Introduction
The high morbidity and mortality of gastric cancer
makes it particularly concerning. Recent advances in tumor
immunology have led to the development of novel immunotherapies for
cancer. Adoptive cellular immunotherapy (ACI), a developing cancer
therapeutic, can mobilize and strengthen the body’s immune system
to kill cancer cells in both a specific and non-specific manner.
Furthermore, adverse effects stemming from ACI are milder than both
radiotherapy and chemotherapy. While clinical trials using cancer
vaccines have yielded low objective response rates (1), recent approaches based on ACI have
shown significantly higher efficacy. Dudley et al
demonstrated partial and complete responses in ∼50% of patients
with metastatic melanoma treated with adoptive transfer of ex
vivo-expanded autologous tumor-infiltrating lymphocytes and a
lymphodepleting host conditioning regime (2,3).
While this method was demonstrated to improve quality of life and
prolong survival time, the distribution of immune cells injected
into a tumor-bearing body has yet to be described. Furthermore, the
ability of intraperitoneally infused immune cells to target in
situ gastric cancer is also unknown. Thus, it would be
worthwhile to find visual evidence of the tumor-targeting ability
of immune cells.
With modern fluorescence imaging techniques, we are
able to trace the migration of cells in living animal models.
Unfortunately, the results of monitoring immune cells injected into
the gastric cancer orthotopic model were unsatisfactory due to a
low signal-to-noise ratio (4). In
recent years, however, the development of a near-infrared
fluorescence imaging technique has made it possible to better trace
living cells in deep tissue (5).
In the present study, we established an orthotopic
gastric carcinoma nude mouse model and dynamically monitored
cytokine-induced killer (CIK) cells and cytotoxic T lymphocytes
(CTLs) labeled with the near-infrared fluorescent dye, DiR
(1,1′-dioctadecyl-3,3,3′,3′-tetramethyl indotricarbocya-nine
iodide).
Materials and methods
Cell line
Human gastric adenocarcinoma cell line BGC-823
(maintained in our laboratory) was grown in Dulbecco’s modified
Eagle’s medium (DMEM) (Sigma, St. Louis, MO, USA) with 10% fetal
bovine serum and 1 U/ml gentamicin and maintained in a humidified
atmosphere of 5% CO2 at 37°C. CIK cells were generated
from the peripheral blood mono-nuclear cells (PBMCs) of
volunteers.
Animals
Female BALB/c-nu/nu nude mice (6- to 8-weeks old)
were obtained from the animal center of the Academy of Military
Medical Science (Beijing, China). All animals were housed under
specific pathogen-free conditions and all animal protocols followed
the experimental procedures of the National Institutes of Health
Guide for the Care and Use of Laboratory Animals. Water and food
were available ad libitum. Sterile supplies and techniques
were applied whenever animals underwent surgery.
Preparation of immune cells
From volunteers who signed an informed consent,
10–20 ml of peripheral blood was drawn into sterile centrifuge
tubes containing heparin. PBMCs were obtained from buffy coats by
Ficoll-Hypaque density centrifugation, washed with saline and then
transferred to sterile Petri dishes with fresh serum-free Cellix
901 medium and incubated at 37°C in a humidified atmosphere of 5%
CO2.
CIK cells
Three hours later, cells in suspension were
transferred to fresh dishes containing serum-free Cellix 601 medium
with 1,000 U/ml recombinant human IFN-γ at a concentration of
2×106 cells/ml. The next day, immobilized anti-CD3
antibody (2 μg/ml), anti-CD28 antibody (1 μg/ml) and recombinant
human IL-2 (1,000 U/ml) were added to the incubation medium. On Day
5, fresh serum-free Cellix 602 medium containing recombinant human
IL-2 (1,000 U/ml) was added to the cell suspension and replenished
every 2–3 days over 9 more days of incubation. During the
generation period, cell number was maintained at approximately
5×106/ml.
CTLs
Following 3 h of PBMCs incubation in a sterile Petri
dish, as previously described, the adherent cells were incubated in
Cellix 901 medium containing recombinant human IL-4 (1,000 IU/ml)
and GM-CSF (1,000 IU/ml) after being washed with Cellix 901 medium.
On Day 7, BGC-823 cell lysate (20 μg/ml), which was obtained by
repeated freezing-thawing, and Pseudomonas aeruginosa (3
μl/ml) were added to the incubation medium. The next day, the cells
were obtained and co-cultured with the PBMC suspension cells
following the same incubation conditions as that of the CIK
cells.
Fluorescent labeling of immune
cells
DiR (1,1′-dioctadecyl-3,3,3′,3′-tetramethyl
indotricarbocyanine iodide) is a lipophilic, near-infrared
fluorescent cyanine dye useful for labeling the cytoplasmic
membrane. Our laboratory found that the proliferation rate and
tumor-killing activity of immune cells were not significantly
affected by the use of 10 μg of DiR/106 cells at a
concentration of 1×106 cells/ml. Thus, this is a useful
labeling technique for immune cell tracing experiments.
CIK cells and CTLs were put in suspension at a
concentration of 1×106 cells/ml. The DiR working
solution (1 μg/μl) was added into the cell suspension and incubated
for 30 min at 37°C with 10 μl of dye/106 cells. The dye
was then cleared away with two washes of Cellix 602 medium followed
by centrifugation at 1,700 rpm for 8 min. Flow cytometry (Beckman
Coulter) was applied to verify staining using near-infrared
excitation. A trypan blue exclusion test was run to determine
viability, and the viable cells were brought to the desired
concentration in sterile saline.
Cell viability assay
Proliferation assay
An MTT assay was used to detect the proliferation of
immune cells. Single-cell suspensions of immune cells were made
both before and after fluorescent labeling at a cell concentration
of 5×105 cells/ml in Cellix 602 medium containing
recombinant human IL-2 (1,000 U/ml). Each suspension was added to a
96-well culture plate at 100 μl/well with 4 groups/suspension and
triplicate wells/group then cultured in a humidified atmosphere of
5% CO2 at 37°C. On Days 1, 3, 6 and 10 one group from
each suspension was tested respectively as follows: i) 30 μl of MTT
solution was added into each test well and incubated for 4 h, ii)
the suspension was centrifuged at 1,500 rpm for 5 min and the
supernatant was discarded, iii) 100 μl of DMSO was added to each
well and agitated gently for 20 min and iv) the absorbance value of
each well was measured by an automatic ELISA reader at a wavelength
of 492 nm.
Cytotoxicity assay
A lactate dehydrogenase (LDH) release assay was used
to determine the cytotoxicity of the immune cells. The ratios of
effect to target (E/T) cells were set at 5:1, 10:1, 20:1 and 40:1.
Target cells (BGC-823 cells) and effect cells (CIK cells and CTLs,
before and after labeling) were added to a 96-well culture plate
with triplicate wells/suspension ratio and incubated for 24 h in a
humidified atmosphere of 5% CO2 at 37°C. After
centrifugation of the suspension at 1,700 rpm for 4 min, 50 μl was
removed and mixed with 50 μl of substrate solution and then
incubated for 30 min in the dark. Finally, 50 μl of stop solution
was added and the absorbance value of each well was measured using
an automatic ELISA reader at a wavelength of 492 nm.
Preparation of the gastric carcinoma
orthotopic model
One female nude mouse was injected subcutaneously
(s.c.) with 1×107 BGC-823 cells. When a tumor formed one
week later, we anesthetized the mouse and surgically excised the
tumor. The tumor tissue was further cut into fragments of ∼1
cm3. Another nude mouse was then anesthetized and a left
para-median abdominal incision was made by which we carefully drew
out the stomach, which was almost covered by the liver, and made a
few slight scarifications with a needle on the serosal surface near
the greater curvature. One piece of tumor fragment was placed over
the scarifications and a single 10 μl drop of fibrinogen solution
was applied to cover it followed by another single 10 μl drop of
thrombin solution ∼5 sec later. When the gelatinous material
formed, the stomach was carefully returned to its position in the
body and the incision was closed. All steps were carried out
aseptically.
Adoptive transfer and fluorescence live
imaging
Experimental design
Nude mice bearing in situ gastric carcinomas
(4–6 weeks after being implanted with tumor fragments) were
randomized into groups: (i) intraperitoneal injection of CTLs
(CTL-i.p.) and (ii) intraperitoneal injection of CIK cells
(CIK-i.p.). Saline suspensions of CTLs and CIK cells labeled with
DiR were constructed at a concentration of 1×108
cells/ml and each tumor-bearing mouse received an infusion of 0.1
ml (1×107) of labeled cells.
Fluorescence live imaging (FLI)
After infusion of mice with CTLs or CIK cells, each
mouse was anesthetized with isoflurane and FLI was performed using
the Xenogen IVIS-Spectrum Imaging System (Xenogen; Caliper Life
Sciences, Inc.). Imaging examination times were set as follows:
immediately after infusion (Day 0), 24 h (Day 1), 48 h (Day 2), 72
h (Day 3), 6 days, 10 days and 14 days after infusion.
Statistical analysis
Data were analyzed with a Mann-Whitney U test or
ANOVA with Bonferroni post-test correction using GraphPad Prism v.5
software (GraphPad Software).
Results
Generation of near-infrared
fluorescent-labeled immune cells with DiR
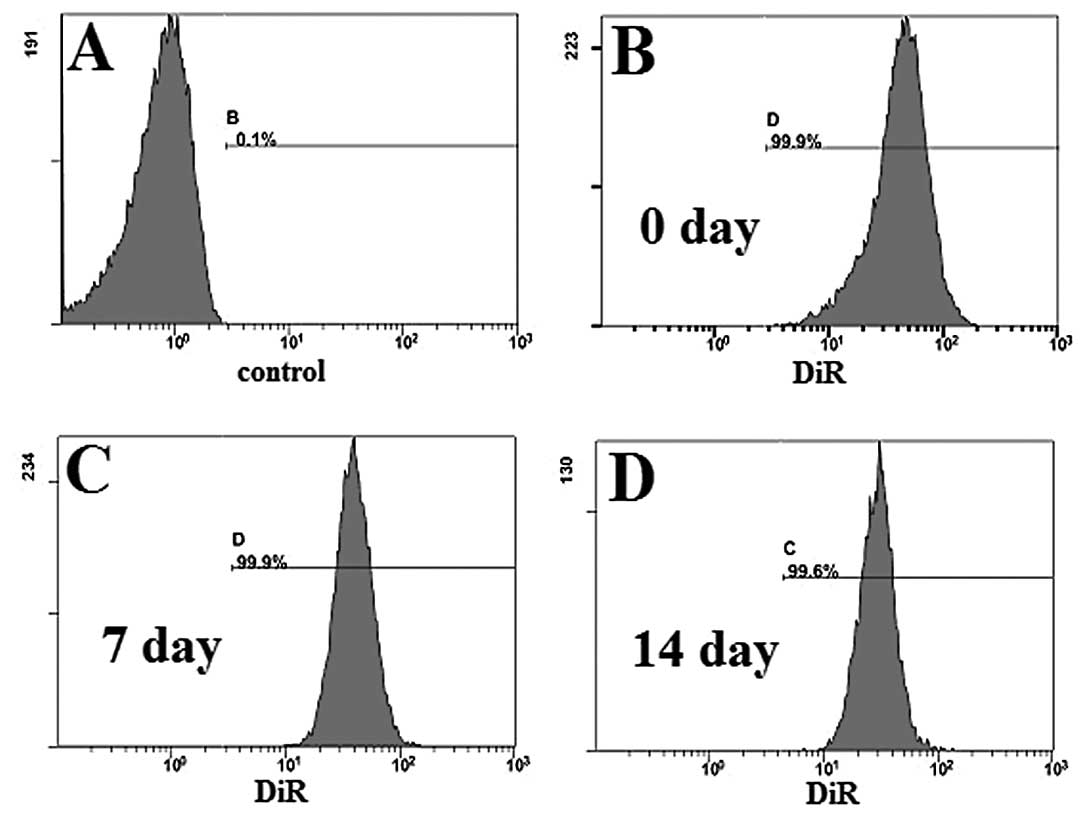
To determine the labeling efficiency of our DiR
system, we used a five channel flow cytometer (Beckman Coulter) to
detect and calculate the percentage of labeled immune cells
(Fig. 1). Fluorescence was
detected, and the labeling efficiency was as high as 99.9%, when
compared to the control group (unlabeled immune cells), in the cy7
channel (Fig. 1A and B). On Days 7
and 14, labeling efficiency remained as high as 99.9% (Fig. 1C) and 99.6% (Fig. 1D), respectively.
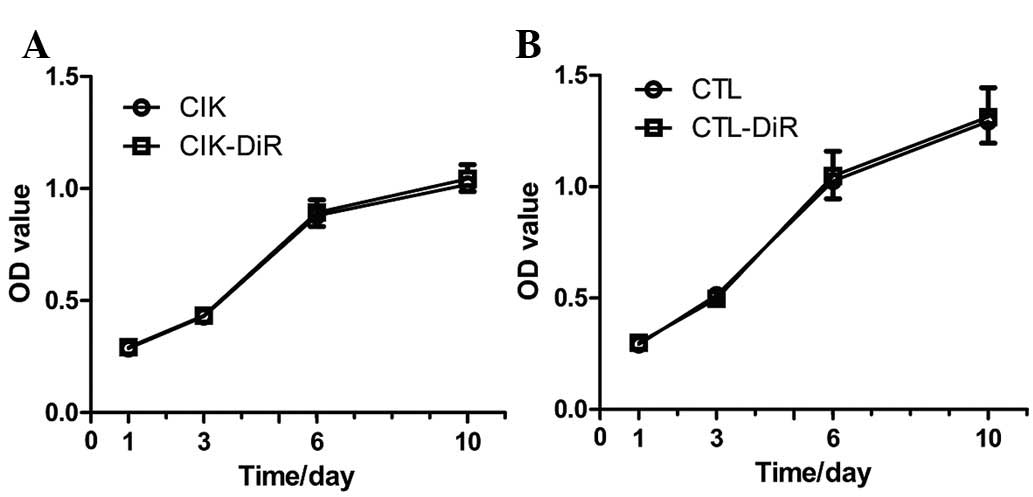
In order to determine whether DiR influences the
proliferation of immune cells, an MTT assay was used. CIK cells and
CTLs were stained by DiR on Day 0, and on Days 1, 3, 6 and 10 we
detected and compared the OD value of labeled and unlabeled immune
cells. Proliferation curves were drawn according to these OD values
(Fig. 2). Proliferation was not
significantly altered after labeling with DiR.
The antitumor effect of immune cells was determined
by an LDH release assay. CIK cells and CTLs were tested at E/T
ratios of 5:1, 10:1, 20:1 and 40:1. The tumor cell-killing rates
were not significantly different between labeled and unlabeled CIK
cells or between labeled and unlabeled CTLs at each E/T ratio
(P>0.05) (Table I). However,
CTLs exhibited a stronger antitumor activity than CIK cells at each
E/T ratio (P<0.05).
 | Table I.Antitumor effects of CIK cells and
CTLs prior to and after labeling in vitro. |
Table I.
Antitumor effects of CIK cells and
CTLs prior to and after labeling in vitro.
| 5:1 | 10:1 | 20:1 | 40:1 |
|---|
| CIK | 20.75±0.67 | 41.03±2.84 | 60.81±3.06 | 69.49±6.06 |
| CIK-DiR | 20.7±1.78 | 41.44±2.52 | 61.7±2.64 | 69.35±5.29 |
| CTL | 25.32±2.40 | 49.99±4.14 | 69.55±3.82 | 86.47±5.28 |
| CTL-DiR | 26.89±0.56 | 49.3±2.92 | 68.91±3.94 | 87.95±4.61 |
Tumor targeting of immune cells in
vivo
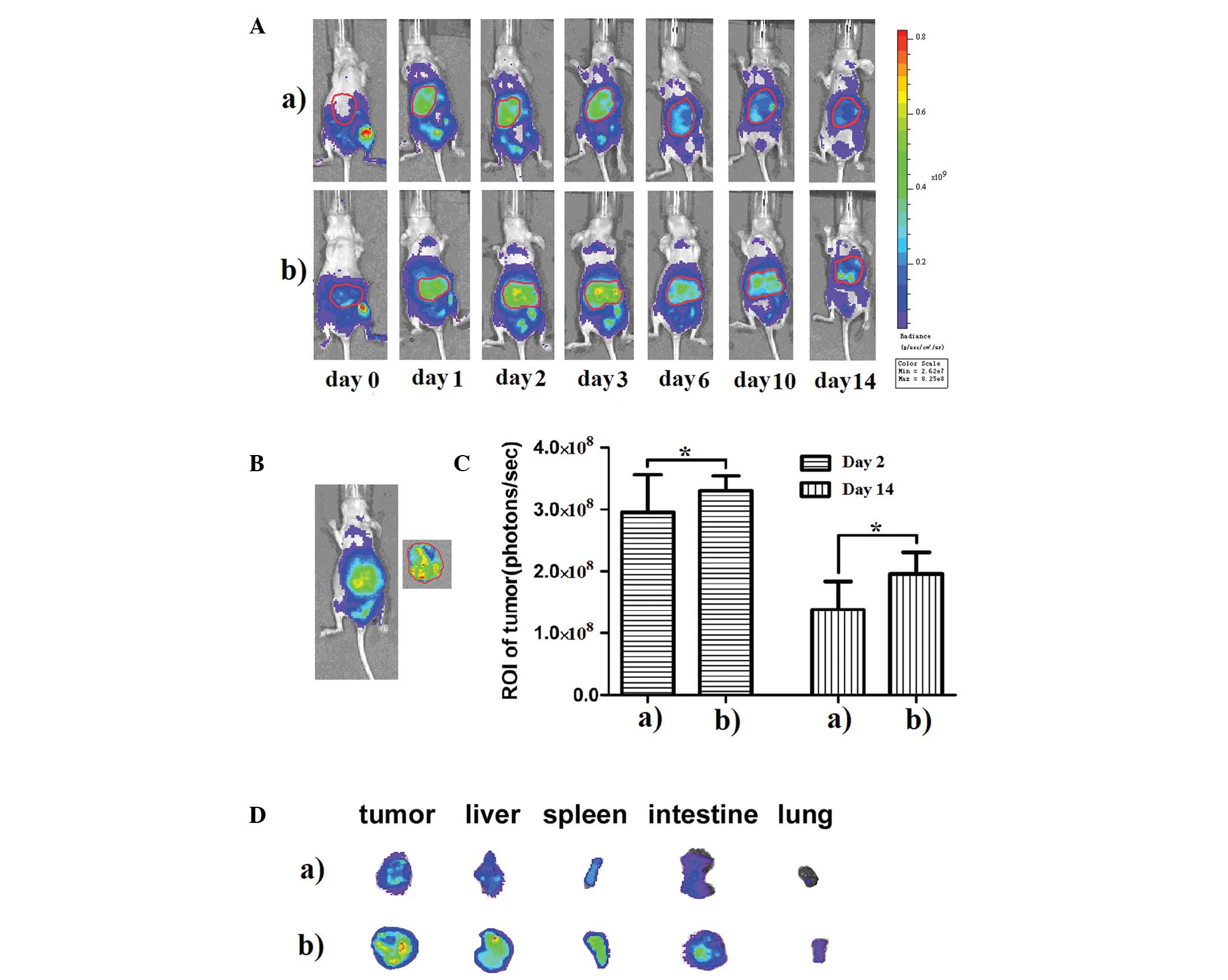
DiR-labeled CTLs and CIK cells were injected
intraperitoneally into tumor-bearing nude mice. FLI was performed
at different time points over the next two weeks (Fig. 3A). The signal was limited to the
abdominal area during the initial period (Day 0). The strongest
signal was detected at the injection site with a slight signal also
observed in the intestines. We focused our attention on the tumor
area. By the time we employed FLI 24 h after the initial
intraperitoneal injection (Day 1), the strongest signal had
migrated to the tumor area, although a slight signal was still
detected in other parts of the mouse. The signal in the tumor area
increased and remained strong through 72 h post-injection (Days 2
and 3). From Day 6 on, the signal in the tumor area began to
gradually subside. The distributions of CIK cells and CTLs were
similar, in general; however, CTLs exhibited a stronger
tumor-targeting ability than CIK cells at each time point. In order
to further compare the tumor-targeting ability of CIK cells and
CTLs, several mice were sacrificed and their tumors were separated
and imaged on Days 2 and 14 (Fig.
3B). Living Image v.4.1 software was used to draw and calculate
the region of interest (ROI). We found that the signal from the
tumor tissue in the CTL group was higher than that of the CIK group
on Days 2 and 14 (P<0.05) (Fig.
3C). We also imaged other organs, such as the liver, spleen,
intestines and lung on Day 14 and confirmed the signals of these
organs (Fig. 3D).
Discussion
This study demonstrates the tumor-targeting capacity
of CIK cells and CTLs following intraperitoneal infusion. There is
a need to visualize the migration of immune cells injected into
living animals and we achieved this by using a new near-infrared
fluorescent dye, DiR (1,1′-dioctadecyl-3,3,3′,3′-tetramethyl
indotricarbocyanine iodide), although the use of DiR as an in
vivo cell tracer is still in its initial experimental stages.
Kalchenko et al (6)
successfully used DiR to stain human leukemia G2L cells, mouse
lymphocytes and rat red blood cells for in vivo tracer
experiments. Granot et al (7) also used DiR to mark fibroblast cells,
which were then observed targeting to an ovarian cancer tumor some
distance away from the injection site. The properties of
near-infrared wavelengths make them ideal for imaging in deep
tissue. Our results showed that labeling with DiR had no
significant influence on the biological properties of CIK cells and
CTLs, suggesting DiR to be suitable for use in living animal
experiments. This finding is consistent with recent studies
employing a similar lipophilic carbocyanine dye DiI (8,9).
Adoptive cellular immunotherapy (ACI), a modern
treatment strategy for cancer, has lately received increased
attention (10–14). CIK cells, a new generation of
antitumor adoptive immune cells following the steps of
lymphokine-activated killer cells (LAK cells), tumor-infiltrating
lymphocytes (TILs) and anti-CD3 monoclonal antibody-activated
killer cells (CD3AK cells), are one of the most widely used ACI
cell type. With their characteristic rapid rate of proliferation
and high efficiency and broad spectrum tumor cell-killing ability,
CIK cells are generally regarded as a safe and efficient cell type
for use in tumor immunotherapy (15). CIK cells have been shown to kill
tumor cells in a non-MHC-restricted manner, similar to NK cells
requiring no specific antigen recognition (16–18).
The mechanism of CIK cells’ antitumor activity has not yet been
described; however, evidence shows that BLT
(N-benzylcarbonyl-L-lysinethiobenzyl ester), perforin, cytolysin
and other cytokines released by CIK cells play an important role
(19). Our results indicate that
CIK cells have a strong capacity to kill BGC-823 gastric cancer
cells, with killing rates of 20.75±0.67, 41.03±2.84, 60.81±3.06 and
69.49±6.06% at the effective target ratios of 5:1, 10:1, 20:1 and
40:1, respectively. These results suggest that the number of immune
cells migrating to the tumor site directly influence the antitumor
effect.
The tumor-targeting capacity of tumor-specific T
cells has been well described. Koya et al (20) utilized T cell receptor (TCR)
engineering of mouse splenocytes to create specificity for
tyrosinase, which is commonly expressed in melanoma cells. They
genetically labeled the splenocytes with bioluminescence imaging
(BLI) and positron emission tomography (PET) reporter genes to
visualize the distribution and antigen-specific tumor-homing of
these TCR transgenic T cells. Using a mouse tail vein infusion,
they found that after an initial brief stage of systemic
distribution, TCR-redirected T cells demonstrated an early pattern
of specific distribution to antigen-matched tumors and locoregional
lymph nodes followed by a more promiscuous distribution one week
later with additional accumulation in antigen-mismatched tumors.
Shu et al (21) used
Micro-PET to detect and observe the movement of transplanted
specific CD8+ T cells. They found that the tumor
response could be predicted as early as three days following
adoptive transfer via the tail vein and an increased signal was
detected in mice exhibiting adoptive transfer cell proliferation.
Adoptive transfer of CTLs has been performed in a number of
clinical tests with impressive antitumor effects in patients with
melanoma, breast cancer and renal carcinoma (22–25);
however, research involving CTLs in gastric cancer is still
lacking.
In our study, systemic distribution of CIK cells and
CTLs injected intraperitoneally did not appear until 24 h
post-injection, suggesting that CIK cells and CTLs can indeed
infiltrate the circulatory system via the abdominal cavity,
although the exact mechanism is not clear yet. After 24 h, we
observed systemic distribution of the immune cells with the
strongest signal at the tumor site, indicating that CIK cells as
well as CTLs can effectively migrate to the in situ gastric
tumor with an excellent tumor-targeting capacity following
intraperitoneal infusion. Furthermore, the signal at the tumor site
gradually increased at 48 and 72 h post-injection, indicating that
human immune cells could propagate after adoptive transfer into
nude mice. Although CIK cells and tumor-specific CTLs were equally
adept at targeting the tumor, ROI calculations of tumor tissue
showed that the number of CTLs was significantly higher than the
number of CIK cells at the tumor site 48 h and 14 days following
adoptive transfer. We observed a similar distinction in other
organs, such as the liver, spleen, intestines and lungs. These
results suggest that tumor-specific CTLs are still the optimal
immune cells for adoptive immunotherapy.
In conclusion, we provided visual evidence of the
tumor-targeting capacity of immune cells in live animals. This
defined distribution pattern of adoptively transferred cells
eliciting robust antitumor activity can be used to further analyze
individual components of this combinatorial approach prior to the
initiation of clinical trials.
References
|
1.
|
Rosenberg SA, Yang JC and Restifo NP:
Cancer immunotherapy: moving beyond current vaccines. Nat Med.
10:909–915. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Dudley ME, Wunderlich JR, Robbins PF, et
al: Cancer regression and autoimmunity in patients after clonal
repopulation with antitumor lymphocytes. Science. 298:850–854.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Dudley ME, Wunderlich JR, Yang JC, et al:
Adoptive cell transfer therapy following non-myeloablative but
lymphodepleting chemotherapy for the treatment of patients with
refractory meta-static melanoma. J Clin Oncol. 23:2346–2357. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Frangioni JV: In vivo near-infrared
fluorescence imaging. Curr Opin Chem Biol. 7:626–634. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
He X, Gao J, Gambhir SS and Cheng Z:
Near-infrared fluorescent nanoprobes for cancer molecular imaging:
status and challenges. Trends Mol Med. 16:574–583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kalchenko V, Shivtiel S, Malina V, et al:
Use of lipophilic near-infrared dye in whole-body optical imaging
of hematopoietic cell homing. J Biomed Opt. 11:0505072006.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Granot D, Addadi Y, Kalchenko V, Harmelin
A, Kunz-Schughart LA and Neeman M: In vivo imaging of the systemic
recruitment of fibroblasts to the angiogenic rim of ovarian
carcinoma tumors. Cancer Res. 67:9180–9189. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Hemmrich K, Meersch M, von Heimburg D and
Pallua N: Applicability of the dyes CFSE, CM-DiI and PKH26 for
tracking of human preadipocytes to evaluate adipose tissue
engineering. Cells Tissues Organs. 184:117–127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kikkawa YS and Pawlowski KS: Cochlear
neuronal tracing for frequency mapping with DiI, NeuroVue, and
Golgi methods. Acta Otolaryngol Suppl. 559:19–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Jiang J, Xu N, Wu C, et al: Treatment of
advanced gastric cancer by chemotherapy combined with autologous
cytokine-induced killer cells. Anticancer Res. 26:2237–2242.
2006.PubMed/NCBI
|
|
11.
|
Kim HM, Kang JS, Lim J, et al: Antitumor
activity of cytokine-induced killer cells in nude mouse xenograft
model. Arch Pharm Res. 32:781–787. 2009. View Article : Google Scholar
|
|
12.
|
Toh U, Fujii T, Mishima M, et al:
Conventional chemotherapy combined with the repetitive immune cell
transfer for patients with refractory advanced gastric cancer. Gan
To Kagaku Ryoho. 34:1931–1933. 2007.(In Japanese).
|
|
13.
|
Wongkajornsilp A, Sangsuriyong S, Hongeng
S, Waikakul S, Asavamongkolkul A and Huabprasert S: Effective
osteosarcoma cytolysis using cytokine-induced killer cells
pre-inoculated with tumor RNA-pulsed dendritic cells. J Orthop Res.
23:1460–1466. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Yamagishi H, Ueda Y and Oka T: A case
report of immunotherapy on a patient with advanced gastric cancer
by adoptive transfer of OK-432-reactive HLA-matched allogeneic
lymphocytes. Cancer Immunol Immunother. 46:113–119. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Schmidt-Wolf IG, Lefterova P, Johnston V,
Huhn D, Blume KG and Negrin RS: Propagation of large numbers of T
cells with natural killer cell markers. Br J Haematol. 87:453–458.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Linn YC and Hui KM: Cytokine-induced
NK-like T cells: from bench to bedside. J Biomed Biotechnol.
2010:4357452010.PubMed/NCBI
|
|
17.
|
Linn YC, Lau LC and Hui KM: Generation of
cytokine-induced killer cells from leukaemic samples with in vitro
cytotoxicity against autologous and allogeneic leukaemic blasts. Br
J Haematol. 116:78–86. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Lopez RD, Waller EK, Lu PH and Negrin RS:
CD58/LFA-3 and IL-12 provided by activated monocytes are critical
in the in vitro expansion of CD56+ T cells. Cancer
Immunol Immunother. 49:629–640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Mehta BA, Schmidt-Wolf IG, Weissman IL and
Negrin RS: Two pathways of exocytosis of cytoplasmic granule
contents and target cell killing by cytokine-induced
CD3+ CD56+ killer cells. Blood. 86:3493–3499.
1995.PubMed/NCBI
|
|
20.
|
Koya RC, Mok S, Comin-Anduix B, et al:
Kinetic phases of distribution and tumor targeting by T cell
receptor engineered lymphocytes inducing robust antitumor
responses. Proc Natl Acad Sci USA. 107:14286–14291. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Shu CJ, Radu CG, Shelly SM, et al:
Quantitative PET reporter gene imaging of CD8+ T cells
specific for a melanoma-expressed self-antigen. Int Immunol.
21:155–165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Butler MO, Lee JS, Ansen S, et al:
Long-lived antitumor CD8+ lymphocytes for adoptive
therapy generated using an artificial antigen-presenting cell. Clin
Cancer Res. 13:1857–1867. 2007.PubMed/NCBI
|
|
23.
|
Mackensen A, Meidenbauer N, Vogl S, Laumer
M, Berger J and Andreesen R: Phase I study of adoptive T-cell
therapy using antigen-specific CD8+ T cells for the
treatment of patients with metastatic melanoma. J Clin Oncol.
24:5060–5069. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Yamaguchi Y, Ohshita A, Hironaka K, et al:
Adoptive immuno-therapy using autologous lymphocytes sensitized
with HLA class I-matched allogeneic tumor cells. Oncol Rep.
16:165–169. 2006.PubMed/NCBI
|
|
25.
|
Yee C, Thompson JA, Byrd D, et al:
Adoptive T cell therapy using antigen-specific CD8+ T
cell clones for the treatment of patients with metastatic melanoma:
in vivo persistence, migration, and antitumor effect of transferred
T cells. Proc Natl Acad Sci USA. 99:16168–16173. 2002.PubMed/NCBI
|