Introduction
Acute spinal cord injury (ASCI) is a type of
disability caused by injury of the central nervous system which
results in severe pain in patients and has implications for the
family and society. The disability is not completely caused by
primary injury, as secondary injury also plays a significant role.
If the primary injury is not treated appropriately and promptly, a
secondary injury may result. This secondary injury may be more
severe than the primary injury and often results in long-lasting
disability. Currently, it is unfeasible to target the primary
injury, thus measures should be taken to reduce the incidence of
secondary injury following ASCI. Therefore, the key steps in the
early treatment of ASCI are to protect the uninjured spinal cord,
reduce or prevent secondary injury and promote the re-construction
of spinal cord function. The mechanisms underlying secondary injury
in ASCI include i) inflammation in which calcium, opioids,
excitatory amino acids and other substances mediate injury; ii)
free radicals and vascular factors; and iii) cell apoptosis. In
recent years, studies have demonstrated that cell apoptosis is
crucial in neurological injury following ASCI (1). During apoptosis, members of the
caspase family are major participants and caspase activation is a
key event in apoptosis.
Methylprednisolone (MP) is the only effective agent
used in ASCI treatment, but the therapeutic mechanism of MP remains
unclear. A previous study (2)
revealed that the therapeutic effectiveness of MP may be attributed
to the inhibition of inflammation, attenuation of edema,
suppression of vascular activity and prostaglandin activity,
increase in blood flow in the spinal cord, inhibition of reactive
oxygen species (ROS) and lipid peroxidation, stabilization of cell
and lysosomal membranes, reversal of intracellular calcium
accumulation, increase in Na+K+-dependent
ATPase activity, increase in resting potential and excitability of
spinal motor fibers and promotion of the generation and
transduction of nerve impulses in the spinal cord. However, the
effect of MP on apoptosis in secondary ASCI and its related
mechanisms are poorly understood. The present study aimed to
observe apoptosis in the spinal cord of ASCI animals following MP
treatment and the activities of apoptosis-related factors
(caspase-3, -6, -8 and -9) were also measured in order to
investigate the antiapoptotic effect of MP and its mechanism in the
treatment of ASCI.
Materials and methods
ASCI animal model and grouping
Healthy adult New Zealand rabbits weighing 2.0–3.0
kg (males or females) were purchased from the Experimental Animal
Center (Guangzhou, China). The animals were divided into three
groups. In the sham (S) group, the spinal cord was exposed but not
injured. In the ASCI group (C), the spinal cord was exposed and the
modified Allen’s method was employed to cause injury to the spinal
cord, but the animals were not treated. In the MP (T) group, ASCI
was introduced to animals using the same procedures and the animals
were then intravenously (ear vein) treated with MP at 30 mg/kg
immediately following injury and thereafter at 5.4 mg/(kg·h) every
2.5 h within the subsequent 23 h. Following surgery, the animals
were housed independently and the bladder was massaged regularly
(4–5 times daily until urination recovered). For all animals,
intramuscular injection with penicillin (800,000 U) was performed
for 3 consecutive days to prevent infection.
Sample collection
At 8 h and 1, 3, 7, 14 and 28 days after injury, the
animals were sacrificed and the lesioned spinal cords were
collected. In brief, the animals were weighed, anesthetized, placed
in a prone position and fixed followed by skin preparation. An
incision was made in the back, the skin and subcutaneous tissues
were isolated and the spinal cord was exposed. The lesioned spinal
cord 1.5–2.0 cm in length was collected. The animals were then
sacrificed by injection of air through the ear vein. The spinal
cord was then added to lysis buffer (5 mg/100 μl) followed by
homogenation on ice. The homogenate was then transferred into a
1.5-ml centrifuge tube followed by lysis on ice for a further 5
min. Centrifugation was performed at 14,000 rpm at 4°C for 10–15
min. The supernatant was collected and transferred into a
pre-cooled centrifuge tube which was stored at −70°C prior to the
detection of caspase activities.
Detection of the activities of caspase-3,
-6, -8 and -9 by ELISA
The detection of caspase activities was performed
according to the manufacturer’s instructions. For example, for the
detection of caspase-3 activity, the procedure was as follows. i)
The dilution solution was prepared according to the ratio of 0.9 ml
of detection buffer to 0.1 ml of lysis buffer; ii)
p-nitroaniline (pNA) (10 mM) was diluted to 0, 10,
20, 50, 100 and 200 μM with the dilution solution and used as
standard samples. iii) A volume (100 μl) of each standard sample
was collected and the absorbance was measured at 405 nm (A405). The
actual A405 of pNA, based on which the pNA standard
curve was delineated, was acquired by subtracting the A405 of the
blank control (without pNA) from that of each standard
sample. iv) The pNA was mixed in Ac-DEVD-pNA (2 mM)
of appropriate volume and the mixture was kept on ice until use. v)
The mixture was incubated at 37°C for 1–2 h. When the color
changed, A405 was measured. When the color remained unchanged, the
incubation was prolonged. vi) The absorbance was measured at 405
nm. The A405 of pNA generated by caspase-3 was calculated by
subtracting the A405 of the blank control from that of the samples.
The content of pNA was determined according to the standard
curve on the basis of the A405 of pNA. vii) The definition
of caspase-3 activity (Chemicon, Temecula, CA, USA) was that when
the substrate was saturated; one unit of enzyme activity is the
amount of caspase-3 that catalyzes 1 nmol of Ac-DEVD-pNA
into 1 nmol of pNA at 37°C. The caspase-3 activity of each
sample was calculated.
Statistical analysis
Statistical analysis was performed using SPSS
version 16.0 statistical software (SPSS, Inc., Chicago, IL, USA).
Comparisons between the T and C groups were performed using a
t-test, and P<0.05 was considered to indicate a statistically
significant result.
Results
In the S group, the activities of caspase-3, -6 and
-8 were 3.127, 0.029 and 2.733×104 U, respectively, at 8
h after surgery; 3.173, 0.029 and 2.777×104 U,
respectively, at 24 h after surgery; 3.14, 0.028 and
2.787×104 U at 3 days after surgery, respectively;
3.143, 0.028 and 2.777×104 U, respectively, at 7 days
after surgery; 3.16, 0.029 and 2.747×104 U,
respectively, at 14 days after surgery; 3.13, 0.287 and
2.787×104 U, respectively, at 28 days after surgery. The
caspase-9 activity was extremely low at 24 h after surgery and was
undetectable at the remaining time points. In the C group, the
activities of caspase-3, -6, -8 and -9 were markedly increased at 8
h after surgery and peaked at 24 h. At 3 days after injury, the
activities of the caspases remained high, but were lower than those
at 24 h. At 7 days after injury, the activities were markedly
reduced and remained at a low level at 14 and 28 days after ASCI.
In the T group, the activities of caspase-3, -6, -8 and -9 were
significantly increased at 8 h after surgery and reached a maximal
level at 24 h. At 3 days after surgery, the activities remained
high but were lower than those at 24 h after surgery and those in
the C group. At 7 days after surgery, the activities were markedly
reduced and remained at a low level at 14 and 28 days after
surgery. The activities of caspase-3, -6, -8 and -9 are shown in
Tables I–IV. When compared with the C group, the
caspase activities were markedly lowered in the T group
(P<0.05). However, no marked difference in caspase activities
was noted between the two groups at 14 and 28 days after surgery
(P>0.05; Tables I–IV and Fig.
1).
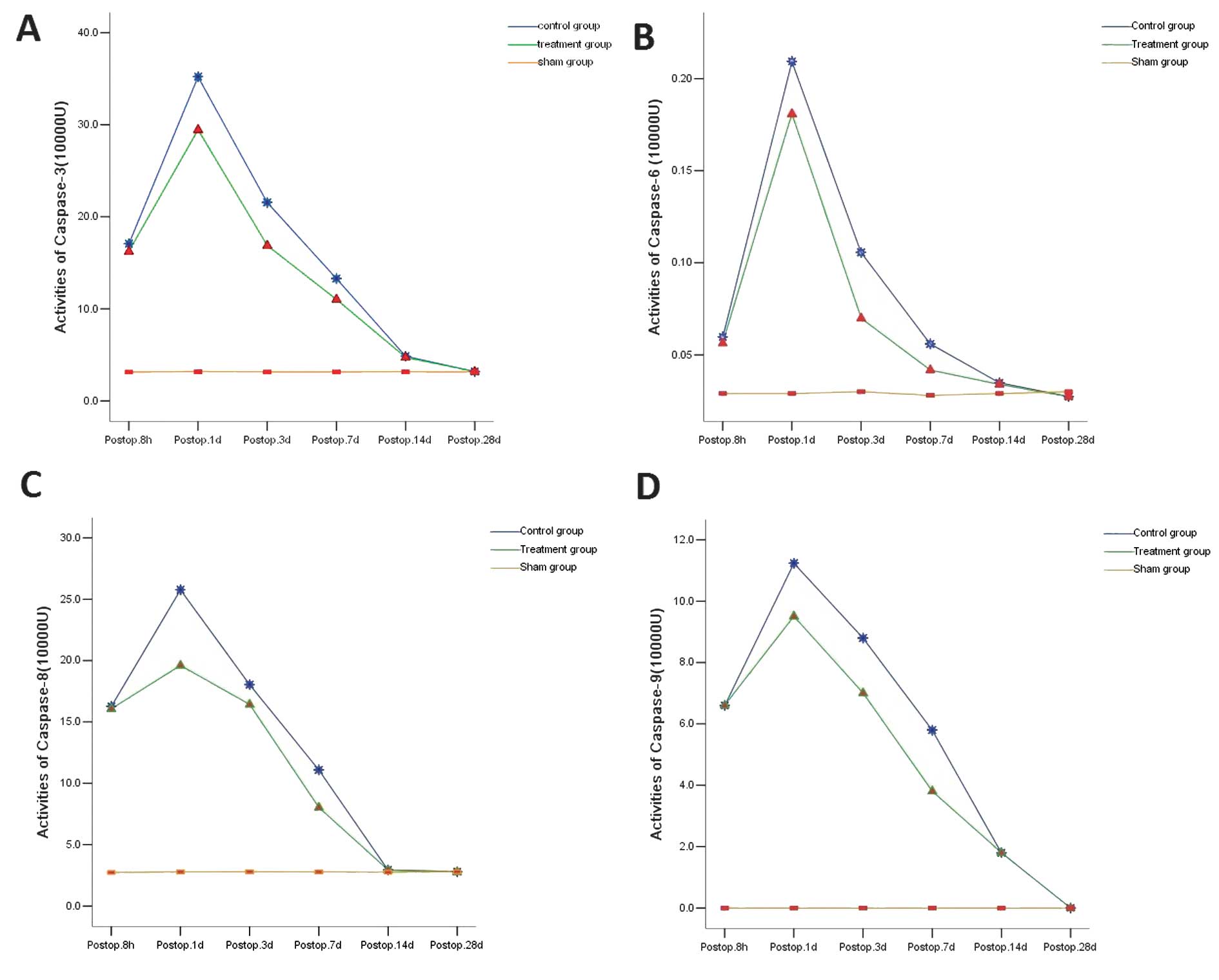 | Figure 1.Caspase-3, -6, -8 and -9 activities in
different groups. (A) Caspase-3; (B) caspase-6; (C) caspase-8; (D)
caspase-9. The caspase-3, -6, -8 and -9 activity increased
significantly at 8 h after surgery, but the increase in the T group
was markedly lower than that in the C group (P<0.05). At 24 h
and 3 and 7 days, the caspase-3, -6, -8 and -9 activity in the T
group was markedly lower than that in the C group (P<0.01). At
14 and 28 days after injury, the caspase-3, -6, -8 and -9 activity
was markedly reduced when compared with that at other time points
but no significant difference was found between the C and T groups
(P>0.05). At 28 days after injury, the caspase-9 activity was
undetectable and comparable to that in the blank control group. T,
treatment; C, control. |
 | Table I.Caspase-3 activity in T group and C
group. |
Table I.
Caspase-3 activity in T group and C
group.
| Time point | Group | Activity (x̄ ±
SD) | t | P-value |
|---|
| 8 h | C | 17.0789±0.86519 | 2.705 | 0.016 |
| T | 16.2244±0.38636 |
| 24 h | C | 35.2322±0.33559 | 22.908 | 0.000 |
| T | 29.4311±0.68157 |
| 3 days | C | 21.5644±0.86794 | 14.126 | 0.000 |
| T | 16.8678±0.49157 |
| 7 days | C | 13.3011±0.27310 | 23.532 | 0.000 |
| T | 11.0100±0.10356 |
| 14 days | C | 4.8633±0.17407 | 1.834 | 0.085 |
| T | 4.7133±0.17292 |
| 28 days | C | 3.1856±0.04246 | 0.553 | 0.588 |
| T | 3.1744±0.04275 |
 | Table IV.Caspase-9 activity in T group and C
group. |
Table IV.
Caspase-9 activity in T group and C
group.
| Time point | Group | Activity (x̄ ±
SD) | t | P-value |
|---|
| 8 h | C | 6.6189±0.05776 | 2.243 | 0.039 |
| T | 6.5689±0.03371 |
| 24 h | C |
11.2422±0.15123 | 15.017 | 0.000 |
| T | 9.5289±0.30706 |
| 3 days | C | 8.7822±0.23679 | 18.515 | 0.000 |
| T | 6.9700±0.17364 |
| 7 days | C | 5.8211±0.47635 | 7.401 | 0.000 |
| T | 3.8078±0.66272 |
| 14 days | C | 1.8178±0.10837 | 0.931 | 0.366 |
| T | 1.7533±0.17720 |
| 28 days | C | 0.0011±0.00333 | 1 | 0.332 |
| T | 0.0000±0.00000 |
Discussion
Apoptosis may occur in a death receptor-,
mitochondria- or endoplasmic reticulum-dependent manner. Caspase
activation has been regarded as a key process in apoptosis. To
date, a total of 14 caspases have been identified, termed caspases
1-14. On the basis of the role they play in apoptosis, caspases are
divided into initiator and effector caspases. The initiator
caspases include caspase-2, -8, -9 and -10, which are located
upstream of the apoptosis cascade reaction. These caspases have
long N-terminals, initiate apoptosis and caspase activation and
regulate downstream caspases. Effector caspases are located
downstream of the apoptosis cascade reaction and include caspase-3,
-6 and -7. Following the activation of caspase-3, -6 and -7 by
upstream initiators, these caspases act on substrates resulting in
the changes in cellular biochemistry and morphology which are
characteristic of apoptosis. Other caspases, including caspase-1,
-4, -5, -13 and -14 are mainly involved in inflammation and exert
an adjunctive effect on apoptosis. Apoptosis occurs in different
ways as a result of the interaction of multiple factors (3,4). In
the present study, we measured the activities of initiator caspases
(caspase-8 and -9) and effector caspases (caspase-3 and -6) aiming
to investigate the antiapoptotic effect of MP in the treatment of
ASCI.
Caspase-3 is a critical participant in apoptosis. In
mammals, caspase-3 corresponds to CED-3 in nematodes (5). Caspase-3 is an important effector
caspase and is the final executive caspase in various apoptosis
pathways (6). The activated
caspase-3 is able to digest or cleave proteins and kinases which
are involved in cell structure, cell cycle and DNA replication,
resulting in the activation or inactivation of these kinases and
proteins and subsequent apoptosis. Sîrbulescu and Zupanc applied
1,3-cyclohexanedione to inhibit caspase activation and the results
revealed that the number of apoptotic cells at 24 h and 5 and 30
days after surgery was markedly lower than that in the control
group (7). Caspase-3 is activated
by endogenous and exogenous pathways. In the exogenous pathway, the
death receptor carrying death signals binds to the corresponding
death ligand in the cell membrane, resulting in its
oligomerization. This then recruits Fas-related protein and
caspase-8 to form the death-inducing signaling complex. Caspase-8
then self-activates and the activated caspase-8 directly activates
caspase-3 (8). Caspase-3 is also
activated by cytochrome C (CytC) leaked from mitochondria, which is
known as the endogenous pathway. The activation of caspase-3 not
only leads to apoptosis but acts on pro-caspase-8 resulting in
caspase-8 activation, which then forms a positive feedback loop and
leads to a cascade reaction (9).
In the present study, our results revealed that at 8 and 24 h and 3
and 7 days after the MP treatment of ASCI, caspase-3 activity was
markedly reduced. That is, the activity of the executive caspases
was inhibited, which then alleviated the cleavage of other proteins
and kinases by caspase-3 and reduced the activation or inactivation
of these proteins and kinases.
Caspase-6 was first identified and cloned by
Fernandes-Alnemri et al (10). Caspase-6 is encoded by the gene
located at 4q25 and has a molecular weight of 34 kDa. Caspase-6
mainly exists in the form of zymogen in the cytoplasm and a
fraction of caspase-6 is located in the nucleus. In apoptosis,
caspase-6 is located downstream of the caspase cascade reaction, as
is caspase-3. Caspase-6 cleaves lamin A, leading to chromosome
condensation and resulting in apoptosis. Although caspase-6 and -3
are located downstream of the apoptosis cascade pathway, the
interaction between caspase-6 and -3 remains unclear (11). There is evidence which indicates
that the activation of caspase-3 is earlier than that of caspase-6
and is closely correlated with the cleavage of procaspase-6 into
caspase-6. In addition, activated caspase-6 may in turn activate
caspase-3, forming a positive feedback loop in the caspase-3
activity. Caspase-6 and -3 are effector caspases and are located
downstream of the apoptosis cascade reaction, but they are not in
the same reaction chain. The two proteins function independently
but are correlated with each other. In the absence of caspase-6
activation, caspase-3 activation alone may also induce apoptosis.
Furthermore, caspase-6 activation alone is also sufficient to
induce apoptosis in the absence of caspase-3 activation. However,
apoptosis in cells usually occurs via more than one pathway. In the
present study, our results showed that the activities of caspase-6
and -3 were inhibited following MP treatment, which suggests that
MP suppresses more than one apoptosis-related pathway, exerting an
antiapoptotic effect. The inhibition of one pathway may also block
the interaction of this pathway with other pathways. That is, the
interaction between caspase-6 and -3 is inhibited, which also
indirectly attenuates apoptosis.
Caspase-8 is regarded as a significant member of the
caspase family that induces apoptosis in mammals (12). Following activation, caspase-8
cleaves and activates caspase-3 downstream of the caspase cascade
reaction. Caspase-3 serves as a substrate of caspase-8 and
activated caspase-3 then acts on certain proteinases in the cell,
resulting in their activation or inactivation. This may cause
damage to lamin and lead to apoptosis (13,14).
In addition, caspase-3 in turn activates pro-caspase-8, forming a
positive feedback loop which leads to the caspase cascade reaction.
In the present study, at 8 and 24 h and 3 and 7 days after MP
treatment of ASCI, the caspase-3 and -8 activities were markedly
decreased and, furthermore, the interaction between caspase-8 and
-3 was also attenuated, which compromised the activation of
caspase-3 by caspase-8 and the positive feedback loop between
caspase-3 and -8. This inhibited the caspase cascade reaction and
resulted in the attenuation of apoptosis. The activated caspase-8
also cleaves the Bid, which is a member of the Bcl-2 family. The
cleaved tBid exposes the C terminal which then binds to the
mitochondria. This transmits the apoptosis signals from the
cytoplasm to the mitochondria, resulting in the release of CytC
from the mitochondria (15), which
is a key step in the endogenous apoptosis pathway. The results of
previous studies have confirmed that CytC expression is positively
correlated with apoptosis. There is evidence which suggests that
fluorouracil promotes the release of CytC from mitochondria to
induce the apoptosis of liver cancer cells (16). The CytC released from mitochondria
is able to form complexes with caspase-1 and -9, which may activate
caspase-3, -6 and -7 in the presence of dATP and ATP and induce
apoptosis. The present study found that the caspase-8 activity was
markedly inhibited following the MP treatment of ASCI, which may
attenuate the interaction between members of the Bcl-2 family and
mitochondria, reduce the transmission of apoptosis signals into the
mitochondria and finally compromise mitochondria-dependent
apoptosis. MP inhibits the activity of caspase-8, which not only
suppresses the caspase cascade reaction but indirectly inhibits
mitochondria-dependent apoptosis.
Caspase-9 is a key proteinase in the
mitochondria-dependent pathway and a significant initiator caspase
in the caspase cascade reaction. When tissues are injured, cells
undergo ischemia and hypoxia, which may increase mitochondrial
permeability. The proteins in the mitochondrial inner and outer
membranes (including CytC) can then be released into the cytoplasm
(16). CytC in the cytoplasm forms
a poly-complex with Apaf-1 which then activates pro-caspase-9. The
activated caspase-9 may further activate caspase-3, initiating the
caspase cascade reaction. Caspase-3 then cleaves substrates,
resulting in apoptosis. A previous study has demonstrated that
activated caspase-3 acts on pro-caspase-9, resulting in caspase-9
activation and forming a feedback loop which may lead to the
caspase cascade (18). Colak et
al (19) applied z-LEHD-fmk, a
caspase-9 inhibitor, in the treatment of spinal cord injury in
rats. Electron microscopy confirmed that z-LEHD-fmk was able to
protect neurons, glial cells, myelin, axons and intracellular
organelles. In the present study, the results showed that caspase-9
activity was markedly reduced following MP treatment of ASCI, which
may inhibit the activation of downstream caspases and attenuate
apoptosis. On the other hand, MP also inhibited caspase-3 activity,
which may suppress the interaction between caspase-3 and the other
caspases. All these effects finally result in the attenuation of
apoptosis in ASCI following MP treatment.
In the present study, we applied MP in the treatment
of ASCI and the activities of caspase-3, -6, -8 and -9 were
measured at different time points following the injury. The results
showed that at 8 and 24 h and 3 and 7 days after injury, the
activities of all caspases were markedly increased but the increase
in the MP treatment group was lower than that in the untreated
animals (P<0.05). At 14 days after injury, the activities of all
caspases were markedly reduced when compared with those at the
former four time points, but a marked difference was not noted
between the MP treatment and the untreated groups (P>0.05). At
28 days after injury, the activities of all the caspases were
comparable to those in the blank control group or were
undetectable. These findings demonstrate that MP is able to inhibit
the activity of these caspases and suppress their interaction,
which indirectly inhibits the mitochondria-dependent apoptosis
pathway and the caspase cascade reaction in apoptosis, resulting in
an antiapoptotic effect. However, MP was not capable of delaying
the activation or causing the inactivation of these caspases.
Thus, we speculate that MP reduces the activities of
certain significant molecules in the caspase cascade reaction
(including caspase-3, -6, -8 and -9) and compromises the
interaction between these molecules, which leads to the inhibition
of the apoptosis cascade and indirectly inhibits the
mitochondria-dependent apoptosis pathway, resulting in an
antiapoptotic effect. Finally, secondary spinal cord injury is
attenuated. However, MP cannot completely inactivate or delay the
activation of these caspases.
Acknowledgements
This study was supported by the
Science and Technology Project of Guangdong Province
(2010/170,2010B03160010) and Science and Technology Development
Fund of Macao (026/2010/A).
References
|
1
|
Lou J, Lenke LG, Ludwig FJ and O’Brien MF:
Apoptosis as a mechanism of neuronal cell death following acute
experimental spinal cord injury. Spinal Cord. 6:683–690. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torres S, Salgado-Ceballos H, Torres JL,
et al: Early metabolic reactivation versus antioxidant therapy
after a traumatic spinal cord injury in adult rats. Neuropathology.
30:36–43. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakagawa T, Zhu H, Morishima N, Li E, Xu
J, Yankner BA and Yuan J: Caspase-l2 mediates
endoplasmic-reticulum-specific apoptosis and cytotoxicity by
amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Srinivasula SM, Hegde R, Saleh A, et al: A
conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO
regulates caspase activity and apoptosis. Nature. 409:112–116.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Breckenridge DG, Kang BH, Kokel D, Mitani
S, Staehelin LA and Xue D: Caenorhabditis elegans drp-1 and
fis-2 regulate distinct cell-death execution pathways downstream of
ced-3 and independent of ced-9. Mol Cell. 31:586–597. 2008.
View Article : Google Scholar
|
|
6
|
Faubel S and Edelstein CL: Caspases as
drug targets in ischemic organ injury. Curr Drug Targets Immune
Endocr Metabol Disord. 5:269–287. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sîrbulescu RF and Zupanc GK: Inhibition of
caspase-3-mediated apoptosis improves spinal cord repair in a
regeneration-competent vertebrate system. Neuroscience.
171:599–612. 2010.PubMed/NCBI
|
|
8
|
Lahiry L, Saha B, Chakraborty J, et al:
The aflavins target Fas/caspase-8 and Akt/pBad pathways to induce
apoptosis in p53-mutated human breast cancer cells. Carcinogenesis.
31:259–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Micheau O, Thome M, Schneider P, et al:
The long form of FLIP is an activator of caspase-8 at the Fas
death-inducing signaling complex. J Biol Chem. 277:45162–45171.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fernandes-Alnemri T, Litwack G and Alnemri
ES: Mch2, a new member of the apoptotic Ced-3/Ice cysteine protease
gene family. Cancer Res. 55:2737–2742. 1995.PubMed/NCBI
|
|
11
|
Warby SC, Doty CN, Graham RK, et al:
Activated caspase-6 and caspase-6-cleaved fragments of huntingtin
specifically colocalize in the nucleus. Hum Mol Genet.
17:2390–2404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng G, Wei L, Zhi-Dan S, Shi-Guang Z and
Xiang-Zhen L: Atorvastatin ameliorates cerebral vasospasm and early
brain injury after subarachnoid hemorrhage and inhibits
caspase-dependent apoptosis pathway. BMC Neurosci. 21:72009.
View Article : Google Scholar
|
|
13
|
Li YH, Wang C, Meng K, Chen LB and Zhou
XJ: Influence of survivin and caspase-3 on cell apoptosis and
prognosis in gastric carcinoma. World J Gastroenterol.
10:1984–1988. 2004.PubMed/NCBI
|
|
14
|
Mandal D, Mazumder A, Das P, Kundu M and
Basu J: Fas-, caspase 8-, and caspase 3-dependent signaling
regulates the activity of the aminophospholipid Translocase and
phosphatidylserine externalization in human erythrocytes. J Biol
Chem. 280:39460–39467. 2005. View Article : Google Scholar
|
|
15
|
Miller CP, Ban K, Dujka ME, McConkey DJ,
Munsell M, Palladino M and Chandra J: NPI-0052, a novel proteasome
inhibitor, induces caspase-8 and ROS-dependent apoptosis alone and
in combination with HDAC inhibitors in leukemia cells. Blood.
110:267–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang JQ: The role of cytochrome C in the
apoptosis of hepatoma cell induced by fluorouracil. Zhougguo Pu
Tong Waike Zazhi. 6:1081–1084. 2007.(In Chinese).
|
|
17
|
Wu KL, Hsu C and Chan JY: Impairment of
the mitochondrial respiratory enzyme activity triggers sequential
activation of apoptosis-inducing factor-dependent and
caspase-dependent signaling pathways to induce apoptosis after
spinal cord injury. J Neurochem. 101:1552–1566. 2007. View Article : Google Scholar
|
|
18
|
Fujita E, Egashira J, Urase K, Kuida K and
Momoi T: Caspase-9 processing by caspase-3 via a feed-back
amplification loop in vivo. Cell Death Differ. 8:335–344. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Colak A, Karaoğlan A, Barut S, Köktürk S,
Akyildiz AI and Taşyürekli M: Neuroprotection and functional
recovery after application of the caspase-9 inhibitor z-LEHD-fmk in
a rat model of traumatic spinal cord injury. J Neurosurg Spine.
2:327–334. 2005. View Article : Google Scholar : PubMed/NCBI
|















