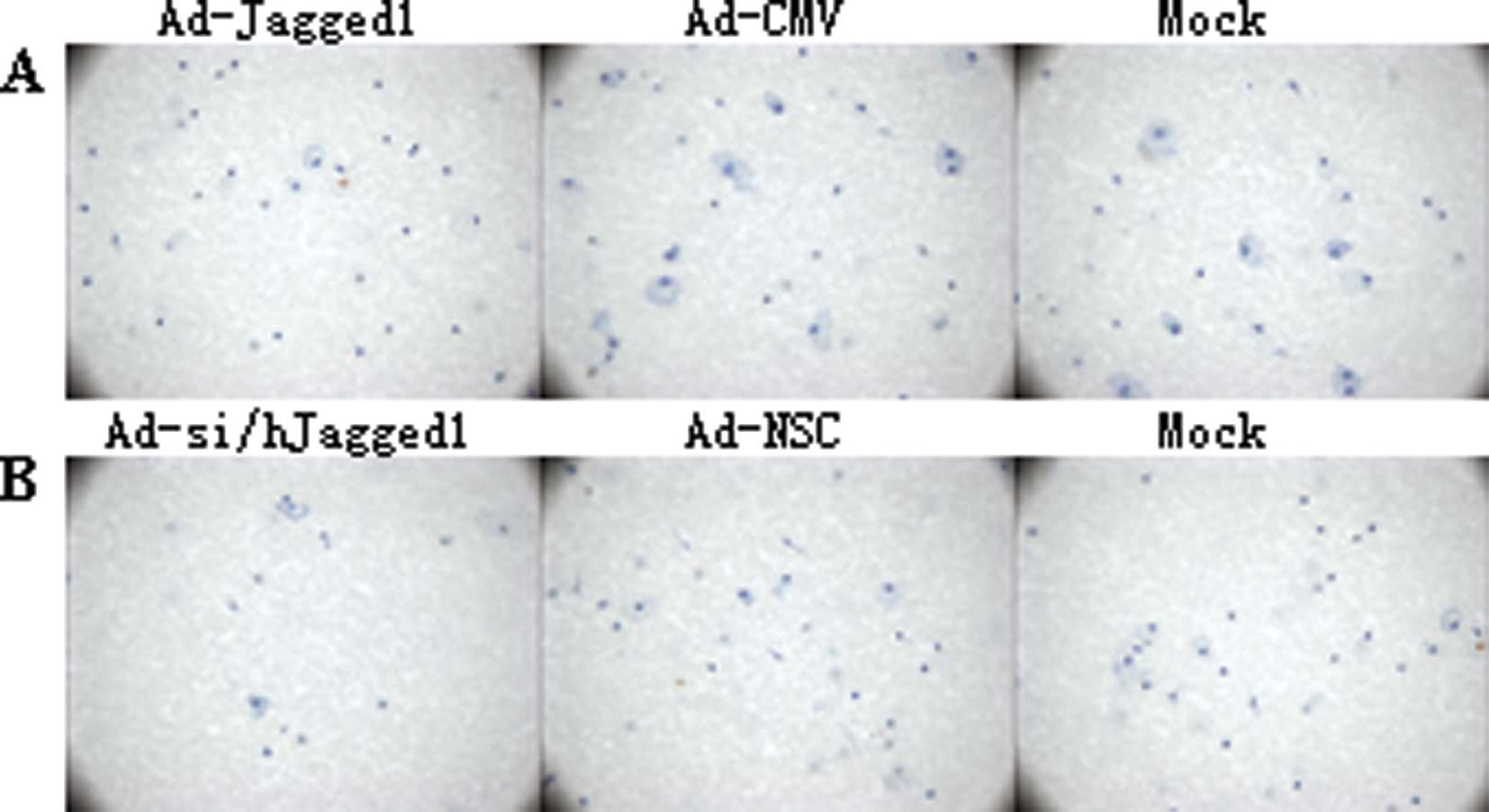
Effect of Jagged1 on the migration of
colon cancer cells
A modified Boyden chamber assay was conducted to
determine the migration of the colon cancer cells. When compared
with the mock group, the migration in the Ad-Jagged1 group was
significantly enhanced (42.24±2.37 cells vs. 21.22±1.95 cells,
P<0.01), but there was no marked difference between the Ad-CMV
group and the mock group. Moreover, the migration in the
Ad-si/hJagged1 group was markedly increased when compared with the
mock group (14.89±1.45 cells vs. 20.89±1.56 cells, P<0.01), but
a significant difference was not observed between the Ad-NSC group
and the mock group (21.32±2.04 cells vs. 20.89±1.56 cells,
P>0.05) (Fig. 2B).
Effect of Jagged1 on Akt expression in
colon cancer cells
A western blot assay was performed to detect the
expression of Akt and p-Akt. The results demonstrated that the
p-Akt expression in the Ad-Jagged1 group was markedly higher than
that in the Ad-CMV group and the mock group (1.06±0.13 vs.
0.82±0.09 vs. 0.81±0.07, P<0.01). In addition, p-Akt expression
in the Ad-si/hJagged1 group was markedly lower than that in the
Ad-NSC group and the mock group (0.61±0.05 vs. 0.80±0.03 vs.
0.79±0.06, P<0.01). However, no significant difference was
observed in the Akt expression between the different groups
(Fig. 3).
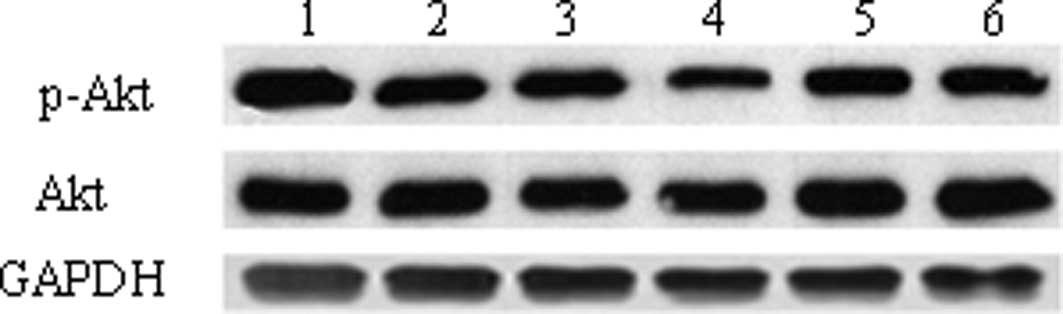 | Figure 3.Detection of p-Akt and Akt in the
colon cancer cells revealed by western blot assay. Lane 1, cells
transfected with Ad-Jagged1; lane 2, cells transfected with Ad-CMV;
lanes 3 and 6, cells without transfection; lane 4, cells
transfected with Ad-si/hJagged1; lane 6, cells transfected with
Ad-NSC. p-Akt, phosphorylated Akt; Ad-CMV, blank adenovirus;
Ad-Jagged1, Jagged1-carrying adenovirus; Ad-NSC, adenovirus without
Jagged1 siRNA; Ad-si/hJagged1, adenovirus carrying siRNA targeting
Jagged1. |
Discussion
Colon cancer is a common type of gastrointestinal
malignancy. In recent years, the incidence of colon cancer has
increased due to the changes in diet structure, and therefore it
has become a significant malignancy threatening human health. A
previous study has demonstrated that over-proliferation and
compromised apoptosis are crucial for the occurrence and
development of cancer (4). In
clinical practice, chemotherapeutics, including 52Fu, cisplatin and
mitomycin have the biological activity to reduce the proliferation
of cancer cells, exerting an anti-tumor effect. However, the
evident side-effects of these drugs, and the resistance of cancer
cells to these drugs significantly limits the application of these
chemotherapeutics (5). To identify
the endogenous pro- and antitumor molecules from the molecular
biological point of view may be a solution to this dilemma.
Invasion and metastasis are significant biological characteristics
of cancers. During invasion, cancer cells migrate from the primary
site to the surrounding or distant tissues. The degree of migration
of the cancer cells determines the degree of metastasis of the
cancer (6). In certain types of
cancer, studies have demonstrated that Jagged1 is crucial in the
regulation of proliferation and migration of cancer cells (3). Moreover, in colon cancer, Jagged1
expression has been reported to be markedly increased when compared
with that in adjacent normal tissues (2). However, the specific role of the
increased expression of Jagged1 in colon cancer remains
unknown.
Our results reveal that Jagged1 overexpression can
promote the proliferation and migration of colon cancer cells,
while Jagged1 silencing using siRNA significantly reduces
proliferation and migration. These findings indicate that Jagged1
is a key molecule involved in the in vitro proliferation and
migration of colon cancer cells. The Notch signaling pathway is
widely conserved in a variety of animals, and is involved in the
determination of cell fate. Studies have revealed that the Notch
signaling pathway is closely involved with the occurrence and
development of certain types of cancer. Jagged1 is a significant
ligand in the Notch signaling pathway, where it activates Notch1, 2
and 4. The binding of Jagged1 to Notch may activate the
transcription factors, including Hes and Hey, and initiate the
proliferation and migration-related signaling pathways, including
MAPK and PI3K/Akt (7), exerting
the biological effects of Jagged1. In the present study, our
results demonstrate that Jagged1 can increase p-Akt expression, but
has no effect on Akt expression. The PI3K/Akt signaling pathway is
a classic pathway involved in the proliferation and differentiation
of cells. Studies have confirmed that the PI3K/Akt signaling
pathway plays a crucial role in the occurrence and development of
breast cancer (8). Akt is a
downstream target protein and also a key molecule in the PI3K/Akt
signaling pathway. The activation of PI3K may lead to the
phosphorylation of Akt which can regulate the proliferation,
apoptosis and migration of several types of cancer cells. There is
evidence demonstrating that the phosphorylation of Akt is increased
in a number of malignancies, including lung, gastric, breast,
cervical and prostate cancer (9,10),
which was consistent with our findings.
In conclusion, the overexpression of Jagged1, a key
component in the Notch signaling pathway, is capable of promoting
the proliferation and migration of colon cancer cells, which may be
related to the phosphorylation of Akt by Jagged1. Our findings
suggest that Jagged1 plays a pivotal role in the occurrence and
development of colon cancer, and thus may be a promising target in
the prevention and treatment of this disease. However, the detailed
mechanisms of the antitumor effect of Jagged1 require further
study.
References
|
1
|
Peto J: Cancer epidemiology in the last
century and the next decade. Nature. 411:390–395. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liao W, Li G and Chen J: Expression of
Notch-1 and Jagged-1 protein in human colorectal cancer and its
significance. J Shandong Med. 50:12–14. 2010.
|
|
3
|
Farnie G and Clarke RB: Mammary stem cells
and breast cancer: role of Notch signalling. Stem Cell Rev.
3:169–175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gongora C, Candeil L, Vezzio N, et al:
Altered expression of cell proliferation related and interferon
stimulated genes in colon cancer cells resistant to SN38. Cancer
Biol Ther. 7:822–832. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shafaee Z, Schmidt H, Du W, et al:
Cyclopamine increases the cytotoxic effects of paclitaxel and
radiation but not cisplatin and gemcitabine in Hedgehog expressing
pancreatic cancer cells. Cancer Chemother Pharmacol. 58:765–770.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaplan RN, Riba RD, Zacharoulis S, et al:
VEGFR1-positive haematopoietic bone marrow progenitors initiate the
pre-metastatic niche. Nature. 4380:820–827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shimojo H, Ohtsuka T and Kageyama R:
Oscillations in notch signaling regulate maintenance of neural
progenitors. Neuron. 58:52–64. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wickenden JA and Watson CJ: Key signaling
nodes in mammary gland development and cancer. Signalling
downstream of PI3 kinase in mammary epithelium: a play in 3 Akts.
Breast Cancer Res. 12:202–204. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Priulla M, Calastretti IA, Bruno P, et al:
Preferential chemosensitization of PTEN mutated prostate cells by
silencing the Akt kinase. Prostate. 67:782–789. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang D and Fan D: Multidrug resistance in
gastric cancer: recent research advances and ongoing therapeutic
challenges. Expert Rev Anticancer Ther. 7:1369–1378. 2007.
View Article : Google Scholar : PubMed/NCBI
|