Introduction
Growth of malignant lesions surrounding the vena
cava or large veins often results in stenosis or obstruction of the
venous vascular lumen and symptoms of venous congestion. Some
malignant tumors may invade the vascular wall to form a tumor
embolus or induce thrombosis in the adjacent vein. Based on the
location and extent of the venous obstruction, this may result in
pain and severe edema of the face, neck, torso and limbs. Other
severe symptoms such as dyspnea, dysphagia and ascites may also
occur. The severity of symptoms is increased by liver dysfunction
and gastrointestinal bleeding from portal tumor embolus, renal
dysfunction from inferior vena cava (IVC) occlusion, airway
obstruction from laryngeal or bronchial edema and coma from
cerebral edema.
Traditional conservative therapy with
anticoagulation may help to slow the progression of symptom
aggravations, but may not be efficient for rapid symptom relief,
and severe symptoms frequently persist. In addition, surgical
treatment of these symptoms in patients with malignant disease
generally has poor results, and therefore more microinvasive
therapies are advocated. Endovascular intervention provides an
effective method for venous vascular complications of malignant
tumors, with good tolerance and few injuries. Catheter-directed
thrombolytic therapy has become widely accepted as a valuable
treatment option in patients with acute deep vein thrombosis (DVT)
(1–5). Self-expandable metallic stent
placement in the vena cava and large veins at the site of such
lesions is widely accepted as a means to improve the quality of
life of patients with advanced malignant disease (6–8).
The purpose of this study was to evaluate the
efficacy and safety of our experience with vena cava filter
implantation, catheter-directed thrombolytic therapy,
recanalization, percutaneous transluminal angioplasty (PTA) and
stent placement in patients with venous vascular complications of
malignant tumors.
Materials and methods
Patients
Between May 2002 and May 2009, 61 consecutive
patients with venous vascular complications of malignant tumors (37
male, 24 female; mean age, 57.8 years; age range, 33–82 years) from
the First Hospital of China Medical University (Shenyang, China)
were enrolled in this study. After providing a complete description
of the study to the patients, written informed consent was obtained
in accordance with the National Health and Medical Research Council
guidelines. The study was approved by the Ethics Committee of our
hospital.
All patients experienced venous obstruction by
malignant tumors and the symptoms included pain and swelling of the
torso and limbs (n=38), pain and swelling of the face and neck
(n=25), varicosis of the torso (n=22), headache (n=8), ascites
(n=6), dyspnea (n=5), dysphagia (n=2), liver dysfunction (n=2),
gastrointestinal bleeding (n=2) and renal dysfunction (n=1).
Vascular ultrasound or computed tomography (CT) scans were
performed before interventional therapy and revealed unresectable
malignant venous obstruction in all patients. Venous stenosis or
occlusion was detected in 32 cases, acute deep vein thrombosis in
18 cases and tumor embolus in the vein in 11 cases. Involved veins
included the superior vena cava (SVC) (29 cases), upper extremity
veins (9 cases), iliac veins (9 cases), IVC (7 cases), portal veins
(4 cases) and jugular veins (3 cases). Underlying obstructing
tumors included lung cancer and lymph node metastasis (n=25),
primary hepatic carcinoma (n=11), colorectal cancer (n=9), lymph
node metastasis (n=7), malignant thymoma (n=6) and thyroid
carcinoma (n=3). All malignancies were diagnosed by pathological
and/or imaging findings in addition to clinical features.
Procedure
Filter implantation and
catheter-directed thrombolytic therapy
Utilizing a femoral vein approach, a 5-Fr pig-tail
catheter was placed into the SVC or IVC. A cavogram was performed
to ensure that the vena cava was free of thrombus and to document
the diameter of the vena cava. An OptEase filter (Cordis
Corporation, Miami Lakes, FL, USA) or a Günther Tulip filter (Vena
Cava MReye filter set; William Cook Europe, Bjaeverskov, Denmark)
was implanted into the SVC or IVC.
The presence of contraindications to percutaneous
thrombolysis, including metastatic central nervous system
malignancy, coagulopathy, occurrence of stroke <3 months prior,
surgery <1 month prior, or gastrointestinal bleeding was
assessed and patients were excluded. Patients at risk for
cerebrovascular accidents or cerebral metastasis were further
evaluated by CT before thrombolysis treatments. After excluding
pulmonary embolism by pulmonary arteriogram, a 5-F curved catheter
over a 0.035-inch hydrophilic guide wire was inserted near the
thrombosed region. The guide wire was pushed firstly towards and
into the thrombus with the support from the catheter. Then, the
catheter entered the thrombus over the guide wire. A veinogram was
performed to ensure the extent of the thrombus and collateral
veins. Subsequently, a thrombolytic catheter (Unifuse,
Angiodynamics, Inc., Queensbury, NY, USA) with a 20-cm- or 30-cm
long side-hole was replaced into the thrombus. Urokinase (500,000
IU) was infused for 2 h twice every day through the thrombolytic
catheter. Systemic anticoagulant therapy was administered by
intravenously injecting 50 IU/kg heparin every 6 h. Repeated
veinogram was performed every 3–4 days during thrombolysis therapy.
According to the extent of the residual thrombus, the location of
the thrombolytic catheter was adjusted.
After thrombolysis therapy, oral warfarin was
administered to maintain an international normalized ratio of 2–2.5
during follow-up.
Recanalization, PTA and stent
In order to demonstrate the extent of the obstructed
vein, a 5-Fr catheter was percutaneously delivered from the femoral
vein or fluent portal vein branch and introduced into the distal
region of the obstructed vein after a 0.035-inch hydrophilic guide
wire was placed beyond the obstructing lesion. In the event that it
was impossible to cross the SVC with a guide wire via the femoral
route, the lesion was crossed with a hydrophilic guide wire via the
brachial route. After injection of heparin (50 IU/kg), balloon
angioplasty was performed (balloon, 8x40 mm) to allow easy
transition of the introducer sheath carrying the stent.
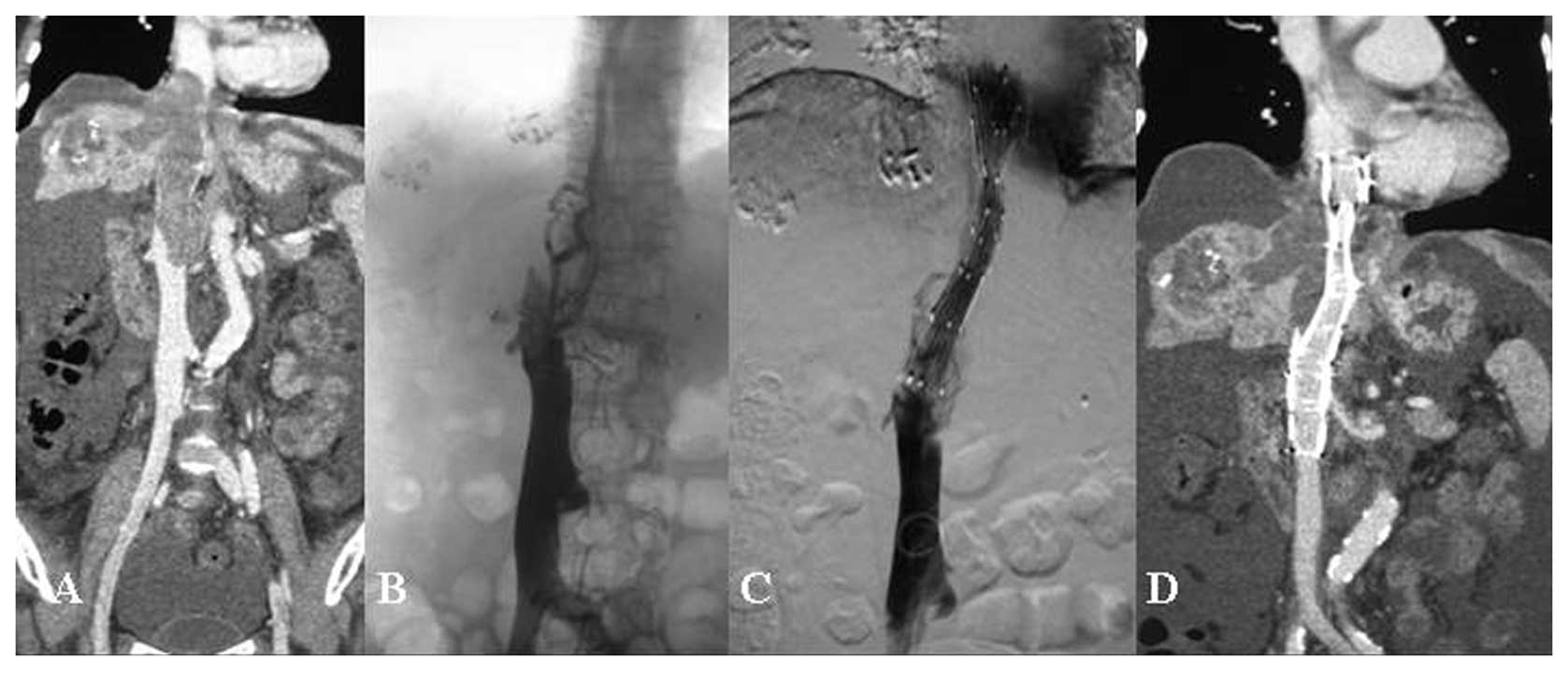
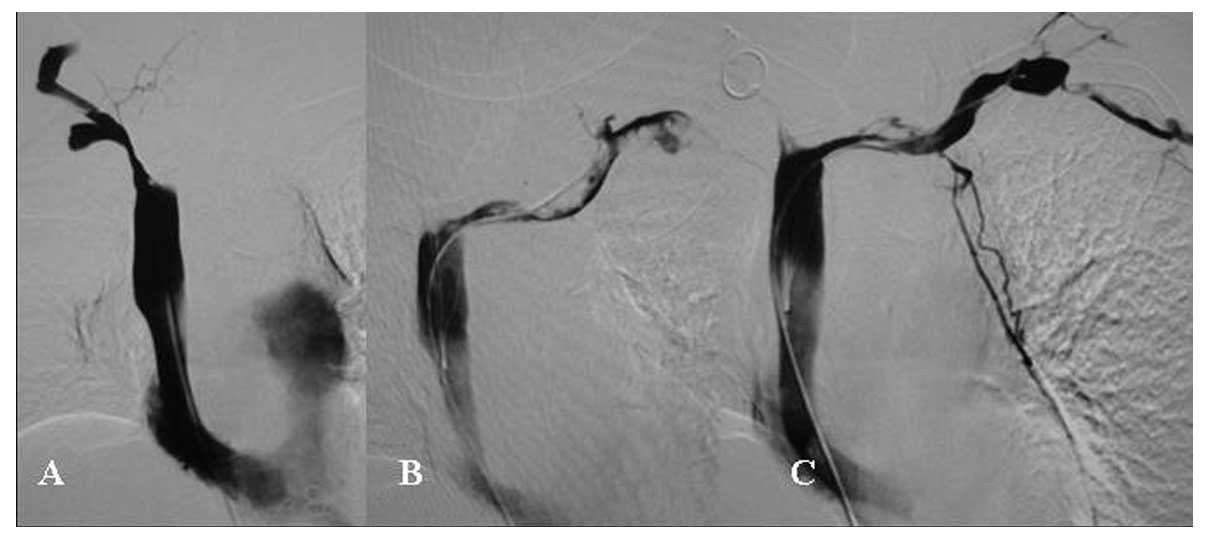
The diameter of the Z-stent implanted in the vena
cava was 20–24 mm and the length of the single Z-stent was 70 mm
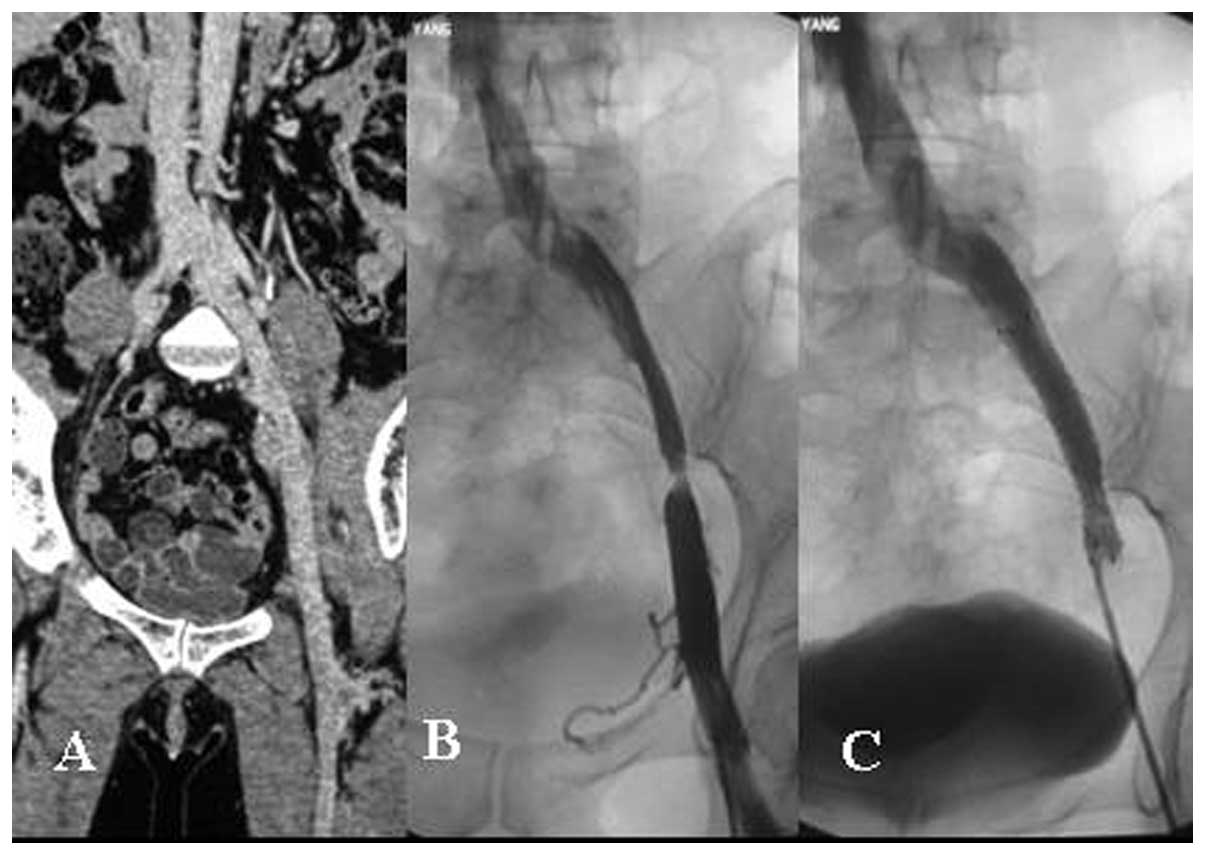
(Fig. 1). The diameter of the
Luminexx self-expandable stent implanted in the iliac vein was
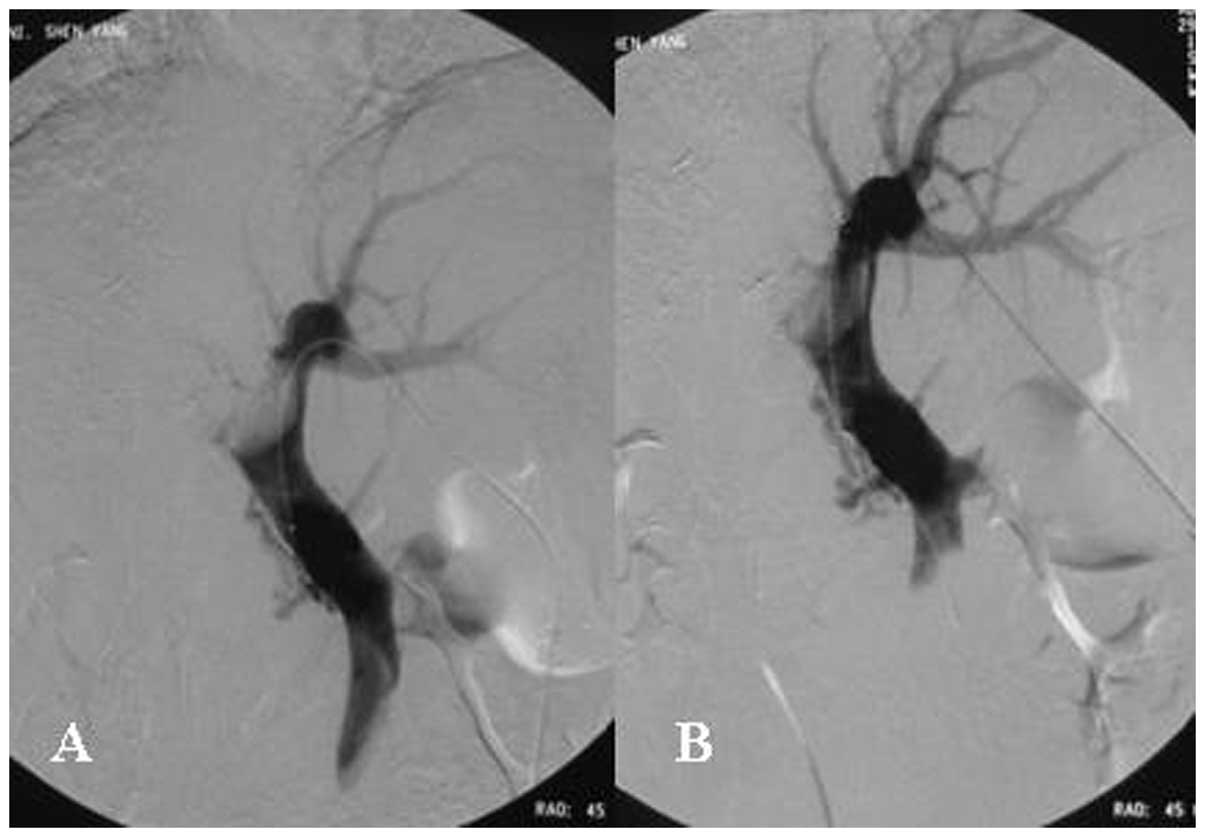
10–12 mm and the length of the stent was 40–80 mm (Fig. 2). The diameter of the Fluency
stent-graft implanted in the portal vein was 10 mm and the length
of the stent was 60–80 mm (Fig.
3). The stent was customized for each patient, taking into
consideration the length and diameter of the obstructing lesions
and the diameter of the normal regions of the vein near the
obstructing lesion. The stent was implanted accordingly so that it
extended over both sides of the obstructing lesion by 1–2 cm. For
longer lesions, two or three stents were implanted. If stent
dilatation was insufficient, post-dilatation was performed with
balloon.
After the procedure, systemic anticoagulant therapy
was administered by intravenously injecting 50 IU/kg heparin every
6 h for 5 days. Subsequently, oral warfarin was administered to
maintain an international normalized ratio of 2–2.5 at least for 3
months.
Study endpoints and definitions
The study endpoints were the success rate of
thrombolysis and stent implantation, the total urokinase dosage,
the clinical success rate, the operation-related complication rate,
the recurrence rate of the treated region and the survival duration
without recurrence of symptoms.
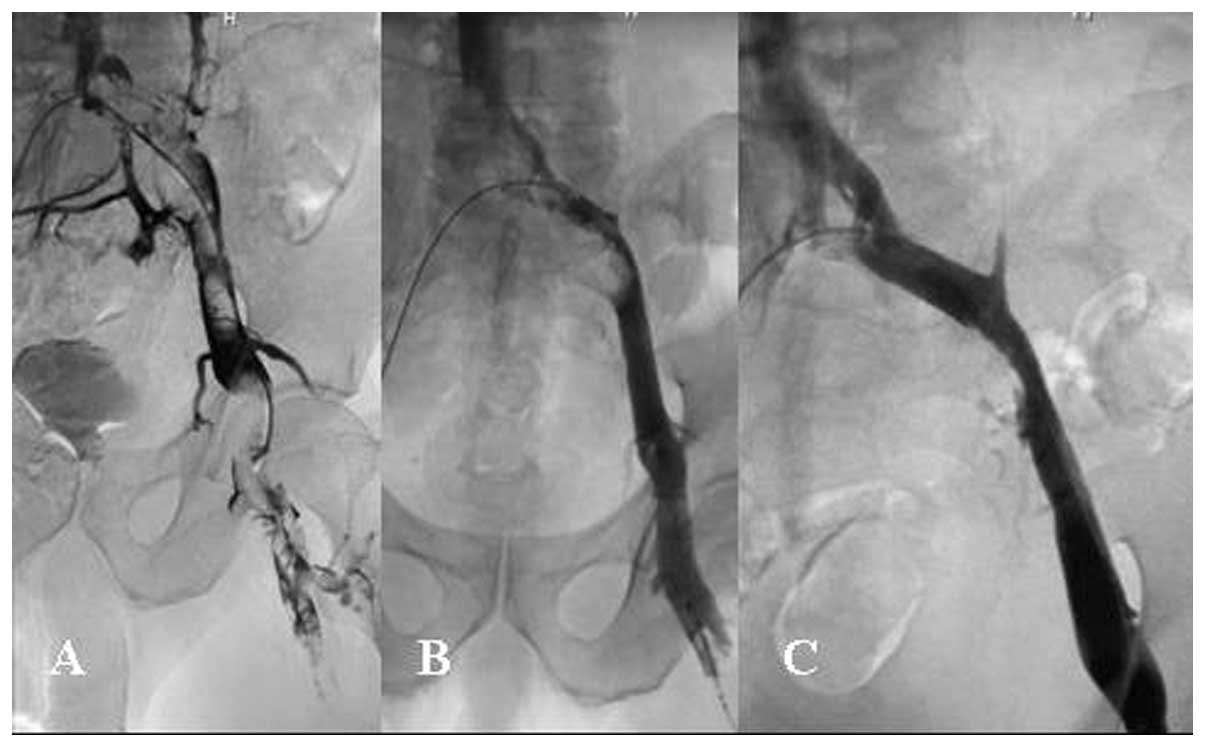
Thrombolysis was defined as complete (grade III)
(Fig. 4), nearly complete
(>90%, grade IIa), partial (50–90%, grade IIb) or no (<50%,
grade I) (Fig. 5) thrombolytic
effect at the final veinogram (1).
Technical success of the stent implantation was defined as
successful placement of the stent into the obstructing lesion.
Clinical success was defined as the complete alleviation of
symptoms.
Follow-up
Patency of the treated region was assessed by
performing Doppler ultrasound every 3 months after operation or at
any time if the symptoms of venous obstruction recurred. The
clinical courses after operation and the causes of death were
studied from clinical records until October 1, 2011.
Results
Initial clinical results
Technical success was achieved in all 18 patients
who accepted filter and thrombolytic therapy (100%). The total
urokinase dosage was 7.42±1.49 (4.5–10) million units. Thrombi in 2
patients were completely dissolved (grade III), in 8 patients
nearly completely dissolved (grade IIa), in 6 patients partially
dissolved (grade IIb) and in 2 patients not dissolved (grade I)
(Fig. 5). No pulmonary embolism
emerged after operations. The clinical success rate was 83.3%
(15/18) when thrombolytic therapy was terminated. The symptoms of 3
patients were palliated.
Among the 43 patients who accepted recanalization,
PTA and stent therapy, the technical success rate was 95.3%
(41/43). SVC obstructions in 2 patients were not successfully
recanalized through femoral and brachial approaches. The symptoms
of venous obstruction were persistent. One patient died due to
respiratory failure secondary to hemoptysis and one due to
suffocation secondary to the invasion of the lung cancer into the
bronchus 2 weeks and 1 month after operation. The clinical success
rate was 61.0% (25/41) within 3 days after recanalization, PTA and
stent therapy. The symptoms of 16 patients were palliated.
Follow-up results
All 61 patients died, and the longest follow-up
period was 32 months. Among the 18 patients who received filter and
thrombolytic therapy, the survival rate of 14 of these patients in
whom there was no recurrence of venous obstruction until death
ranged from 6 to 32 months (median, 16.5 months). The survival rate
of 4 patients in whom recurrence occurred ranged from 5 to 20
months (median, 9.5 months). Three patients recurred after stopping
warfarin and 1 patient recurred during oral warfarin. The causes of
death included lung metastasis (n=5), liver metastasis (n=5),
cerebral metastasis (n=3), cachexia (n=2), suffocation (n=2) and
enterobrosis (n=1).
Among the 41 patients who successfully accepted
recanalization, PTA and stent therapy, the survival rate of 33
patients in whom there was no recurrence of venous obstruction
until death ranged from 3 to 21 months (12.9±4.6; median, 13.0
months). The survival rate of 8 patients in whom recurrence
occurred ranged from 2 to 18 months (10.1±4.5, median, 10.0
months). The causes of death included respiratory failure (n=12),
suffocation (n=8), cerebral metastasis (n=6), liver failure (n=5),
lung metastasis (n=4), hepatic encephalopathy (n=2), hepatorenal
syndrome (n=2), enterobrosis (n=2) and gastrointestinal bleeding
(n=1).
Complications
There were no severe complications during the
procedure. Six patients (9.8%) experienced complications, including
5 patients with temporary local pain and 1 patient with hematuria
who revived after stopping thrombolytic therapy.
Discussion
Although vascular complications of malignancies and
concomitant hemodynamic changes are rarely direct causes of death,
they are usually facilitative factors of tumor deterioration and
predictors of poor prognosis. The aim of treatments for malignant
venous complications is to relieve venous obstructive symptoms,
improve the quality of life of patients and increase the survival
rate. Present therapies for this condition include radiotherapy,
chemotherapy, surgical therapy, endovascular therapy and
anticoagulant therapy. Radiotherapy and chemotherapy may induce the
reduction in tumor bulk and reduce the extent of venous invasion
and compression through killing and injuring tumor cells. These
therapies may radically cure malignant tumors and their venous
complications, but symptoms of venous obstructive are usually noted
at the early stage due to tumor-cell swelling. Endovascular therapy
may reconstruct fluent lumen, remove the thrombus and quickly
relieve the venous congestion. The ideal therapeutic regimen for
malignant venous complications is endovascular therapy followed by
radiotherapy, chemotherapy and anticoagulant therapy (9,10).
Our study demonstrates that catheter-directed
thrombolytic therapy for thrombosis of large veins is quite
effective with thrombus dissolution of grade II or III in 88.9% of
cases. This result is in accordance with the results obtained by
Kee et al (2) for SVC
syndrome, Kim et al (11)
for upper and lower limb DVT and Maleux et al (12) for thoracic deep vein
thrombosis.
Due to concerns of a risk of bleeding, the presence
of malignancy has been considered an exclusion criterion in several
clinical trials dealing with catheter-directed thrombolytic therapy
(1,13). However, Kim et al (11) and Maleux et al (12) found no significant difference
between cancer and non-cancer patients in the safety of
thrombolytic therapy for DVT in the lower and upper limbs and in
the thoracic cavity.
Maleux et al (12) reported that an intracranial
hemorrhage occurred in a cancer patient with an unknown and
asymptomatic cerebral metastasis during catheter-directed
thrombolytic therapy for thoracic deep vein thrombosis in a
retrospective trial. This fatal complication underlines the need
for brain imaging before starting thrombolytic therapy, especially
for cancer patients. No intracranial bleeding occurred in our study
as patients with cerebral metastasis and cerebrovascular accidents
were excluded from thrombolytic therapy.
Patients with malignancy often encounter a
hypercoagulable state, so long-term anticoagulant therapy is
essential after successful catheter-directed thrombolytic therapy.
In our study, all 3 patients who ceased anticoagulant therapy
encountered re-thrombosis. Only 1 of 15 patients who continued
anticoagulant therapy encountered re-thrombosis. No bleeding
occurred during follow-up and anticoagulant therapy. Hutten et
al (14) and Prandoni et
al (15) found a 2- to 6-fold
increased risk of bleeding in cancer patients compared with
non-cancer patients during systemic anticoagulation.
Venous obstructions significantly impact the quality
of life patients, and palliative therapies may be indicated even
for patients with malignancies with relatively short life
expectancies. Radiation and chemotherapy are only modestly
effective for vein obstruction secondary to malignant tumors, thus
stent placement represents a less invasive but effective therapy
for relieving vein obstruction and increasing the quality of life
of these patients (2,8,10,16–23).
Due to risk factors, SVC occlusion is usually more serious than
that of IVC and iliac veins for similar venous obstructive
symptoms. The difficulty in the recanalization of the SVC is
greater than that in other veins. To increase the success rate of
recanalization, the brachial route should be used when the lesion
is crossed with a hydrophilic guide wire via the femoral route. In
the present study, all 2 unsuccessful recanalizations occurred in
SVC occlusion patients.
If dilatation of the implanted stent in the superior
vena cava is insufficient, post-dilatation should be moderately
performed with balloon. Excess dilatation may induce adjacent lumen
compression or collapse for example presenting as dyspnea due to
tracheal collapse. There are various complications (24–27)
related to vena cava stent implantation, including pulmonary edema
resulting from a high venous return, stent migration, pulmonary
embolus, cardiac tamponade and local pain. To avoid stent
migration, the diameter of the stent should be greater than that of
the normal vein, and the length of the stent should be 2–4 cm
longer than that of the lesion.
The objective of stent-graft implantation for
intrahepatic portal vein tumor embolus is to assure adequate blood
supply to the non-tumor side of the liver lobe, to support normal
hepatic function and to avoid tumor embolus to intrude the
contralateral portal branch. In our study, the portal right or left
branch was occluded completely by a tumor embolus and the embolus
intruded the contralateral portal branch and/or portal major branch
in all 4 patients who received portal vein stent-graft
implantation. Stent-grafts were implanted between the portal major
branch and the contralateral portal branch. Due to the 10-mm
diameter, the distal segment of the stent-graft could not be
attached tightly to the vascular wall. The anchoring area of the 10
mm stent-graft was located at the contralateral portal branch. In
order to prevent tumor embolus overgrowth, the stent-graft was
placed over both sides of the obstructing lesion by 2 cm. The
diameter of the uncovered stents for malignant extrinsic portal
vein stenosis or occlusion were determined according to the
diameter of the involved portal vein with caution to oversize the
stent diameter by 1–2 mm (23).
To assure enough space for anchoring area of the
stent, the puncture entrance of the portal branch should be >2
cm from the tumor embolus. To show the relationship between the
left portal branch and the main trunk, the portogram should be
performed on right anterior oblique 30–45°. To measure exactly the
extent of the tumor embolus, a marked catheter should be used
during portogram. To prevent intraperitoneal hemorrhage, it is
crucial to embolize the liver parenchymal tract when the sheath is
withdrawn.
The main limitation of the present study include the
small number of patients, which limits the statistical analysis of
the survival rate after operation between the patients with and
without recurrence. Another limitation of this single-centre case
series is its retrospective nature over a long time period. In
addition, the cause of recurrence was not determined by
pathological examination. Finally, this study does not objectively
assess improvement in the quality of life of the patients.
In conclusion, interventional therapy has advantages
of smaller injuries, well-tolerance, high success rate, quick
palliation of symptoms and superior clinical efficacy in the
treatment of venous vascular complications as a result of malignant
tumors. However, whether interventional therapy increases the
survival time of malignancy patients with venous vascular
complications should be further investigated.
Acknowledgements
This study was supported by research
grants from the Scientific Research Fund of Liaoning Science and
Technology Agency, China (no. 2008225010-5) and the Scientific
Research Fund of Liaoning Education Agency, China (no. 2007T183)
and the Scientific Research Fund of First Hospital of China Medical
University (no. FSFH1006).
References
|
1.
|
Mewissen MW, Seabrook GR, Meissner MH,
Cynamon J, Labropoulos N and Haughton SH: Catheter-directed
thrombolysis for lower extremity deep venous thrombolysis: report
of a national multicenter registry. Radiology. 211:39–49. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Kee ST, Kinoshita L, Razavi MK, Nyman UR,
Semba CP and Dake MD: Superior vena cava syndrome: treatment with
catheter-directed thrombolysis and endovascular stent placement.
Radiology. 206:187–193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
AbuRahma A and Robinson PA: Effort
subclavian vein thrombosis: evolution of management. J Endovasc
Ther. 7:302–308. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kreienberg PB, Chang BB, Darling RC III,
et al: Long-term results in patients with thrombolysis, thoracic
inlet decompression, and subclavian vein stenting for
Paget-Schroetter syndrome. J Vasc Surg. 33:S100–S105. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kearon C, Kahn SR, Agnelli G, Goldhaber S,
Raskob GE and Comerota AJ; American College of Chest Physicians:
Antithrombotic therapy for venous thromboembolic disease: American
College of Chest Physicians - evidence-based clinical practice
guidelines, 8th edition. Chest. 133:S454–S545. 2008. View Article : Google Scholar
|
|
6.
|
Furui S, Sawada S, Kuramoto K, et al:
Gianturco stent placement in malignant caval obstruction: analysis
of factors for predicting the outcome. Radiology. 195:147–152.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Fletcher WS, Lakin PC, Prommier RF and
Wilmarth T: Results of treatment of inferior vena cava syndrome
with expandable metallic stents. Arch Surg. 133:935–938. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lanciego C, Chacon JL, Julian A, et al:
Stenting as first option for endovascular treatment of malignant
superior vena cava syndrome. AJR Am J Roentgnenol. 177:585–593.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Lanciego C, Pangua C, Chacón JI, et al:
Endovascular stenting as the first step in the overall management
of malignant superior vena cava syndrome. AJR Am J Roentgnenol.
193:549–558. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Nagata T, Makutani S, Uchida H, et al:
Follow-up results of 71 patients undergoing metallic stent
placement for the treatment of a malignant obstruction of the
superior vena cava. Cardiovasc Intervent Radiol. 30:959–967. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kim HS, Preece SR, Black JH, Pham LD and
Streiff MB: Safety of catheter-directed thrombolysis for deep
venous thrombosis in cancer patients. J Vasc Surg. 47:388–394.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Maleux G, Marchal P, Palmers M, et al:
Catheter-directed thrombolytic therapy for thoracic deep vein
thrombosis is safe and effective in selected patients with and
without cancer. Eur Radiol. 20:2293–2300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Heymans S, Verhaeghe R, Stockx L and
Collen D: Feasibility study of catheter-directed thrombolysis with
recombinant staphylokinase in deep venous thrombosis. Thromb
Haemost. 79:517–519. 1998.PubMed/NCBI
|
|
14.
|
Hutten BA, Prins MH, Gent M, Ginsberg J,
Tijssen JG and Büller HR: Incidence of recurrent thromboembolic and
bleeding complications among patients with venous thromboembolism
in relation to both malignancy and achieved international
normalized ratio: a retrospective analysis. J Clin Oncol.
18:3078–3083. 2000.
|
|
15.
|
Prandoni P, Lensing AW, Piccioli A, et al:
Recurrent venous thrombo-embolism and bleeding complications during
anticoagulant treatment in patients with cancer and venous
thrombosis. Blood. 100:3484–3488. 2002. View Article : Google Scholar
|
|
16.
|
Nicholson AA, Ettles DF, Arnold A,
Greenstone M and Dyet JF: Treatment of malignant superior vena cava
obstruction: metal stents or radiation therapy. J Vasc Interv
Radiol. 8:781–788. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Miller JH, McBride K, Little F and Price
A: Malignant superior vena cava obstruction: stent placement via
the subclavian route. Cardiovasc Intervent Radiol. 23:155–158.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
de Gregorio Ariza MA, Gamboa P, Gimeno MJ,
et al: Percutaneous treatment of superior vena cava syndrome using
metallic stents. Eur Radiol. 13:853–862. 2003.PubMed/NCBI
|
|
19.
|
Stambo GW, Leto J, Van Epps K, Woeste T
and George C: Endovascular treatment of intrahepatic inferior vena
cava obstruction from malignant hepatocellular tumor thrombus
utilizing Luminexx self-expanding nitinol stents. South Med J.
99:1148–1149. 2006. View Article : Google Scholar
|
|
20.
|
Zamora CA, Sugimoto K, Mori T, et al: Use
of the wallstent for symptomatic relief of malignant inferior vena
cava obstructions. Radiat Med. 23:380–385. 2005.PubMed/NCBI
|
|
21.
|
Kishi K, Sonomura T, Fujimoto H, et al:
Physiologic effect of stent therapy for inferior vena cava
obstruction due to malignant liver tumor. Cardiovasc Intervent
Radiol. 29:75–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Nio Y, Iguchi C, Itakura M, et al:
Placement of an expandable metallic stent improves the efficacy of
chemoradiotherapy for pancreatic cancer with malignant portal vein
stenosis or obstruction. Anticancer Res. 29:3329–3335.
2009.PubMed/NCBI
|
|
23.
|
Novellas S, Denys A, Bize P, et al:
Palliative portal vein stent placement in malignant and symptomatic
extrinsic portal vein stenosis or occlusion. Cardiovasc Intervent
Radiol. 32:462–470. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Kishi K, Sonomura T, Mitsuzane K, et al:
Self-expandable metallic stent therapy for superior vena cava
syndrome: Clinical observations. Radiology. 189:531–535. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Smayra T, Otal P, Chabbert V, et al:
Long-term results of endovascular stent placement in the superior
cava venous system. Cardiovasc Intervent Radiol. 24:388–394. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Smith SL, Manhire AR and Clark DM: Delayed
spontaneous superior vena cava perforation associated with a SVC
Wallstent. Cardiovasc Intervent Radiol. 24:286–287. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Dinkel HP, Mettke B, Schmid F, Baumgartner
I, Triller J and Do DD: Endovascular treatment of malignant
superior vena cava syndrome: Is bilateral Wallstent placement
superior to unilateral placement? J Endovasc Ther. 10:788–797.
2003. View Article : Google Scholar : PubMed/NCBI
|