Introduction
Anastomotic dehiscence following gastrointestinal
surgery, particularly colorectal surgery, is a significant cause of
morbidity and mortality, and leakage from colonic anastomosis is a
major concern for surgeons. The incidence of reported anastomotic
leakage varies between 10 and 13% (1,2).
Presently, the use of mechanical staplers for colorectal
anastomoses has been increasing and is becoming widely accepted in
Western countries due to the short procedure time and reliability
afforded by this technique. It has reduced the operation time, has
facilitated the performance of gastrointestinal anastomoses at
sites with a poor field of view and has decreased the likelihood of
suture failure (3,4). Several prospective randomized
controlled studies have compared hand suturing with mechanical
stapling in patients undergoing total gastrectomy and low anterior
colorectal resection (5–7). However, hand-sutured anastomoses are
still popular due to economic conditions in developing countries,
and few experimental studies have yet been reported comparing wound
healing of stapled anastomosis with hand-sutured anastomosis,
particularly in conditions of peritonitis.
Many factors contribute to wound healing and the
integrity of an anastomosis, such as blood supply, tension of the
anastomosis, bowel preparation, patient condition and inflammation
(8). In addition, peptide growth
factors (PGFs) play a significant role in wound healing. These
molecules have been shown to mediate the stages of wound healing,
including neovascularization and synthesis, deposition and
maturation of collagen. Among these PGFs, transforming growth
factor-β1 (TGF-β1) has been found to play the
most important role in anastomotic wound healing, including
inflammation, fibroplasia and deposition of collagen (9,10).
Similarly, the most important cytokine implicated in
neovascularization processes is vascular endothelial growth factor
(VEGF) (11).
The aim of the present study was to evaluate the
safety and effectiveness of mechanical stapled anastomosis vs.
hand-sutured anastomosis in an animal model of peritonitis by
comparison of wound healing between these two styles of
anastomoses.
Materials and methods
Study design
Adult male Sprague-Dawley rats were allowed to
acclimate to laboratory conditions for 1 week prior to experimental
use. Animals were housed at 21°C with a 12-h day-night cycle. They
had free access to water and standard laboratory chow. The study
protocol was approved by the Animal Ethics Review Committee of the
Oita University, Faculty of Medicine (Oita, Japan).
Surgical procedure
In 48 rats weighing 250–300 g each, bacterial
peritonitis was induced using a cecal ligation and puncture (CLP)
model, with minor modification (12). Rats were fasted for 24 h and
anesthetized with ether. The abdomen was shaved and disinfected
with 70% alcohol and a 2-cm midline incision was made. The cecum
was dissected without damaging its vascular supply and was filled
with feces by milking stool back from the ascending colon.
Thereafter, the cecum was ligated 5 mm below the ileocecal valve
with 3-0 silk suture and punctured twice with an 18-gauge needle at
the antimesenteric site. The abdominal wall was closed in two
layers. Immediately after the operation, rats received saline
subcutaneously (5 ml/100 g body weight) and were placed in cages.
After 24 h, the abdomen was reopened and cecal resection was
performed. Rats were randomized into two groups (n=24 each): the
stapler group, in which the cecum was resected just below the
ileocecal valve with a 6-row linear stapler (Endo-GIA Universal;
Covidien, Mansfield, MA, USA) and the hand-sutured group, in which
the cecum was resected just above the ligation followed by closure
of the stump with four interrupted two-row Albert-Lembert suture
using 6-0 polydioxanone sutures (PDS II; Ethicon, Somerville, NJ,
USA). The abdomen was closed in two layers. The operative time was
recorded in all surgical procedures. Rats were maintained on
standard laboratory chow and water ad libitum after surgery.
They were sacrificed on postoperative day (POD) 0 (immediately
after surgery) and on PODs 3, 5 and 7 after surgery under full
inhalant anesthesia with ether.
The abdominal wall was disinfected with 70% alcohol
and reopened. For measurement of bursting pressure, the anastomoses
were carefully resected by transecting at the ileum and the
ascending colon so as not to injure the intestinal wall. After
determination of the bursting pressure, colonic tissue samples
consisting of a 0.5-cm segment of perianastomotic tissue were
obtained 0.5 cm proximal to the anastomotic line. The colonic
tissue samples were snap-frozen in liquid nitrogen and then stored
at −80°C for later use.
Anastomotic bursting pressure
The anastomotic bursting pressure (ABP) was measured
on postoperative days (PODs) 0, 3, 5 and 7. Fecal material was
removed from the ileal and colonic segments. A tube was inserted
into the cecum from the ileal segment. Then, two ligations were
made at the ascending colon and the ileum. The tube was connected
to an infusion pump and a pressure transducer was linked to a
recorder. Pressure was measured during infusion of normal saline
solution through the tube at a constant rate of 1 ml/min. The
pressure recorded immediately before abrupt loss of pressure was
considered the ABP.
Total-RNA isolation
Total-RNA was isolated from frozen intestinal
samples with an EZ1 RNA Tissue Mini kit (Qiagen, Tokyo, Japan)
following the manufacturer's protocol. Complementary DNA (cDNA) was
synthesized as described previously (13). The RNA was resuspended in
DEPC-treated water and stored at −80°C until the reverse
transcription step. The concentration of the RNA was determined
with a spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Total-RNA (1.0 μg) was reverse-transcribed at 37°C for
60 min in the presence of a 25-μl reaction containing 80
pmol random primer (Takara Bio, Inc., Shiga, Japan) and 200 U
Moloney murine leukemia virus reverse transcriptase (M-MLV Reverse
Transcriptase; Invitrogen, Tokyo, Japan) according to the
manufacturer's protocol. The cDNA was used as a template for
subsequent real-time polymerase chain reaction (PCR).
Real-time PCR
Quantitative real-time PCR was carried out with a
Light-Cycler System (Roche Diagnostics, Lewes, East Sussex, UK).
Primer sets for TGF-β1 and VEGF were purchased from
Search-LC (Heidelberg, Germany) (rat TGF-β1 set no.
410636 and rat VEGF set no. 4410874). Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH primer set; Search-LC) was amplified according
to the manufacturer's protocol as an internal control to allow
quantitation of the TGF-β1 and VEGF amplification
products. A fresh standard dilution series was prepared. The PCR
mix, which contained 9.4 μl PCR-grade water, 1 μg of
each TGF-β1- and VEGF-specific primers (10 μM),
1.6 μl of 25 mM MgCl2 and 2 μl LightCycler
FastStart DNA Master SYBR Green I, was added to a 1.5-ml
light-protected LightCycler capillary and 5 μl cDNA template
(diluted 10X) were added. The PCR mix (15 μl) was pipetted
into 4 precooled LightCycler capillary tubes, and 5 μl
undiluted and 5 μl freshly diluted standard were then added
to each capillary. Each capillary was sealed with a stopper and
centrifuged at 700 x g for 15 sec. The capillaries were placed into
the rotor of the LightCycler and the samples were amplified. PCR
cycles were monitored continuously with SYBR Green I dye. After
amplification, melting curve analysis permitted accurate
identification of the PCR amplicons. Data were analyzed with
LightCycler analysis software (Roche Diagnostics) and a standard
curve that correlated cycle number with the amount of product
formed was plotted for each sequence of interest. TGF-β1
and VEGF expression levels were then normalized to that of
GAPDH.
Hydroxyproline assay
Hydroxyproline concentrations in the anastomotic
segment on the operative day and PODs 3 and 7 were measured as an
indicator of collagen accumulation (14). This assay was performed on tissue
samples frozen in liquid nitrogen and kept at −80°C in a deep
freezer. After measuring the wet tissue weight, the samples were
hydrolyzed by adding 6 N HCl at 110°C for 24 h in sealed test
tubes. Hydrolysates were transferred to flasks and dried overnight
in desiccators to remove the hydrochloric acid by evaporation.
After dissolving the hydrolysates in 3 ml of 0.02 N HCl, 0.1 ml of
the solution was extracted and added to 0.9 ml of 0.02 N HCl. Final
solutions were applied in 50-μl aliquots to the amino acid
analyzer to determine the hydroxyproline concentration.
Statistical analysis
All data are expressed as means ± standard
deviation. Statistical analysis was performed by means of the
Mann-Whitney U test. A value of P<0.05 was considered to
indicate statistical significance. The Dr SPSS II for Windows
11.0.1J program (IBM Japan, Tokyo, Japan) was used for all
statistical analyses.
Results
Operative findings and ABP
All animals tolerated the surgical intervention well
and no animal died prematurely during the study. The operative time
was significantly shorter in the stapler group than in the
hand-sutured group (9.1±2.8 vs. 15.9±2.4 min, P<0.001,
respectively) (Table I). Both
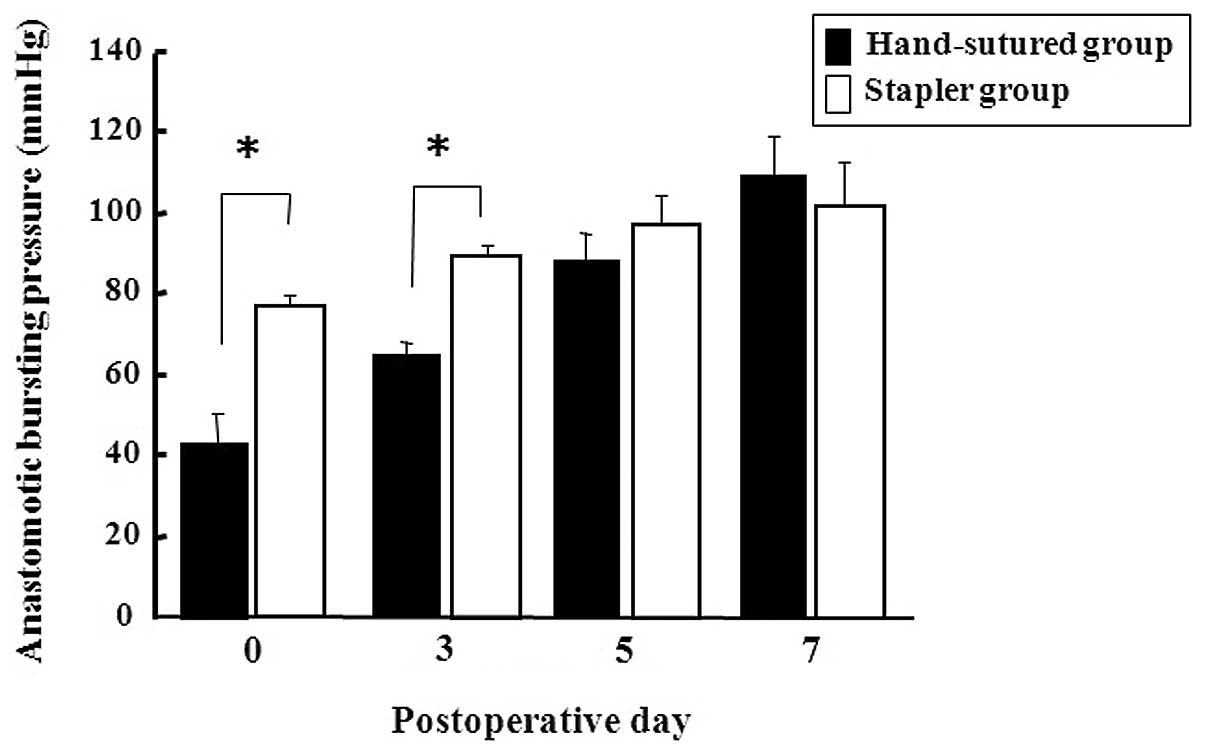
groups showed progressive increases in ABP over the postoperative
period (Fig. 1). The stapler group
had a significantly higher ABP than the hand-sutured group on PODs
0 and 3, but the difference was not statistically significant on
PODs 5 and 7.
 | Table I.Operative times in the stapler and
hand-sutured groups. |
Table I.
Operative times in the stapler and
hand-sutured groups.
| Stapler group | Hand-sutured
group | P-value |
|---|
| Operative
timea | 9.1±2.8 | 15.9±2.4 | <0.001 |
Local temporal gene expression of the
PGFs
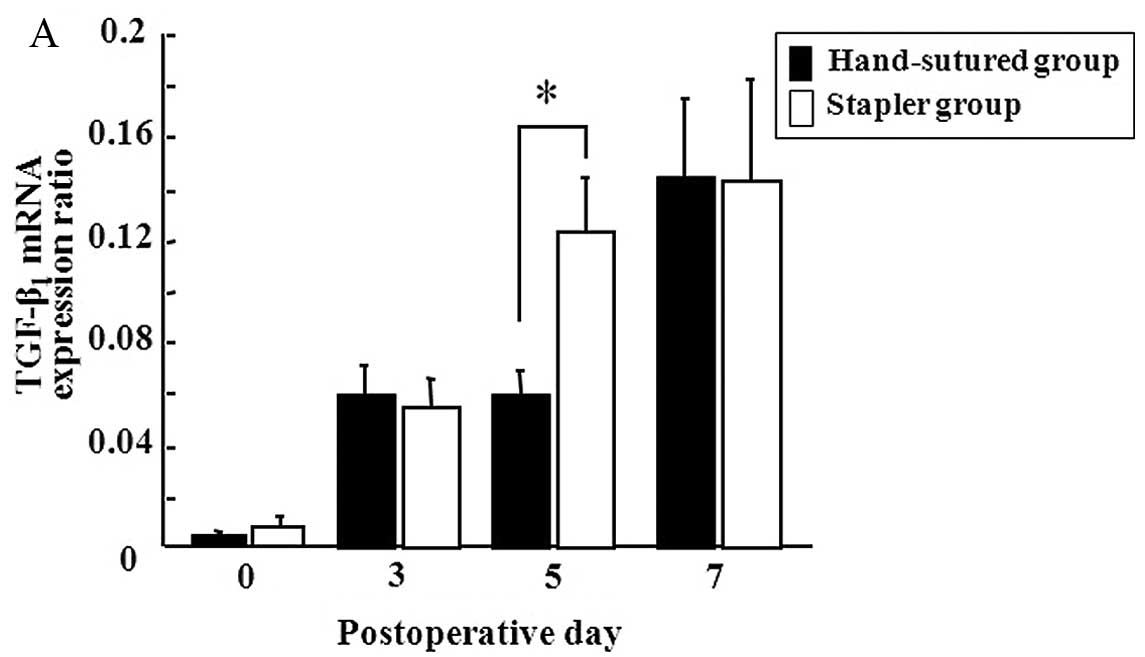
Gene expression of TGF-β1 and VEGF on the
days studied during the first 7 PODs is shown in Fig. 2. Both groups showed progressive
increases in the expression of TGF-β1 and VEGF over the
7-day postoperative period. On POD 5, the stapler group showed a
higher level of gene expression of TGF-β1 than that of
the hand-sutured group (Fig. 2A).
Gene expression of VEGF was identical in both groups (Fig. 2B).
Hydroxyproline assay
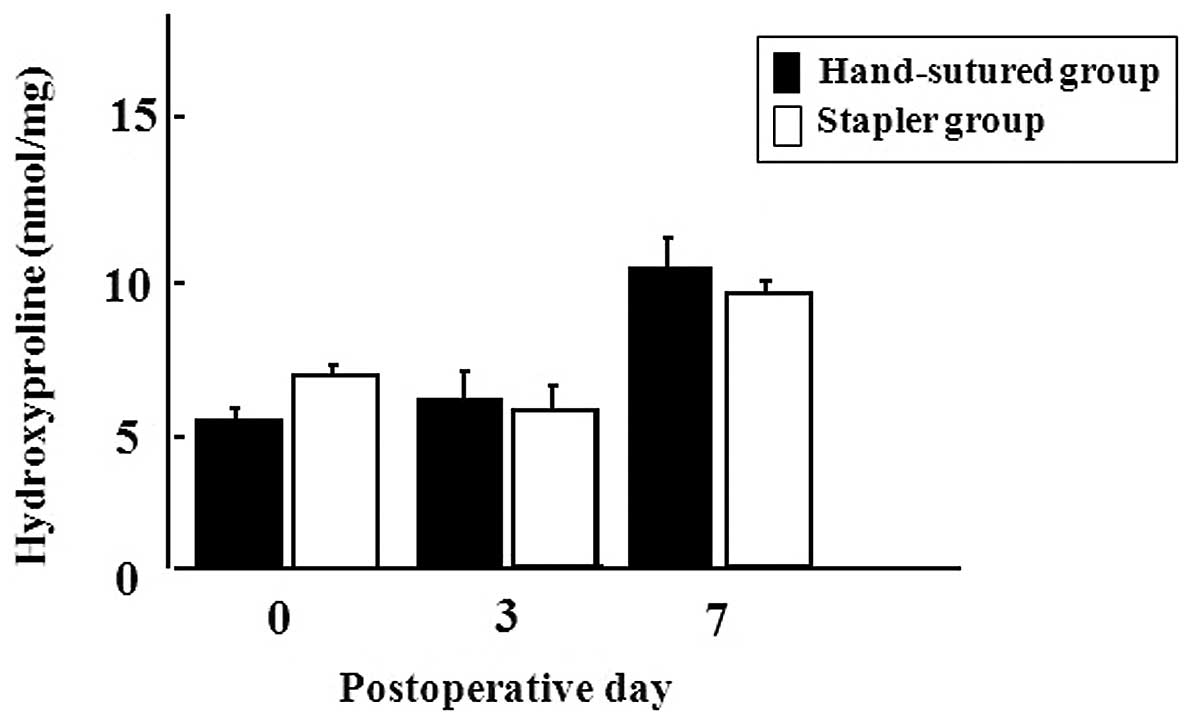
Tissue hydroxyproline concentration around the
anastomotic segments on PODs 0, 3 and 7 is shown in Fig. 3. Although the concentration of
hydroxyproline began to increase from POD 7 in both groups, there
was no significant difference between the two groups.
Discussion
Mechanically stapled anastomoses are clinically
common in Western countries. However, to our knowledge, there are
no reports comparing wound healing of intestinal mechanically
stapled anastomosis with that of hand-sutured anastomosis in an
animal model of peritonitis. The model of CLP, as reported by
Wichterman et al (12),
produces an early and late phase of sepsis. In the first 16 h
(early phase), animals show typical hyperdynamic features, whereas
those in the late phase of sepsis show hypodynamic features as in
peritonitis. In our experiment, the anastomosis was constructed
during the late phase of sepsis, but the ongoing source of
infection (ligated perforated cecum) was removed to allow the
animals to recover. To compare the integrity and healing process of
the anastomoses, we determined the bursting pressure of the
anastomoses, concentration of hydroxyproline and local temporal
expression of TGF-β1 and VEGF. Our present results
showed that the anastomosis made by stapler is safer and more
effective than that made by hand suturing in an animal model of
bacterial peritonitis, because of the stronger anastomosis created
in the early postoperative period and the shorter operating time
afforded by the stapler.
In the gastrointestinal tract, mechanical wall
strength resides in the collagenous fibrous network of the
submucosa. After creation of an anastomosis, reconstitution of the
submucosal layer occurs through the deposition of new collagen and
degradation of existing collagen (15).
After the initial inflammatory phase, a
proliferative phase ensues consisting of the formation of
granulation tissue, including synthesis of noncollagenous protein
such as fibronectin, and loss of colonic wall collagen in the
tissue adjacent to the anastomosis (16). During the first few postoperative
days, anastomotic strength is low as collagen is degraded secondary
to collagenase activity at the anastomosis site. Early anastomotic
strength is therefore dependent on the suture- or staple-holding
capacity of the existing collagen until large amounts of new
collagen can be newly synthesized by both fibroblasts and smooth
muscle cells (17). Although
neither type of anastomosis in the present study led to anastomotic
leakage, our present results showed that mechanical staples offer
better holding capacity compared to hand sutures. Ahrendt et
al (18) found a decrease in
the absolute amount of bowel wall structural collagen in intact
uninjured colon following 24 h of sepsis induced by CLP. An
anastomosis created under these conditions may have impaired suture
holding capacity from the beginning. Therefore, we believe that the
use of a mechanical stapler in conditions of peritonitis is
feasible to avoid anastomotic leakage.
TGF-β1 is an important regulator of bowel
anastomosis healing. In normal bowel tissue, TGF-β1 is
mainly produced by epithelial cells (19). In injured tissues,
TGF-β1 is produced by both inflammatory cells in the
lamina propria and epithelial cells (20). Migaly et al (21) reported that there is a crucial
switch from collagen degradation to collagen deposition, with
TGF-β1 identified in wound healing as being trophic and
chemotactic for fibroblasts around the POD 5. The upregulation of
TGF-β1 is temporally related to the transcription of
procollagen I. The transcription of TGF-β1 increases
from the time of wounding through POD 5 and during the crucial
switch from the inflammatory to the fibroplasia phase. Experimental
studies have shown that in bowel anastomoses, maximum production of
TGF-β1 molecules occurs on POD 7 with subsequent
diminution of TGF-β1 levels (9). Although our results also showed this
progressive increase until POD 7 in both groups, on POD 5 the
stapler group showed a higher level of gene expression of
TGF-β1 than that of the hand-sutured group. We believe
that in the stapler group, the crucial switch from collagen
degradation to deposition may have come earlier than in the
hand-sutured group because of less surgical damage resulting from
less manipulation and a shorter operating time.
The formation of new vessels, or angiogenesis, is
one of the key components in anastomotic healing. Vessels sprouting
from capillaries, venules and arterioles help supply ischemic
tissues with substrates essential for growth and repair. In an
experimental analysis of angiogenesis, Seifert et al
(22) demonstrated a significant
increase in vessel growth at colonic anastomoses from PODs 3–7.
VEGF is a member of the platelet-derived growth factor family of
glycoproteins. It is the most potent endothelial growth factor and
its role in angiogenesis is well-established (23). Upregulated by ischemia, VEGF
enhances vascular permeability and vasodilatation and promotes the
growth, proliferation and migration of endothelial cells (24). In the present study, gene
expression of VEGF gradually increased until POD 7 and was
identical in both groups. This result indicates that the degree of
ischemia at postoperative anastomotic sites in both groups was
homogenous, even though the sites were sutured with different
material and in a different manner.
Although the concentration of hydroxyproline around
the anastomotic segment was not changed on POD 3, on POD 7 an
increase was observed in both groups; however, the differences were
not significant. Christensen et al (25) reported that collagen fibrils are
visible in the anastomotic healing zone after 4 days of healing and
emphasized the importance of the sutures in the early healing
state. Our results support their opinion and show that in our
study, neither the suture material nor manner of suturing affected
collagen synthesis of the healing process at the anastomosis.
In the clinical setting, many randomized studies
have been performed to evaluate stapling methods in elective
surgery and none revealed any difference in the incidence of
anastomotic leakage (26).
Particularly, a few reports have compared stapling and hand
suturing in an emergency setting. Catena et al (27) randomized 201 patients to receive
stapled or hand-sutured anastomoses and aimed to elucidate whether
staplers could be used in an emergency setting and in unprepared
patients. Except for significantly shorter operating times for the
stapled anastomoses, no other differences were found. In our animal
study, in addition to shorter operating times, we also showed that
the anastomosis made by stapler is safer than that made by hand
suturing in the early postoperative period in peritonitis, a type
of emergency setting. In contrast to elective surgery, anastomoses
in emergency surgery are usually performed in critically ill
patients under difficult situations. Specifically in peritonitis,
the intestine used for anastomosis is damaged by edema, congestion
and intraperitoneal abscess. Even though we could not find any
differences in the incidence of anastomotic leakage, we would
choose the method offering higher holding capacity in the early
postoperative days, the most dangerous period.
The only apparent disadvantage of stapled
anastomosis is cost. Further improvements in mechanical stapling
devices are necessary that can compensate for this disadvantage.
Moreover, further investigation of the wound healing process,
particularly the activity of PGFs, is necessary to extend the
indications for stapled anastomosis in an emergency setting.
In conclusion, we found that the anastomosis made by
stapler is safer and more effective than that made by hand suturing
in an animal model of bacterial peritonitis as it creates a
stronger anastomosis in the early postoperative period and requires
less operating time. Mechanical anastomosis is feasible in
intestinal surgery in the presence of diffuse peritonitis since it
offers substantial holding capacity and a shorter procedure without
adversely affecting the healing process.
References
|
1.
|
Guenaga KF, Matos D, Castro AA, Atallah AN
and Willejorgensen P: Mechanical bowel preparation for elective
colorectal surgery. Cochrane Databese Syst Rev. 2:CD0015442003.
|
|
2.
|
Peeters KC, Tollenaar RA, Marijnen CA, et
al: Risk factors for anastomotic failure after total mesorectal
excision of rectal cancer. Br J Surg. 92:211–216. 2005. View Article : Google Scholar
|
|
3.
|
Everett WG, Friend PJ and Forty J:
Comparison of stapling and hand suture for left sided large bowel
anastomosis. Br J Surg. 73:345–348. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kataoka M, Masaoka A, Hayashi S, et al:
Problems associated with the EEA stapling technique for
esophagojejunostomy after total gastrectomy. Ann Surg. 209:99–104.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
West of Scotland and Highland Anastomosis
Study Group: Suturing or stapling in gastrointestinal surgery: a
prospective randomized study. Br J Surg. 78:337–341. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Brennan SS, Pickford IR, Evans M and
Pollok AV: Staplers or sutures for colonic anastomoses - a
controlled clinical trial. Br J Surg. 69:722–724. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Didolkar MS, Reed WP, Elias EG, Schnaper
LA, Brown SD and Chaudhary SM: A prospective randomized study of
sutured versus stapled bowel anastomoses in patients with cancer.
Cancer. 57:456–460. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Fielding LP, Stewart-Brown S, Blesovsky L
and Kearny G: Anastomotic integrity after operations for
large-bowel cancer: a multicentre study. Br Med J. 281:411–414.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Buckmire MA, Parquet G, Greenway S and
Rolandelli RH: Temporal expression of TGF-beta1, EGF, and PDGF-BB
in a model of colonic wound healing. J Surg Res. 80:52–57. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Cromack DT, Sporn MB, Roberts AB, Merino
MJ, Dart LL and Norton JA: Transforming growth factor beta levels
in rat wound chambers. J Surg Res. 42:622–628. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Bauer SM, Bauer RJ, Liu ZJ, Chen H,
Goldstein L and Velazquez OC: Vascular endothelial growth factor-C
promotes vasculogenesis, angiogenesis, and collagen constriction in
three-dimensional collagen gels. J Vasc Surg. 41:699–707. 2005.
View Article : Google Scholar
|
|
12.
|
Wichterman KA, Baue AE and Chaudry IH:
Sepsis and septic shock a review of laboratory models and a
proposal. J Surg Res. 28:189–201. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ninomiya S, Inomata M, Tajima M, et al:
Effect of Bevacizumab, a humanized monoclonal antibody to vascular
endothelial growth factor, on peritoneal metastasis of MKN-45P
human gastric cancer in mice. J Surg Res. 154:196–202. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Stegemann H and Stalder K: Determination
of hydroxyproline. Clin Chim Acta. 18:267–273. 1967. View Article : Google Scholar
|
|
15.
|
Blasken P, Renvall S and Sandberg M:
Fibronectin and collagen gene expression in healing experimental
colonic anastomoses. Br J Surg. 78:1048–1052. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Fukuchi SG, Seeburger JL, Parquet G and
Rolandelli RH: Influence of 5-fluorouracil on colonic healing and
expression of transforming growth factor-beta 1. J Surg Res.
84:121–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Sarah KT, Eugene YC and Blair AJ: Clinical
review: healing in gastrointestinal anastomosis, part 1.
Microsurgery. 26:131–136. 2006. View Article : Google Scholar
|
|
18.
|
Ahrendt GM, Gardiner K and Barbul A: Loss
of colonic structural collagen impairs healing during
intra-abdominal sepsis. Arch Surg. 129:1179–1183. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Barnard JA, Warwick GJ and Gold LI:
Localization of transforming growth factor beta isoforms in the
normal murine small intestine and colon. Gastroenterology.
105:67–73. 1993.PubMed/NCBI
|
|
20.
|
di Mola FF, Friess H, Scheuren A, et al:
Transforming growth factor-betas and their signaling receptors are
coexpressed in Crohn's disease. Ann Surg. 229:67–75.
1999.PubMed/NCBI
|
|
21.
|
Migaly J, Lieberman J, Long W, et al:
Effect of adenoviral-mediated transfer of transforming growth
factor-β1 on colonic anastomotic healing. Dis Colon Rectum.
47:1699–1705. 2004.
|
|
22.
|
Seifert WF, Verhofstad AAJ, Wobbes T, et
al: Quantitation of angiogenesis in healing anastomoses of rat
colon. Exp Mol Pathol. 64:31–40. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kleespies A, Guba M, Jauch K and Burns CJ:
Vascular endothelial growth factor in esophageal cancer. J Surg
Oncol. 87:95–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Henry TD: Therapeutic angiogenesis. BMJ.
318:1536–1539. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Christensen H, Chemnitz J, Christensen B
and Oxlund H: Collagen structural organization of healing colonic
anastomoses and the effect of growth hormone treatment. Dis Colon
Rectum. 38:1200–1205. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Korolija D: The current evidence on
stapled versus hand-sewn anastomosis in the digestive tract. Minim
Invasive Ther Allied Technol. 17:151–154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Catena F, La Donna M, Gagliardi A, et al:
Stapled versus hand-sewn anastomosis in emergency intestinal
surgery: Results of a prospective randomized study. Surg Today.
34:123–126. 2004. View Article : Google Scholar : PubMed/NCBI
|