Introduction
Major surgery is associated with a transient severe
inflammatory response involving the release of proinflammatory
mediators and leads to systemic inflammation (1). Esophagectomy for esophageal carcinoma
is one of the most invasive surgical procedures and is associated
with a generalized systemic inflammatory response characterized by
the activation of proinflammatory cytokines and other chemical
mediators (2). After inflammatory
stimulus, the pattern of plasma proteins synthesized by the liver
changes significantly. In general, the concentration of positive
acute phase proteins (APPs), including C-reactive protein (CRP),
increases. These proteins are widely used as indicators in rapid
diagnosis and the assessment of the response to therapy in
inflammatory diseases. In addition, it has been reported that
polymorphonuclear cell elastase (PMNE) is released from
granulocytes during surgery and that the postoperative serum level
of PMNE is a good indicator of surgical stress. More importantly,
the monitoring of PMNE was found to be more useful than that of
serum CRP for estimation of the inflammatory status and early
detection of an acute-phase response (3–5).
The urinary trypsin inhibitor (UTI) is one of the
Kunitz-type trypsin inhibitors found in human urine and is
synthesized by the inter-α-trypsin inhibitor (ITI) family (6). As previously reviewed by Fries and
Blom (7), UTI is responsible for
most of the antitryptic activity in urine and is excreted in
increased amounts in urine under certain pathological conditions
such as cancer and bacterial infections (8–10).
Thus, UTI is an important anti-inflammatory substance, and is also
considered to be a positive APP (11,12).
UTI has previously been suggested as a possible screening test for
bacterial and/or viral infections (13). More importantly, the urine
concentration of UTI could be useful for predicting the risk of
complications and outcome of bone marrow transplantation (14).
We previously reported that preoperative
administration of methylprednisolone successfully suppresses the
release of certain indicators of response to surgical injury such
as IL-6 and IL-8, and plays a significant role in the prevention of
systemic inflammatory responses (15). Postoperative complications,
so-called stress-induced organ dysfunction states, are thought to
be caused by an uncontrolled inflammatory response due to the
overproduction of proinflammatory cytokines and chemokines
(3,16). However, the mechanisms involved in
reduced postoperative complications upon preoperative
administration of methylprednisolone have not been clearly
elucidated (17–19). Focusing on UTI and PMNE, our aim in
the present study was to better understand the relationship between
steroid and inflammatory mediators and thus to better appreciate
the information provided by preoperative administration of
methylprednisolone. We determined serum UTI and PMNE and urine UTI
levels using an enzyme-linked immunosorbent assay (ELISA). We also
evaluated the preoperative administration of methylprednisolone on
the postoperative clinical course and adverse inflammatory
reactions.
Patients and methods
Patients and study design
Thirty-one consecutive patients who underwent
radical esophagectomy for esophageal cancer at Nippon Medical
School Main Hospital between 2002 and 2004 were investigated in
this study. The patients did not receive previous intravenous
treatment with UTI. Patients who had a preoperative inflammatory
reaction within 7 days prior to surgery, preoperative complications
such as liver cirrhosis or diabetes mellitus, or tuberculosis
lesions were excluded from the study. There were no significant
differences in preoperative characteristics between the patients in
the steroid and the non-steroid groups (Table I). Therefore, even though the study
was not performed in a randomized manner, it is reasonable to state
that the data shown here suggest objective results. In the steroid
group, 10 mg/kg body weight of methylprednisolone was administered
intravenously to each patient just before the start of
esophagectomy. Informed consent according to the Declaration of
Helsinki was obtained from all patients.
 | Table I.Comparison of the clinical features
between the steroid and the non-steroid group. |
Table I.
Comparison of the clinical features
between the steroid and the non-steroid group.
| Features | Steroid group
(n=19) | Non-steroid group
(n=12) | P-value |
|---|
| Male/female | 17/2 | 8/4 | 0.12 |
| Age (year), mean ±
SD | 60.9±10.2 | 63.7±9.2 | 0.46 |
| Operative time
(min) | 498.6±49.9 | 421.8±80.0 | 0.08 |
| Intraoperative blood
loss (ml) | 844.3±364.7 | 891.9±369.0 | 0.56 |
| Tumor stage
(I:II:III:IV) | 4:3:5:7 | 5:3:1:3 | 0.40 |
Enzyme immunoassay for PMNE and UTI
Serum and urine samples were obtained and stored at
−80°C until being assayed. Serum PMNE was measured the day prior to
surgery and 24 h, 3, 5, 7 and 14 days after the start of surgery by
enzyme immunoassay (ELISA) using a commercial kit reagent (PMN
Elastase; E. Merck, Germany). Plasma and urine UTI were measured
using a one-step sandwich enzyme-linked immunosorbent assay (ELISA;
Mochida Pharmaceutical Co., Tokyo, Japan). Briefly, purified
anti-ulinastatin immunoglobulins (Igs) were labeled with
horseradish peroxidase (Toyobo Enzymes, Tokyo, Japan). Each well in
the ELISA plates was coated with 100 μl of anti-ulinastatin Igs (5
mg/ml) in carbonate buffer (pH 9.5) at 25°C for 12 h. Coated plates
were washed and subsequently blocked with 0.5% bovine serum albumin
(BSA) in phosphate-buffered saline (PBS) (pH 6.4) for 1 h at room
temperature. Then 20 μl of the diluted samples or standards was
added, after which 80 ml of peroxidase-conjugated anti-ulinastatin
Igs in 10% (v/v) rabbit serum/PBS (pH 6.4) was added. The plate was
incubated for 30 min at 37°C and washed three times with 400 μl of
physiological saline containing 0.05% Tween-20 (Biosciences Inc.,
La Jolla, CA, USA). Then 100 μl of freshly prepared
tetramethylbenzydine containing hydrogen peroxide (0.02%) was added
and incubated for 10 min at room temperature. The reaction was
terminated by the addition of 100 μl of 0.5 mol
H2SO4. The absorbance was read at 450 nm
using a microplate reader (ImmunoMini NJ-2300; Sic, Tokyo, Japan).
All samples were assayed in duplicate.
Definition of postoperative
complications
The postoperative course of each patient was
monitored daily, and the complications were defined as follows.
Pulmonary complications were defined by the presence of massive
atelectasis, pulmonary edema, or pneumonia. Postoperative
hyperbilirubinemia was defined as the peak total bilirubin level
>4 mg/dl. Anastomotic leakage was diagnosed by gastrography and
clinical features. Liver dysfunction was defined as either the
aspartate aminotransferase (AST) or alanine aminotransferase (ALT)
value >200 IU/l.
Statistical analysis
The Mann-Whitney’s U test, Chi-square test, and
repeated measure ANOVA were used to determine significant
differences between the two groups. P-values of <0.05 were
considered significant.
Results
Effect of steroid therapy on the changes
in acute phase parameters
Postoperative changes in the levels of serum UTI,
serum PMNE and urine UTI were compared between both groups.
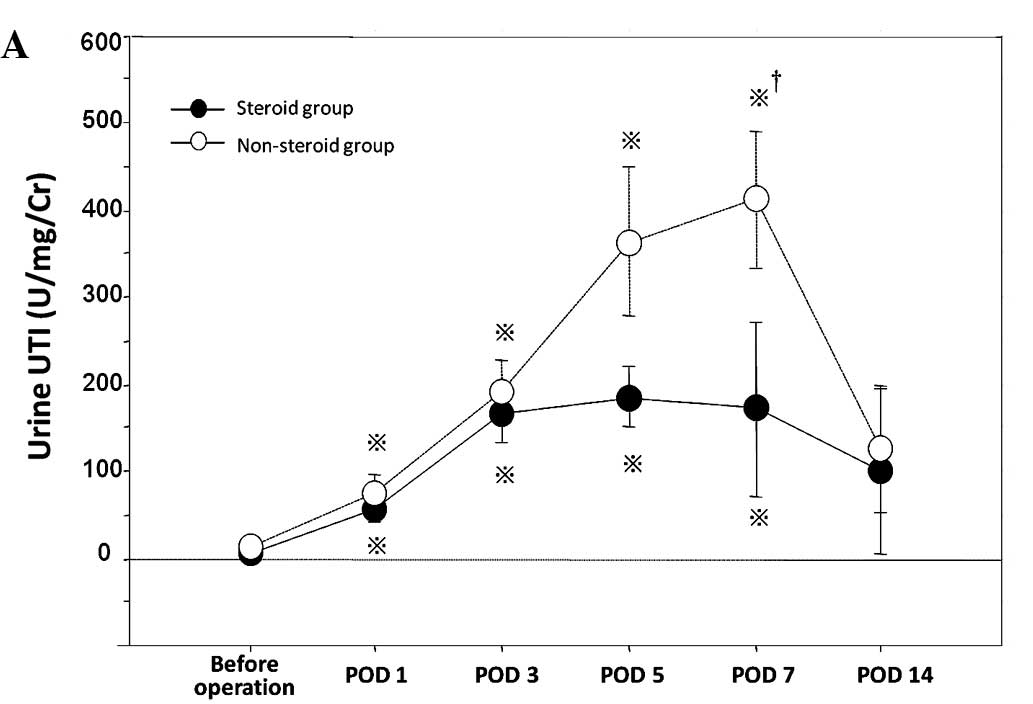
Compared with the non-steroid group, the steroid group had
significantly lower levels of urine UTI and serum PMNE (Fig. 1A and C). Regarding the level of
serum UTI, no significant difference was noted between the groups
(Fig. 1B).
Correlation between urine UTI and serum
levels of aminotransferases
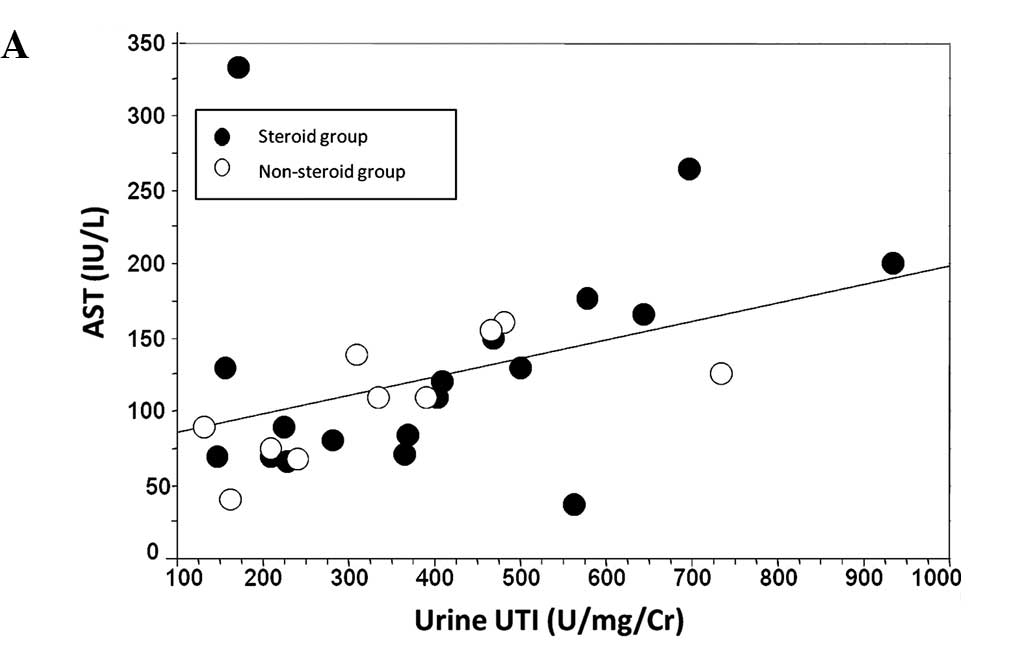
For the evaluation of hepatic injury, the peak
levels of AST and ALT in the serum were correlated with that of
urine UTI. The peak levels of UTI correlated positively with those
of aminotransferases (Fig. 2).
Correlation between urine UTI and
postoperative complications
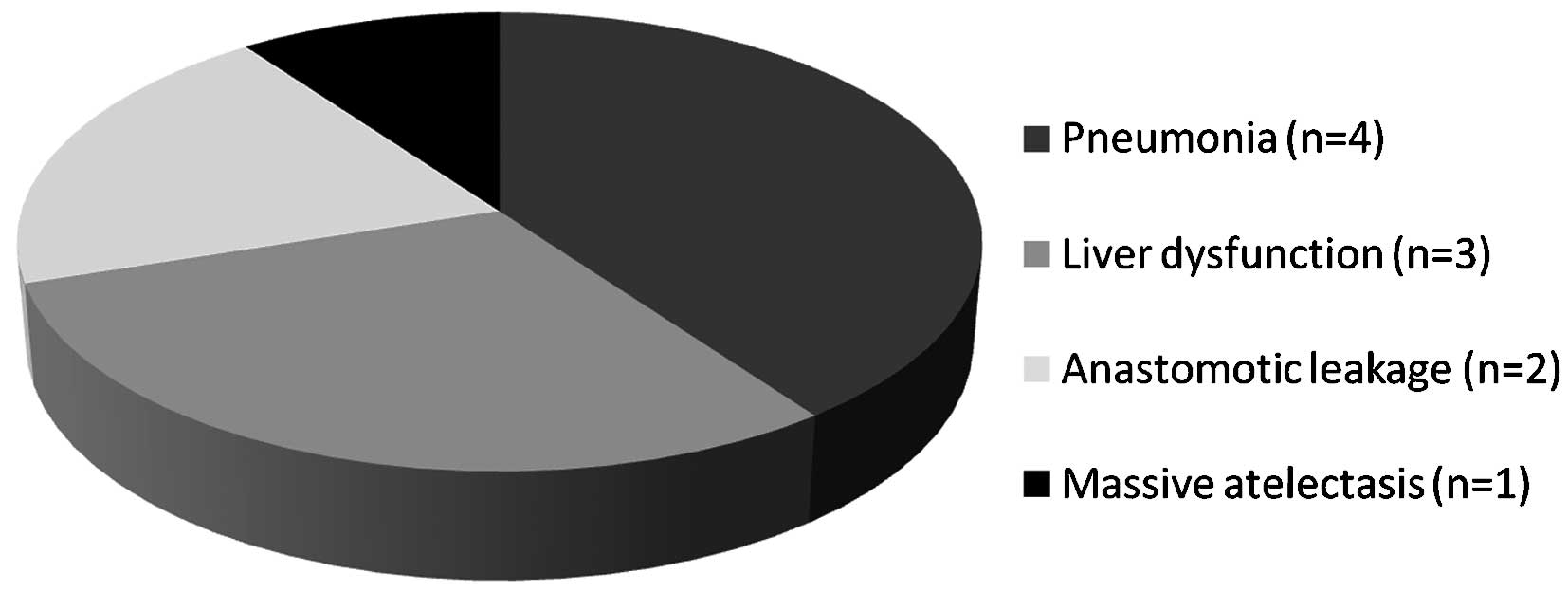
The postoperative complications are shown in
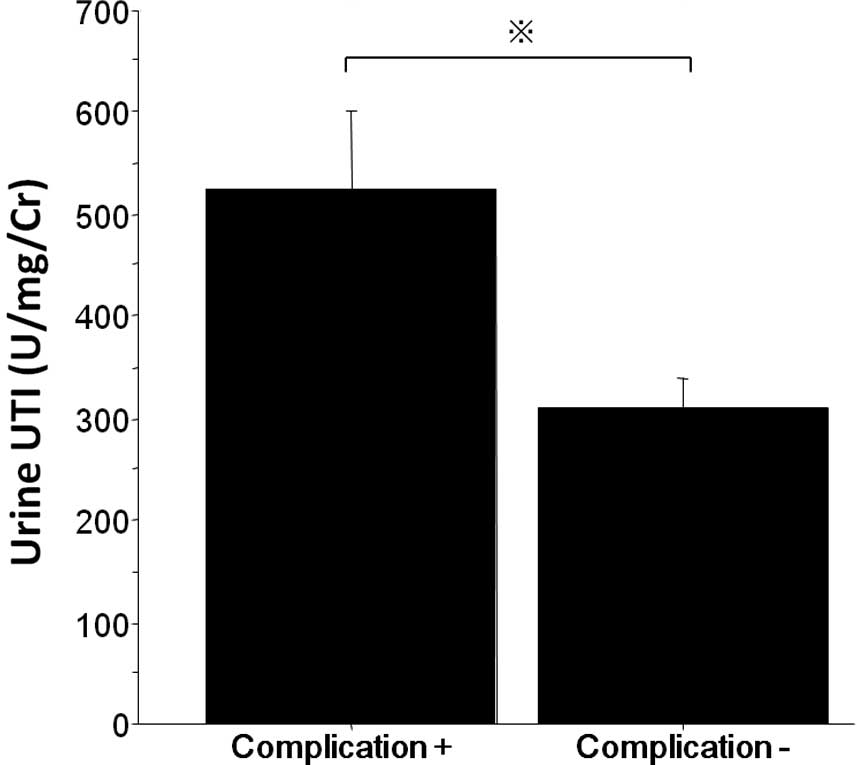
Fig. 3. The maximum level of urine
UTI in patients without complications was lower than that in
patients with complications (Fig.
4).
Discussion
This study was designed to understand the
relationship between steroid and inflammatory mediators and to
evaluate the effects of preoperative administration of
methylprednisolone. Patients with esophageal cancer scheduled for
surgery were selected for the following reasons. While a balance of
proinflammatory and anti-inflammatory mediators is essential for
the appropriate immune response of patients, the postoperative
overproduction of proinflammatory mediators may lead to tissue
damage via the production of free oxygen species and may also
result in systemic inflammatory response syndrome (SIRS) with
subsequent multiorgan failure (20). Surgical treatment of esophageal
cancer is one of the most stressful surgical procedures, and the
frequency of postoperative organ failure remains high (21–23).
Esophageal cancer surgery was therefore selected as one of the most
appropriate procedures for evaluating the effects of steroids on
surgical stress.
UTI is one of the protease inhibitors, and is
produced in the liver and kidneys (24,25).
It is also one of the acute phase reactants (11,13).
The amount of this inhibitor excreted in the urine is considered
proportional to the invasiveness of an insult to the host,
typically major surgery (14). In
the present study, the concentration of serum UTI was significantly
lower than that of urine UTI, and no significant difference was
noted between the steroid and non-steroid group (Fig. 1). These results are in accord with
those from other studies which found that the concentration of
serum UTI of patients undergoing partial hepatectomy was only
slightly increased and even appeared to be unmodified during the
acute phase response (12).
Indeed, owing to its small amount, UTI circulating in the blood
secondarily diffuses into extracellular spaces and reaches tissues.
On the other hand, most of it is quickly excreted in the urine
(26). Therefore, the amount of
UTI present in urine appears to be a better indicator of UTI
released in circulating plasma.
Methylprednisolone has been known to suppress the
release of mediators (autocrine and paracrine), such as IL-6, IL-8
and PMNE, following surgical injury, and was found to play a
significant role in the prevention of developing the subsequent
spread of inflammation. PMNE metabolizes inter-α-inhibitor (IαI) to
UTI, which then suppresses the activity of PMNE, thus forming a
homeostatic negative feedback loop (27). It has been suggested that when the
balance is upset and PMNE activity exceeds that of UTI,
inflammation develops (28). As
mentioned by Faarvang and Lauritsen (29), increased excretion of urine UTI has
been recognized in response to inflammatory conditions. This was
confirmed by our results which demonstrated that levels of urine
UTI and serum PMNE in the steroid group were significantly
decreased compared to those in the non-steroid group (Fig. 1).
During the acute-phase response, a large number of
mediators which can cause postoperative complications are
generated, and all of these may be markers of inflammation. It has
been reported that there is a strong correlation between the
concentrations of CRP in whole blood and urine UTI (10,30).
More importantly, UTI is a better predictor of the subsequent
spread of inflammation compared to CRP (31). In all of our patients, urine UTI
levels correlated positively with serum levels of aminotransferases
(Fig. 2). This result supports the
hypothesis that UTI reflects liver injury as well.
It is of great importance that the possible clinical
effects of preoperative steroid administration include the
prevention of postoperative complications. Yamashita et al
(32) and Shirabe (33) previously reported that the
administration of steroids in liver surgery results in decreased
values of immunosuppressive acid protein and postoperative positive
rate of serum Candida antigen, which is a marker of bacterial
translocation. Similar findings were reported for other surgical
operations. Shimada et al (34) reported that morbidity rates
including hyperbilirubinemia, anastomotic leakage, and liver
dysfunction in patients with esophagectomy were significantly lower
in a steroid group than in a non-steroid group. In addition, the
median hospital stay was reported to be shorter in a steroid group,
and adverse effects of steroid use, such as abnormality in glucose
tolerance and delay in wound healing, did not occur following liver
surgery (35). In the present
study, the level of urine UTI in the steroid group was decreased
compared to that in the non-steroid group (Fig. 1), and the maximum level of urine
UTI in patients without complications was significantly lower than
that in patients with complications (Fig. 4). According to these results, the
UTI concentration predicts complications following esophagectomy,
and the suppression of UTI levels induced by steroid administration
may be one of the reasons for the reduction in postoperative
complications.
A study with a much larger population is required
before any firm conclusions can be drawn. Based on findings of the
present study, it was suggested that urine UTI provides useful
information on postoperative clinical course, and that preoperative
administration of methylprednisolone may contribute to decrease
postoperative complications following esophagectomy.
Acknowledgements
We thank the hospital committees for
endorsing this study.
References
|
1
|
Cruickshank AM, Fraser WD, Burns HJ, Van
Damme J and Shenkin A: Response of serum interleukin-6 in patients
undergoing elective surgery of varying severity. Clin Sci (Lond).
79:161–165. 1990.PubMed/NCBI
|
|
2
|
Ono S, Aosasa S and Mochizuki H: Effects
of a protease inhibitor on reduction of surgical stress in
esophagectomy. Am J Surg. 177:78–82. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakamoto K, Arakawa H, Mita S, et al:
Elevation of circulating interleukin 6 after surgery: factors
influencing the serum level. Cytokine. 6:181–186. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kato M, Suzuki H, Murakami M, Akama M,
Matsukawa S and Hashimoto Y: Elevated plasma levels of
interleukin-6, interleukin-8, and granulocyte colony-stimulating
factor during and after major abdominal surgery. J Clin Anesth.
9:293–298. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sato N, Murakami K, Ishida K, Ikeda K and
Saito K: Pulmonary hypertension and polymorphonuclear leukocyte
elastase after esophageal cancer operations. Ann Thorac Surg.
51:754–758. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salier JP, Rouet P, Raguenez G and Daveau
M: The inter-alpha-inhibitor family: from structure to regulation.
Biochem J. 315:1–9. 1996.
|
|
7
|
Fries E and Blom AM: Bikunin - not just a
plasma proteinase inhibitor. Int J Biochem Cell Biol. 32:125–137.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mizon C, Balduyck M, Bonneterre JP and
Mizon J: Urinary trypsin inhibitory capacity determination: study
in patients with disseminated cancers. Bull Cancer. 70:266–270.
1983.PubMed/NCBI
|
|
9
|
Chawla RK, Rausch DJ, Miller FW, Vogler WR
and Lawson DH: Abnormal profile of serum proteinase inhibitors in
cancer patients. Cancer Res. 44:2718–2723. 1984.PubMed/NCBI
|
|
10
|
Gosset D, Mizon C, Savinel P, et al:
Clinical value of the determination of urinary antitrypsin
activity. Presse Med. 17:329–332. 1988.(In French).
|
|
11
|
Kuwajima S, Matsui T, Kitahashi S, et al:
Automated measurement of trypsin inhibitor in urine with a
centrifugal analyzer: comparison with other acute phase reactants.
Clin Biochem. 23:167–171. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Noie T, Sugawara Y, Harihara Y, et al:
Kinetics of urinary trypsin inhibitor in patients undergoing
partial hepatectomy. Scand J Gastroenterol. 36:410–416. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Piette AM, Saba J, Bernard N, et al:
Urinary trypsin inhibitory activity for the diagnosis of bacterial
infection: a prospective study in 690 patients. Eur J Med.
1:273–276. 1992.PubMed/NCBI
|
|
14
|
Yamada S, Takatsuka H, Takemoto Y, et al:
Urinary trypsin inhibitor concentration can predict the
immunological insult of chemotherapy and complications after bone
marrow transplantation. Bone Marrow Transplant. 27:195–199. 2001.
View Article : Google Scholar
|
|
15
|
Takeda S, Takeda S, Kim C, et al:
Preoperative administration of methylprednisolone attenuates
cytokine-induced respiratory failure after esophageal resection. J
Nippon Med Sch. 70:16–20. 2003. View Article : Google Scholar
|
|
16
|
Sato N, Koeda K, Ikeda K, et al:
Randomized study of the benefits of preoperative corticosteroid
administration on the postoperative morbidity and cytokine response
in patients undergoing surgery for esophageal cancer. Ann Surg.
236:184–190. 2002. View Article : Google Scholar
|
|
17
|
Bone RC, Fisher CJ Jr, Clemmer TP, Slotman
GJ, Metz CA and Balk RA: A controlled clinical trial of high-dose
methylprednisolone in the treatment of severe sepsis and septic
shock. N Engl J Med. 317:653–658. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tonnesen E, Wanscher M, Hohndorf K, et al:
Effect of methylprednisolone on the cytokine response in patients
undergoing lung surgery. Acta Anaesthesiol Scand. 37:410–414. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miyashita M: Controversy of
corticosteroids in septic shock. J Nippon Med Sch. 77:67–70. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
American College of Chest
Physicians/Society of Critical Care Medicine Consensus Conference:
Definitions for sepsis and organ failure and guidelines for the use
of innovative therapies in sepsis. Crit Care Med. 20:864–874. 1992.
View Article : Google Scholar
|
|
21
|
Swisher SG, Hunt KK, Holmes EC, Zinner MJ
and McFadden DW: Changes in the surgical management of esophageal
cancer from 1970 to 1993. Am J Surg. 169:609–614. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Isono K, Sato H and Nakayama K: Results of
a nationwide study on the three-field lymph node dissection of
esophageal cancer. Oncology. 48:411–420. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fok M, Law SY and Wong J: Operable
esophageal carcinoma: current results from Hong Kong. World J Surg.
18:355–360. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaumeyer JF, Polazzi JO and Kotick MP: The
mRNA for a proteinase inhibitor related to the HI-30 domain of
inter-alpha-trypsin inhibitor also encodes alpha-1-microglobulin
(protein HC). Nucleic Acids Res. 14:7839–7850. 1986. View Article : Google Scholar
|
|
25
|
Sugiki M, Sumi H, Maruyama M, Yoshida E
and Mihara H: Clearance and distribution of acid-stable trypsin
inhibitor (ASTI). Enzyme. 42:31–38. 1989.PubMed/NCBI
|
|
26
|
Mizon C, Piva F, Queyrel V, Balduyck M,
Hachulla E and Mizon J: Urinary bikunin determination provides
insight into proteinase/proteinase inhibitor imbalance in patients
with inflammatory diseases. Clin Chem Lab Med. 40:579–586. 2002.
View Article : Google Scholar
|
|
27
|
Kobayashi H: Endogenous anti-inflammatory
substances, inter-alpha-inhibitor and bikunin. Biol Chem.
387:1545–1549. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ogawa M, Nishibe S, Mori T and Neumann S:
Effect of human urinary trypsin inhibitor on granulocyte elastase
activity. Res Commun Chem Pathol Pharmacol. 55:271–274.
1987.PubMed/NCBI
|
|
29
|
Faarvang HJ and Lauritsen OS: Circadian
variations in sensitivity to glucocorticoid evaluated by urinary
trypsin inhibitor excretion. Proc Soc Exp Biol Med. 120:338–340.
1965. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ueki M, Yokono S, Taie S, Nogaya J,
Komatsu H and Ogli K: Changes of urinary ulinastatin and serum CRP
after elective surgery for gastric cancer. Masui. 45:933–936.
1996.(In Japanese).
|
|
31
|
Pugia MJ and Lott JA: Pathophysiology and
diagnostic value of urinary trypsin inhibitors. Clin Chem Lab Med.
43:1–16. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamashita Y, Shimada M, Hamatsu T, et al:
Effects of preoperative steroid administration on surgical stress
in hepatic resection: prospective randomized trial. Arch Surg.
136:328–333. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shirabe K, Takenaka K, Yamatomto K, et al:
Impaired systemic immunity and frequent infection in patients with
Candida antigen after hepatectomy. Hepatogastroenterology.
44:199–204. 1997.PubMed/NCBI
|
|
34
|
Shimada H, Ochiai T, Okazumi S, et al:
Clinical benefits of steroid therapy on surgical stress in patients
with esophageal cancer. Surgery. 128:791–798. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aldrighetti L, Pulitano C, Arru M, et al:
Impact of preoperative steroid administration on
ischemia-reperfusion injury and systemic responses in liver
surgery: a prospective randomized study. Liver Transpl. 12:941–949.
2006. View
Article : Google Scholar
|