Introduction
Gastric cancer (GC) remains a major public health
issue worldwide, with the highest incidence in China, Japan and
Eastern European countries, despite a steady decrease in incidence
and mortality in the past 30 years (1,2). The
etiology of GC has a clear environmental component characteristic
of the geographically varied incidence in its distribution
(1,2). Over the years, several environmental
factors, including diet, tobacco smoking and Helicobacter
pylori infection, have been shown to be responsible for gastric
carcinogenesis (2,3). Besides environmental factors, genetic
factors also play an important role in the development of GC, and
this is confirmed by the fact that only a small proportion of
individuals exposed to known environmental risk factors develop GC
(4,5). Additionally, recent studies have
shown that single-nucleotide polymorphisms (SNPs) in genes encoding
tumor necrosis factor α, COX-2, and CD14 are associated with
increased GC risk (5–7), indicating that genetic variation
contributes to GC carcinogenesis. However, the molecular mechanism
of GC pathogenesis is still unknown.
Located on chromosome 8q24.2, the prostate stem cell
antigen (PSCA) gene encodes a 123-amino acid glycoprotein, which is
a cell surface antigen. PSCA was first identified as a
prostate-specific antigen overexpressed in prostate cancers,
including metastatic and hormone-refractory cancers. However, it is
also expressed in other solid tumors, including ovarian mucinous
and pancreatic cancer, renal-cell carcinoma and bladder cancer
(8,9). In contrast with observations in other
tumors, PSCA expression is downregulated in GC (10). Although an in vitro
cell-proliferation inhibitory activity of PSCA was reported, the
regulation of PSCA expression and its physiological function are
still largely unknown (9,11). Previously, genome-wide association
studies (GWAS) have found that two SNPs in PSCA, rs2976392 and
rs2294008, are significantly associated with GC in Chinese,
Japanese, Korean and Caucasian individuals, particularly for
diffuse-type GC (11–13). However, the sample size of these
studies was small, and the association between SNPs (rs2976392 and
rs2294008) in the PSCA gene and susceptibility to intestinal-type
GC remains controversial (11,13).
In addition, the prognostic value of PSCA gene polymorphisms for GC
patients requires additional study.
The aim of this study was to perform a systematic
review and meta-analysis concerning: i) the association between
PSCA gene polymorphisms and GC risk; ii) the influence of this
polymorphism on survival outcomes in GC patients.
Materials and methods
Search strategy
The literature was searched using PubMed and EMBASE
to identify relevant and available published articles. The upper
limit of the search date was not specified and the lower limit was
July 2011. The following search terms were used: gastric
cancer/carcinoma/tumor/neoplasm, stomach
cancer/carcinoma/tumor/neoplasm, prostate stem cell antigen/PSCA,
and polymorphism (rs2976392 or rs2294008). Free text and MeSH
search for keywords were employed. The language in which the papers
were written was not restricted. To search for more potentially
relevant trials, reference lists from studies included in the
electronic search were screened.
Inclusion and exclusion criteria
The inclusion criteria of this meta-analysis were as
follows: i) independent case-control or cohort studies (for humans
only); and ii) studies providing complete case and control data
regarding the association between PSCA gene polymorphism (rs2976392
or rs2294008) and GC risk (or survival outcomes of GC patients).
The exclusion criteria were: i) no controls; ii) incomplete data;
and iii) duplicate publications.
Data extraction
Information was carefully extracted from all
eligible studies independently by two researchers (Zhen Wang and
Jun-Qiang Chen) according to the above-mentioned inclusion
criteria. The following variables were extracted if available:
first author, publication time, country and ethnicity of the
sample, genotyping method, sample size (numbers of GC patients and
control subjects) and the results of studies. Where a study
reported results on different sub-populations according to
ethnicity, we considered each sub-population as a separate study in
our meta-analysis. Final agreement was obtained through
discussion.
Statistical analysis
The association of PSCA gene polymorphisms with GC
risk and survival outcomes in GC patients were assessed by pooled
odds (ORs) or hazard ratios (HRs) with their corresponding 95%
confidence intervals (CIs), respectively. Statistical heterogeneity
between and within groups were measured by using the Q test, and
P<0.1 was considered to indicate statistical significance. The
fixed-effects model was used to pool the data, but the
random-effects model was used if statistical heterogeneity
(Ph<0.1) was found. Additionally, subgroup analysis
was conducted on the basis of ethnicity, Lauren’s classification,
and tumor location of GC in order to establish the effect of
clinical heterogeneity. Funnel plots were used to assess
publication bias. All of the calculations were performed using
Revman version 5.1 (14), and all
statistical tests were two-sided.
Results
Study selection and description
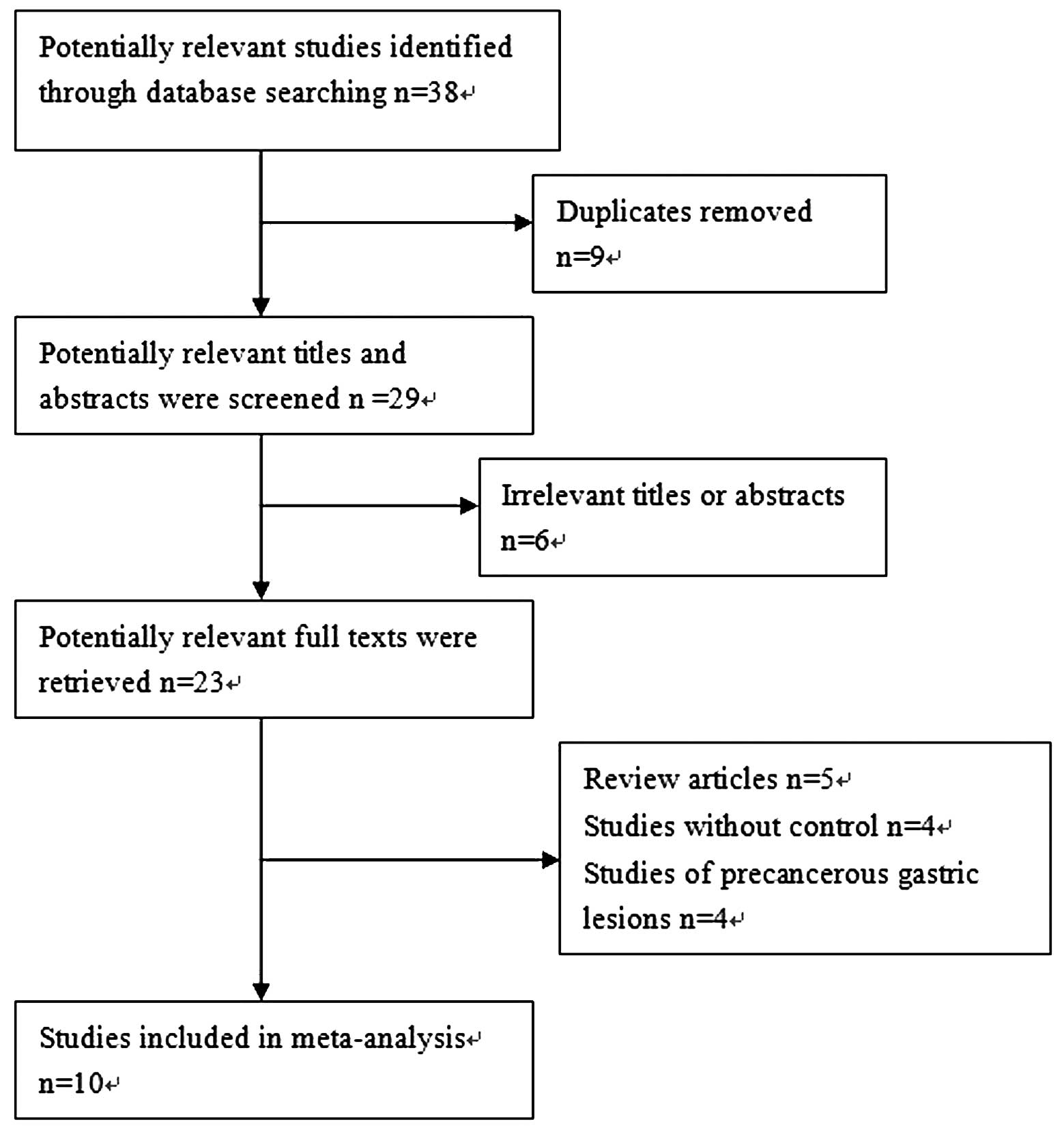
According to the search strategy above, a total of
38 studies were yielded: 23 in Pubmed and 15 in Embase. Through the
step of screening the titles and abstracts, a number of articles
were found to be irrelevant or were identified in more than one
database. Therefore, 23 articles remained for potential inclusion
and were obtained in full-text version. Following review of the
full text, 13 articles were excluded. The main reasons for
excluding studies were as follows: i) review articles; ii) studies
without control; and iii) studies of precancerous gastric lesions.
A total of 10 studies (11–13,15–21)
were eligible for this meta-analysis. The study selection process
is shown in Fig 1. Among the 10
included studies, 9 studies analyzed the association between SNPs
(9 studies of rs2294008 polymorphism and 5 studies of rs2976392
polymorphism) and GC risk (11–13,15–20);
and 2 studies analyzed the association between rs2294008
polymorphism and the survival of GC patients (20,21).
Notably, the study by Wang et al (21) only analyzed the association between
the rs2294008 polymorphism and the survival of GC patients, thus
there was no control in this study.
Table I lists the
main characteristics of the 10 included studies, including first
author, published year, original country, ethnicity, genotyping
method and number of cases and controls. Tables II and III list genotype distribution of
rs2294008 and rs2976392 polymorphisms. The distribution of
genotypes in the controls was consistent with Hardy-Weinberg
equilibrium in all studies except one (which did not report
clearly) (11). Table IV lists the main results of the
meta-analysis of PSCA gene (rs2976392 or rs2294008) polymorphism
and GC risk.
 | Table I.Characteristics of studies included in
the meta-analysis. |
Table I.
Characteristics of studies included in
the meta-analysis.
| First
author/(ref) | Year | Country | Ethnicity | Genotyping
method | Cases | Controls | Genes |
|---|
| Ou (15) | 2010 | China | Asian | PCR/LDR | 196 | 246 | rs2294008 and
rs2976392 |
| Song (16) | 2011 | Korea | Asian | PCR-RFLP | 3245 | 1700 | rs2294008 |
| Lochhead a (12) | 2011 | Poland | Caucasian | PCR | 312 | 383 | rs2294008 |
| Lochhead b (12) | 2011 | USA | Caucasian | PCR | 309 | 211 | rs2294008 |
| Sakamoto a (11) | 2008 | Japan | Asian | PCR | 1531 | 1398 | rs2294008 and
rs2976392 |
| Sakamoto b
(11) | 2008 | Korea | Asian | PCR | 875 | 390 | rs2294008 and
rs2976392 |
| Lu (13) | 2010 | China | Asian | PCR-RFLP | 1053 | 1100 | rs2294008 and
rs2976392 |
| Zeng (20) | 2011 | China | Asian | PCR-RFLP | 460 | 549 | rs2294008 |
| Wu (17) | 2009 | China | Asian | PCR-RFLP | 1736 | 1020 | rs2294008 and
rs2976392 |
| Matsuo (18) | 2009 | Japan | Asian | PCR | 708 | 708 | rs2294008 and
rs2976392 |
| Sala (19) | 2011 | 10 European
countries | Caucasian | PCR | 411 | 1530 | rs2294008 |
| Wang (21) | 2010 | China | Asian | PCR | 943 | no | rs2294008 |
 | Table II.Genotype distribution of
rs2294008. |
Table II.
Genotype distribution of
rs2294008.
| Study/(ref) | Cases
| Controls
| HWE |
|---|
| CC | CT | TT | C (%) | T (%) | CC | CT | TT | C (%) | T (%) |
|---|
| Ou (15) | 85 | 93 | 18 | 263 (67.1) | 129 (32.9) | 132 | 96 | 18 | 360 (73.2) | 132 (26.8) | >0.05 |
| Song (16) | 576 | 1620 | 1049 | 2772 (42.7) | 3718 (57.3) | 414 | 818 | 468 | 1646 (48.4) | 1754 (51.6) | 0.13 |
| Lochhead a
(12) | 47 | 143 | 102 | 237 (40.6) | 347 (59.4) | 101 | 166 | 115 | 368 (48.2) | 396 (51.8) | >0.05 |
| Lochhead b
(12) | 85 | 129 | 94 | 299 (48.5) | 317 (51.5) | 49 | 110 | 49 | 208 (50.0) | 208 (50.0) | >0.05 |
| Sakamoto a
(11) | 96 | 700 | 728 | 892 (29.3) | 2156 (70.7) | 210 | 650 | 536 | 1070 (38.3) | 1722 (61.7) | NR |
| Sakamoto b
(11) | 133 | 461 | 277 | 727 (41.7) | 1015 (58.3) | 122 | 176 | 92 | 420 (53.8) | 360 (46.2) | NR |
| Lu (13) | 547 | 404 | 72 | 1498 (73.2) | 548 (26.8) | 605 | 387 | 77 | 1597 (74.7) | 541(25.3) | 0.166 |
| Zeng (20) | 202 | 216 | 42 | 620 (67.4) | 300 (32.6) | 289 | 223 | 37 | 801 (73.0) | 297 (27.0) | 0.493 |
| Wu (17) | 759 | 819 | 132 | 2337 (68.3) | 1083 (31.7) | 506 | 412 | 77 | 1424 (71.6) | 566 (28.4) | >0.05 |
| Matsuo (18) | 330 | 329 | 49 | 989 (69.8) | 427 (30.2) | 273 | 338 | 97 | 884 (62.4) | 532 (37.6) | 0.64 |
| Sala (19) | 93 | 198 | 118 | 384 (46.9) | 434 (53.1) | 491 | 714 | 310 | 1696 (56.0) | 1334 (44.0) | >0.05 |
 | Table III.Genotype distribution of
rs2976392. |
Table III.
Genotype distribution of
rs2976392.
| Study/(ref) | Cases
| Controls
| HWE |
|---|
| GG | GA | AA | G (%) | A (%) | GG | GA | AA | G (%) | A (%) |
|---|
| Ou (15) | 99 | 85 | 12 | 283 (72.2) | 109 (27.8) | 130 | 102 | 14 | 362 (73.6) | 130 (26.4) | >0.05 |
| Sakamoto a
(11) | 97 | 691 | 737 | 885 (29.0) | 2165 (71.0) | 211 | 650 | 536 | 1072 (38.4) | 1722 (61.6) | NR |
| Sakamoto b
(11) | 134 | 453 | 278 | 721 (41.7) | 1009 (58.3) | 122 | 175 | 93 | 419 (53.7) | 361 (46.3) | NR |
| Lu (13) | 500 | 464 | 79 | 1464 (70.2) | 622 (29.8) | 602 | 402 | 78 | 1606 (74.2) | 558 (25.8) | 0.336 |
| Wu (17) | 789 | 793 | 142 | 2371 (68.8) | 1077 (31.2) | 492 | 429 | 81 | 1413 (70.5) | 591 (29.5) | >0.05 |
| Matsuo (18) | 331 | 328 | 48 | 990 (70.0) | 424 (30.0) | 274 | 337 | 96 | 885 (62.6) | 529 (37.4) | 0.64 |
 | Table IV.Results of the meta-analysis
(OR). |
Table IV.
Results of the meta-analysis
(OR).
| Polymorphism | Overall | Asians | Caucasians | Diffuse | Intestinal | Cardia | Non-cardia |
|---|
| rs2294008 C/T | | | | | | | |
| CT/TT vs. CC
(D) | 1.44 r (1.16,
1.79) | 1.46 r (1.13,
1.90) | 1.37 r (0.87,
2.16) | 1.42 r (0.89,
2.27) | 1.30 r (0.91,
1.86) | 1.21 f (1.03,
1.42) | 1.45 f (1.26,
1.66) |
| TT vs. CT/CC
(R) | 1.20 r (1.01,
1.42) | 1.12 r (0.90,
1.39) | 1.44 f (1.20,
1.72) | 1.04 r (0.60,
1.80) | 1.05 r (0.76,
1.46) | 1.00 f (0.76,
1.31) | 1.36 f (1.11,
1.68) |
| T vs. C (A) | 1.23 r (1.08,
1.40) | 1.22 r (1.04,
1.43) | 1.33 f (1.19,
1.49) | 1.13 r (0.85,
1.52) | 1.14 r (0.89,
1.46) | 1.12 f (0.99,
1.26) | 1.31 f (1.19,
1.45) |
| rs2976392 A/G | | | | | | | |
| GA/AA vs. GG
(D) | 1.41 r (0.98,
2.04) | - | - | 2.80 r (1.43,
5.47) | 1.46 f (1.27,
1.69) | 1.03 f (0.85,
1.25) | 1.20 f (1.01,
1.43) |
| AA vs. GA/GG
(R) | 1.05 r (0.75,
1.48) | - | - | 1.67 f (1.45,
1.92) | 1.22 f (1.05,
1.42) | 0.82 f (0.57,
1.20) | 1.04 f (0.75,
1.42) |
| A vs. G (A) | 1.17 r (0.93,
1.48) | - | - | 2.18 r (1.21,
3.91) | 1.26 f (1.15,
1.38) | 0.99 r (0.85,
1.15) | 1.12 f (0.98,
1.28) |
rs2294008 polymorphism and GC risk
Nine studies were eligible for meta-analysis
(11–13,15–20),
and the results of the meta-analysis indicated that rs2294008 C/T
polymorphism of the PSCA gene was significantly associated with
susceptibility to GC (dominant model: OR, 1.44; 95% CI, 1.16–1.79;
recessive model: OR, 1.20; 95% CI, 1.01–1.42; additive model: OR,
1.23; 95% CI, 1.08–1.40). Significant between-study heterogeneity
was detected in all genetic models (Ph<0.1). We
therefore performed subgroup analysis according to ethnicity,
Lauren’s classification, and tumor location. In the subgroup
analysis based on ethnicity, significant association was detected
in both Asians (dominant and additive model, Table IV) and Caucasians (recessive and
additive model, Table IV).
Notably, in subgroup analysis based on Lauren’s classification, no
significant association was detected for either diffuse-type or
intestinal-type GC (Table IV). In
subgroup analysis based on tumor location, a significantly
increased GC risk was observed in both cardia and non-cardia
subgroups, but the effect was larger in the non-cardia GC (Table IV).
rs2976392 polymorphism and GC risk
Five studies were eligible for meta-analysis
(11,13,15,17–18),
and all the included patients were Asians. The result of the
meta-analysis indicated that rs2976392 A/G polymorphism was
associated with increased risk of GC, but the difference was not
statistically significant (dominant model: OR, 1.41; 95% CI,
0.98–2.04; Table IV). Notably, in
subgroup analysis based on Lauren’s classification, significant
association was detected in diffuse and intestinal subgroups, and
the association was stronger for the diffuse histological type
(Table IV). However, in subgroup
analysis based on tumor location, a significantly increased GC risk
was observed in the non-cardia subgroup (dominant model, Table IV), but not in the cardia
subgroup.
Association of PSCA polymorphisms with
the survival of GC patients
Only two included studies analyzed the association
of PSCA rs2294008 genotypes with GC survival (20,21),
and the results of the two studies were opposing. Therefore, we did
not combine the data here. Wang et al (21) found no significant association
between the genotypes and the survival of GC; however, by using
stratified analysis according to Lauren’s classification they found
that the patients with rs2294008 CT/TT variant genotypes had a 25%
significantly increased survival (HR, 0.75; 95% CI, 0.59–0.96),
compared to the CC homo-zygote among the diffuse-type GC. By
contrast, Zeng et al (20)
revealed that the TT genotype was associated with poor prognosis in
GC patients (HR, 2.12; 95% CI, 1.22–3.69).
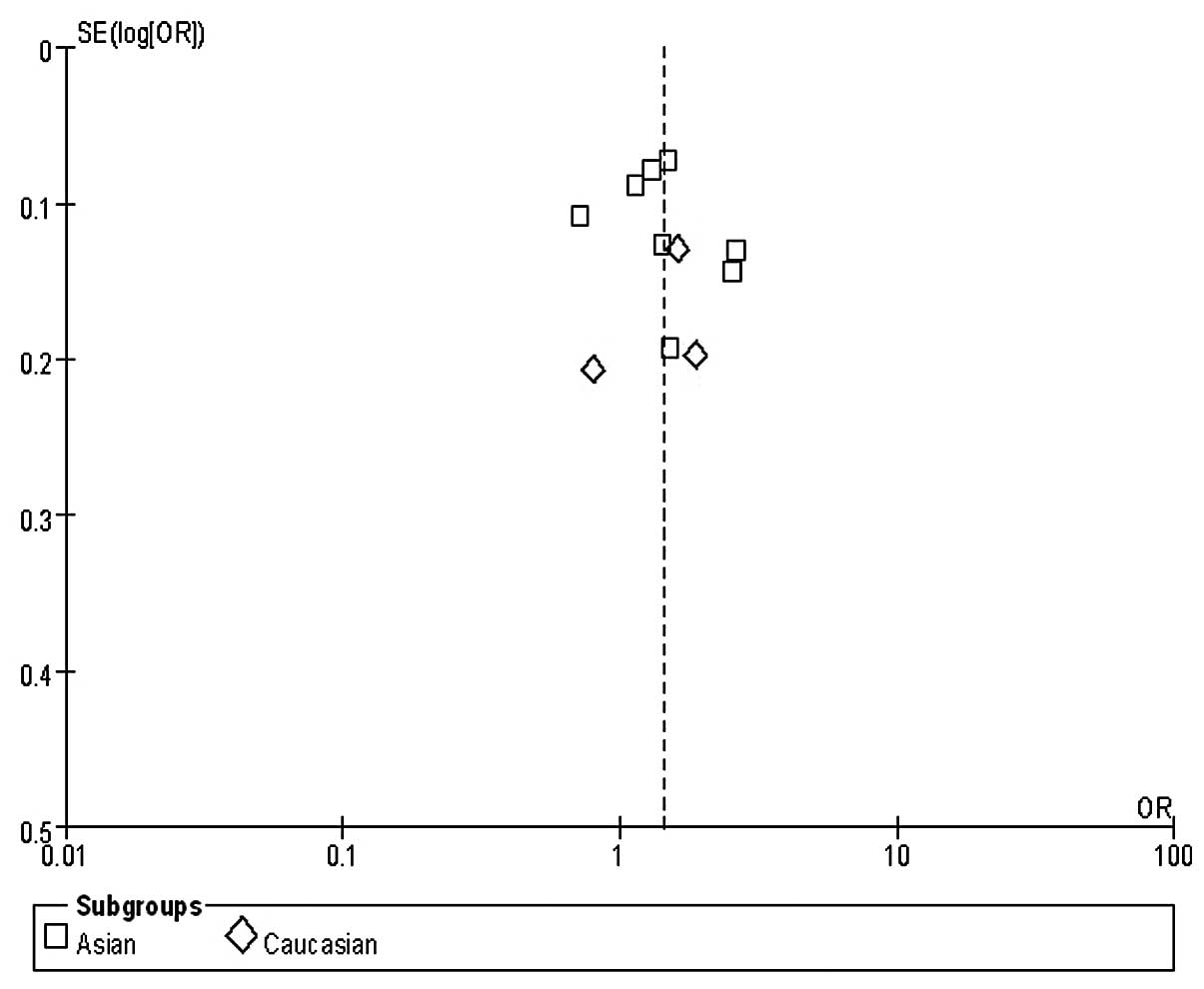
Publication bias
One funnel plot of the outcome of the rs2294008
polymorphism and GC risk in included studies demonstrated symmetry,
indicating no serious publication bias (Fig. 2).
Discussion
Despite recent progress in the treatment of GC, the
prognosis of GC patients remains poor. To the best of our
knowledge, stage is the best available clinical measure of tumor
aggression and prognosis, but there are significant differences
even within the same tumor stage (22). Therefore, discovery of new
biomarkers and their application, in conjunction with traditional
cancer diagnosis, staging and prognosis, may improve early
diagnosis and reasonable care to a large extent. Previous studies
have focused on the detection of genetic variants that are
correlated with the development and progression of GC (23).
Certain Investigators have proposed that PSCA is
involved in intracellular signaling, but much remains unknown
regarding its physiological function and regulatory mechanism in
normal and cancer cells. PSCA is up-regulated in prostate cancer
and its expression is positively correlated with advanced clinical
stage and metastasis in prostate cancer. Therefore, PSCA has been
considered as a biomarker of diagnosis and prognosis, as well as a
target of therapy for prostate cancer (8). By contrast, PSCA is down-regulated in
GC and may have tumor-suppressing function in the gastric
epithelium (10). Promising
observations that genetic variations of PSCA (rs2976392 and
rs2294008) are closely associated with the risk and survival of GC
have opened up a new avenue of research regarding the pathological
function of PSCA (11,20). Here, we conducted a meta-analysis
in order to validate the association between these PSCA
polymorphisms and GC risk and survival. We found that rs2294008 C/T
(dominant model: OR, 1.44; 95% CI, 1.16–1.79) and rs2976392 A/G
(dominant model: OR, 1.41; 95% CI, 0.98–2.04) polymorphisms were
associated with increased risk of GC, although the association of
rs2976392 was not statistically significant.
Two studies from different countries confirmed the
significant association between PSCA polymorphisms and GC risk
(13,18). Notably, the frequency of risk T
allele of rs2294008 and risk A allele of rs2976392 in populations
of Japanese (0.617 and 0.616, respectively) was more common than
those in Chinese (0.256 and 0.244, respectively), indicating the
potential genetic heterogeneity among different populations.
Therefore, we performed subgroup analysis according to ethnicity in
this meta-analysis. We found that rs2294008 polymorphism was
significantly associated with GC risk in Asians (dominant model:
OR, 1.46; 95% CI, 1.13–1.90) and Caucasians (recessive model: OR,
1.44; 95% CI, 1.20–1.72). However, subgroup analysis was not
conducted for rs2976392 as all the included patients were Asians,
but the result of meta-analysis indicated that the rs2976392
polymorphism was not significantly associated with GC risk in
Asians (dominant model: OR, 1.41; 95% CI, 0.98–2.04). This result
differed from those of previous studies, which reported that the
two SNPs were significantly associated with GC risk, and that the
associations were weaker in Chinese than in Japanese patients
(11,13). The possible explanation for the
difference may be the different ethnic population with genetic
heterogeneity. More case-control studies with larger sample sizes,
particularly studies evaluating rs2976392 in Caucasians, are
required to further confirm these.
PSCA polymorphisms were found to be correlated with
intestinal- and diffuse-type GC risk, and the effect was greater in
diffuse-type GC (11,18). Additionally, investigators proposed
that the difference between intestinal- and diffuse-type GC with
respect to genetic susceptibility is congruous with the hypothesis
that two distinct pathways of gastric carcinogenesis exist: One
arising in atrophic gastritis with or without intestinal
metaplasia, which develops into intestinal-type GC at least
initially, and the other originating from stem cells or precursors
for gastric epithelial cells in the background of normal gastric
mucosa, typically leading to diffuse-type cancer (24). In this meta-analysis, we found the
rs2294008T allele was non-significantly associated with risk of
diffuse-type (dominant model: OR, 1.42; 95% CI, 0.89–2.27) or
intestinal-type (dominant model: OR, 1.30; 95% CI, 0.91–1.86) GC;
however, rs2976392A allele was significantly correlated with both
diffuse-type (dominant model: OR, 2.80; 95% CI, 1.43–5.47) and
intestinal-type (dominant model: OR, 1.46; 95% CI, 1.27–1.69) GC.
This result was not consistent with those of the majority of
previous studies, but it was the same as the study by Lu et
al (13). The possible reason
for the difference was that we included patients with different
characteristics, such as ethnicity, sample size, frequencies of
allele and tumor location.
Studies have found that PSCA is mainly expressed in
stomach nerve endocrine cells located in the stomach body and
bottom gland (25), and this was
validated by subsequent clinical trials, which showed that PSCA
rs2294008 T allele was associated with significantly increased risk
of non-cardia, no or decreased risk of cardia GC (12,17,20).
We found that the rs2294008 T allele was significantly associated
with an increased risk of cardia and non-cardia GC, and the effect
was larger in non-cardia (dominant model: OR, 1.45; 95% CI,
1.26–1.66) than in cardia GC (dominant model: OR, 1.21; 95% CI,
1.03–1.42). In addition, our meta-analysis revealed a positive
association between rs2976392 and non-cardia GC but no association
with cardia cancer (Table IV).
These findings were compatible with those of the studies
above-mentioned, suggesting that the germline variations in PSCA
may be specific genetic markers for susceptibility to non-cardia GC
development. A possible explanation is that cardia GC has different
epidemiological characteristics, pathogenesis and clinical
behaviors than non-cardia GC (26).
The majority of previous studies has focused on the
association between PSCA polymorphisms and GC susceptibility, and
few mention the prognostic value of PSCA polymorphisms to GC
patients. In this review, we identified two studies examining the
prognostic value of rs2294008 genotypes to GC patients, and the
results were completely contrary (20,21).
Studies demonstrated that substitution of the C allele with the
risk allele T reduced transcriptional activity of PSCA (11), and that decreased expression of
PSCA may cause a decrease in cell adhesion molecules, which usually
retain tumor cells at the primary site of carcinogenesis, thus
increasing the chance of metastasis formation and decreasing the
survival of GC patients (27,28).
This was consistent with the study by Zeng et al, who
reported that the TT genotype was associated with the poor
prognosis of GC patients (HR, 2.12; 95% CI, 1.22–3.69) (20). By contrast, Wang et al
observed that patients with rs2294008 CT/TT variant genotypes had a
25% significantly increased survival (HR, 0.75; 95% CI, 0.59–0.96)
(21). One potential reason for
their result is that the reduced PSCA expression may increase
sensitivity of GC cells to chemo-/radiotherapy, thus conferring
improved survival; another reason may be due to different
treatments in the two studies. However, the exact regulation
mechanisms of PSCA expression and its biological function are
largely unknown. Clinical trials analyzing the role of PSCA
rs2294008 in GC patients receiving the same protocol of anticancer
drugs are warranted to examine whether PSCA can serve as a
potential prognostic marker.
Located in intron 2 of PSCA, the association of
rs2976392 to GC susceptibility was evaluated by very few studies
compared to rs2294008. Certain investigators found that the
functional relevance of rs2976392G>A change was in strong
linkage disequilibrium (LD) with the rs2294008C>T SNP (11,13).
In this meta-analysis, we found that rs2294008 and rs2976392 were
associated with increased risk of GC, although the association of
rs2976392 was not statistically significant (dominant model: OR,
1.41; 95% CI, 0.98–2.04). This further supports the findings of the
two studies above. However, the functional significance of the SNP
rs2976392 remains unclear. It is also likely that rs2976392 is in
high LD with other potentially functional or causal SNPs
contributing to the development of GC. Therefore, the priority of
research is to find the molecular mechanism by which the PSCA gene
polymorphisms (especially rs2976392) are associated with GC.
In conclusion, our study showed that PSCA gene
polymorphisms were associated with increased risk of GC, and were
correlated with subtypes of clinicopathological features and the
prognosis of GC patients. Therefore, our data indicated that PSCA
may play a significant role in gastric carcinogenesis and
prediction of prognosis of GC patients. Additional studies are
required to evaluate the molecular mechanisms by which PSCA gene
polymorphisms are associated with GC in populations with diverse
clinicopathological features, and to validate the prognostic value
in a larger number of patients.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Crew KD and Neugut AI: Epidemiology of
gastric cancer. World J Gastroenterol. 12:354–362. 2006.
|
|
3
|
Shikata K, Doi Y, Yonemoto K, Arima H,
Ninomiya T, Kubo M, et al: Population-based prospective study of
the combined influence of cigarette smoking and Helicobacter
pylori infection on gastric cancer incidence: the Hisayama
study. Am J Epidemiol. 168:1409–1415. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Machado JC, Figueiredo C, Canedo P,
Pharoah P, Carvalho R, Nabais S, et al: A proinflamatory genetic
profile increases the risk for chronic atrophic gastritis and
gastric carcinoma. Gastroenterology. 125:364–371. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Canedo P, Durães C, Pereira F, Regalo G,
Lunet N, Barros H, et al: Tumor necrosis factor alpha extended
haplotypes and risk of gastric carcinoma. Cancer Epidemiol
Biomarkers Prev. 17:2416–2420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dong J, Dai J, Zhang M, Hu Z and Shen H:
Potentially functional COX-2 -1195G>A polymorphism increases the
risk of digestive system cancers: A meta-analysis. J Gastroenterol
Hepatol. 25:1042–1050. 2010.
|
|
7
|
Zhao D, Sun T, Zhang X, Guo Y, Yu D, Yang
M, et al: Role of CD14 promoter polymorphisms in Helicobacter
pylori infection-related gastric carcinoma. Clin Cancer Res.
13:2362–2368. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reiter RE, Gu Z, Watabe T, Thomas G,
Szigeti K, Davis E, et al: Prostate stem cell antigen: a cell
surface marker overexpressed in prostate cancer. Proc Natl Acad Sci
USA. 95:1735–1740. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saeki N, Gu J, Yoshida T and Wu X:
Prostate stem cell antigen: a Jekyll and Hyde molecule? Clin Cancer
Res. 16:3533–3538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bahrenberg G, Brauers A, Joost HG and
Jakse G: Reduced expression of PSCA, a member of the LY-6 family of
cell surface antigens, in bladder, esophagus and stomach tumors.
Biochem Biophys Res Commun. 275:783–788. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakamoto H, Yoshimura K, Saeki N, Katai H,
Shimoda T, Matsuno Y, et al: Genetic variation in PSCA is
associated with susceptibility to diffuse-type gastric cancer. Nat
Genet. 40:730–740. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lochhead P, Frank B, Hold GL, Rabkin CS,
Ng MTH, Vaughan TL, et al: Genetic variation in the prostate stem
cell antigen gene and upper gastrointestinal cancer in white
individuals. Gastroenterology. 140:435–441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu Y, Chen J, Ding Y, Jin G, Wu J, Huang
H, et al: Genetic variation of PSCA gene is associated with the
risk of both diffuse-and intestinal-type gastric cancer in a
Chinese population. Int J Cancer. 127:2183–2189. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
The Nordic Cochrane Centre, The Cochrane
Collaboration. Review Manager (RevMan) version 5.1. Copenhagen: The
Nordic Cochrane Centre, The Cochrane Collaboration; 2011
|
|
15
|
Ou J, Li K, Ren H, Bai H, Zeng D and Zhang
C: Association and haplotype analysis of prostate stem cell antigen
with gastric cancer in Tibetans. DNA Cell Biol. 29:319–323. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song HR, Kim HN, Piao JM, Kweon SS, Choi
JS, Bae WK, et al: Association of a common genetic variant in
prostate stem-cell antigen with gastric cancer susceptibility in a
Korean population. Mol Carcinogen. 50:871–875. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu C, Wang G, Yang M, Huang L, Yu D, Tan
W, et al: Two genetic variants in prostate stem cell antigen and
gastric cancer susceptibility in a Chinese population. Mol
Carcinogen. 48:1131–1138. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsuo K, Tajima K, Suzuki T, Kawase T,
Watanabe M, Shitara K, et al: Association of prostate stem cell
antigen gene polymorphisms with the risk of stomach cancer in
Japanese. Int J Cancer. 125:1961–1964. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sala N, Muñoz X, Travier N, Agudo A, Duell
EJ, Moreno V, et al: Prostate stem-cell antigen gene is associated
with diffuse and intestinal gastric cancer in Caucasians: Results
from the EPIC-EURGAST study. Int J Cancer. 130:2417–2427. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng Z, Wu X, Chen F, Yu J, Xue L, Hao Y,
et al: Polymorphisms in prostate stem cell antigen gene rs2294008
increase gastric cancer risk in Chinese. Mol Carcinogen.
50:353–358. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang M, Bai J, Tan Y, Wang S, Tian Y, Gong
W, et al: Genetic variant in PSCA predicts survival of diffuse-type
gastric cancer in a Chinese population. Int J Cancer.
129:1207–1213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Catalano V, Labianca R, Beretta GD, Gatta
G, De Braud F and van Cutsem E: Gastric cancer. Crit Rev Oncol
Hematol. 71:127–164. 2009. View Article : Google Scholar
|
|
23
|
Becker KF, Keller G and Hoefler H: The use
of molecular biology in diagnosis and prognosis of gastric cancer.
Surg Oncol. 9:5–11. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smith MG, Hold GL, Tahara E and El-Omar
EM: Cellular and molecular aspects of gastric cancer. World J
Gastroenterol. 12:2979–2990. 2006.
|
|
25
|
Schoeman JH, De Vos V and Van Aswegen G:
Distribution of endocrine cells in the gut of the impala.
Ondersteport J Vet Res. 65:31–35. 1998.PubMed/NCBI
|
|
26
|
Heidl G, Langhans P, Mellin W, Bunte H and
Grundmann E: Adenocarcinoma of esophagus and cardia in comparison
with gastric carcinoma. J Cancer Res Clin Oncol. 120:95–99. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moore ML, Teitell MA, Kim Y, Watabe T,
Reiter RE, Witte ON, et al: Deletion of PSCA increases metastasis
of TRAMP-induced prostate tumors without altering primary tumor
formation. Prostate. 68:139–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Raff AB, Gray A and Kast WM: Prostate stem
cell antigen: a prospective therapeutic and diagnostic target.
Cancer Lett. 277:126–132. 2009. View Article : Google Scholar : PubMed/NCBI
|