Introduction
Radiation therapy (RT) as an initial treatment
option has long been considered standard practice worldwide for
nasopharyngeal carcinoma (NPC) (1). The irradiated volume usually covers
the primary site, positive lymph nodes, as well as areas
potentially involved by the disease. The whole neck is treated
definitively or prophylactically except for patients with N0 stage
(2). Critical organs including the
parotids, spinal cord, brain stem, pituitary gland, temporal lobes,
cranial nerves and middle and inner ears are inevitably exposed to
unnecessary irradiation due to their close proximity to the
targets. An excessive radiation dose to these organs destroys their
partial or whole function, and subsequently impairs the quality of
life (QOL) of patients. In addition, toxicities to normal
structures often restrict dose escalation to the targets, while
insufficient dose to the targets may result in decreased local
and/or regional control.
Apart from the organs mentioned above, the brachial
plexus is another dose-limiting structure. In laryngeal carcinoma
treated with total laryngectomy and left radical neck dissection
followed by intensity-modulated radiation therapy (IMRT), the
maximum dose to the brachial plexus may reach 68 Gy when not
defined as an avoidance structure during treatment planning
(3). As a unique subtype of
head-and-neck cancer, NPC is found to have a higher risk of
metastasis to the neck lymph nodes. Nodal involvement in the
ipsilateral neck occurs in 85–90%, and in the bilateral neck in 50%
of patients. For this reason, all of these nodal regions are
usually covered by the clinical target volume, and the tumoricidal
radiation dose needs to be delivered to the positive lymph nodes
(4). Anatomically, the brachial
plexus is in proximity to the gross neck disease at the levels
between the C5 and T2 vertebral bodies, thus it is assumed that an
increased risk of excessive radiation to the brachial plexus occurs
when the neck is treated with a higher dose and no dose constraints
is placed on the brachial plexus. Based on this hypothesis, we
conducted this retrospective study to evaluate the dose
distribution to the brachial plexus in NPC patients treated with
definitive IMRT.
Materials and methods
Study population
From November 2009 to December 2010, 43 patients
with newly diagnosed NPC were treated with IMRT at the Department
of Radiation Oncology at the People’s Hospital of Guangxi Zhuang
Autonomous Region. Fifteen patients were excluded from the present
study: 5 with distant metastasis, 5 treated with palliative intent
due to extensive local or regional disease, 2 treated with
palliative intent due to uncontrolled medical comorbidities, 3
treated with conventional RT followed by IMRT. The remaining 28
treated with definitive IMRT were included for analysis. Of these
patients, 20 were men and 8 were women with a median age of 42
years (range, 22–76). All patients had histologically confirmed
undifferentiated carcinoma. The staging distribution according to
the 2002 AJCC Staging System is listed in Table I. The study was approved by the
Institutional Review Board (IRB) of the People’s Hospital of
Guangxi Zhuang Autonomous Region.
 | Table I.Baseline characteristics and treatment
details. |
Table I.
Baseline characteristics and treatment
details.
| Characteristic | P-value |
|---|
| Age (years) | |
| Median | 42 |
| Range | 22–76 |
| Gender, no. (%) | |
| Male | 20 (71.4) |
| Female | 8 (28.6) |
| T stage, no. (%) | |
| T1 | 2 (7.1) |
| T2 | 13 (46.4) |
| T3 | 8 (28.6) |
| T4 | 5 (17.9) |
| N stage, no. (%) | |
| N0 | 6 (21.5) |
| N1 | 10 (35.7) |
| N2 | 9 (32.1) |
| N3 | 3 (10.7) |
| AJCC stage group, no.
(%) | |
| IIA | 1 (3.6) |
| IIB | 4 (14.3) |
| III | 15 (53.5) |
| IVA | 5 (17.9) |
| IVB | 3 (10.7) |
| Radiation dose (Gy),
median (range) | |
| Median dose to
PTVnx | 70.0 (66.0–71.6) |
| Median dose to
PTVnd | 69.0 (63.6–70.0) |
| Median dose to
PTV1 | 60.0 (56.1–64.0) |
| Median dose to
PTV2 | 54.0 (50.4–56.1) |
Intensity-modulated radiotherapy
Patients were immobilized in a supine position with
the head in a neutral position, with a tailored thermoplastic mask
covering the head, neck and shoulders. Intravenous
contrast-enhanced CT using 2-mm slices from the vertex to the
manubriosternal joint was performed for planning. The CT data were
imported to the CMS-XiO treatment planning system (CMS Inc., St.
Louis, MO, USA).
The target delineation for NPC patients has been
described previously (5). In
brief, the primary gross tumor volume (GTVnx) and the involved
lymph nodes (GTVnd) included all known gross disease as determined
by the imaging, clinical, and endoscopic findings. Clinical target
volume (CTVnx) included the GTVnx plus a 5- to 10-mm margin, and
CTVnd included the GTVnd plus a 5-mm margin. CTV1 was defined as
the entire nasopharynx, parapharyngeal space, pterygopalatine
fossa, posterior third of the nasal cavity and maxillary sinuses,
inferior sphenoid sinus, posterior ethmoid sinus, skull base, and
anterior half of the clivus. CTV1 also included bilateral
retropharyngeal lymph nodes and ipsilateral level II for
node-negative neck. CTV1 extended to the next ipsilateral level for
node-positive neck, or included the full length of ipsilateral neck
for node-positive in the lower neck. CTV2 was defined as low-risk
node region below the CTV1. Level V was separated by the borderline
between the CTV1 and CTV2 (i.e. regions above the borderline were
covered by the CTV1 and regions below the borderline were covered
by the CTV2). Level Ib was not included unless the ipsilateral
level II was involved. The respective planning target volumes
(PTVs) were generated with a 3-mm margin when daily kilovoltage
cone-beam computed tomography (KV-CBCT) was performed, or with a
4-mm margin when not. The contoured critical structures included
the brain stem, chiasm, optic nerves, spinal cord, eyes, lens,
cochlea, parotid glands, oral cavity, larynx, mandible and
temporomandibular joints. The brachial plexus was not delineated
during the initial planning, and dose to the brachial plexus was
not calculated.
The treatment plans were optimized by using the CMS
inverse treatment-planning system. The median doses delivered to
the PTVnx, PTVnd, PTV1, and PTV2 were 70.0 Gy (66.0–71.6 Gy), 69.0
(63.6–70.0 Gy), 60.0 (56.1–64.0 Gy) and 54.0 Gy (50.4–56.1 Gy),
respectively, in 30–33 fractions. All patients were treated once
daily, with 5 fractions weekly (Table
I). The dose constrains to critical structures were within the
tolerance, according to the Radiation Therapy Oncology Group (RTOG)
0225 protocol, and every effort was made to meet the criteria as
closely as possible. IMRT was delivered via seven fixed-gantry
angles with an Elekta Synergy Linear Accelerator (Elekta Ltd).
Concurrent chemotherapy
Two patients were treated with IMRT alone and 26
were treated with IMRT and concurrent platinum-based chemotherapy.
Of these, 18 patients received platinum alone and 8 patients
received combination chemo-therapy with platinum and
5-fluorouracil.
Brachial plexus contour and dose
recalculation
The left and right brachial plexi were delineated
retrospectively and separately on the initial axial-planning CT
scan. A delineation was performed step-by-step, following the
CT-based atlas for delineating the brachial plexus, a guideline
proposed by Hall et al (6)
and endorsed by the RTOG.
To avoid inter-observer variations, a senior
radiation oncologist was assigned to contour the brachial plexus.
After completing the delineation of the brachial plexus, the
dose-distribution was recalculated and a dose-volume histogram was
generated based on the original treatment plan, without changes in
any dosimetric parameters. The mean volume of, and the dose to the
brachial plexus were compared between the left and right side. Upon
delineation of the brachial plexus, it was determined whether or
not the brachial plexus was adjacent to the positive lymph nodes.
BPAN was defined when the ipsilateral neck from C5 to T2 vertebral
body consisted of positive lymph nodes, whereas BPNAN was defined
when no positive lymph nodes were present. The BPANs were compared
with the BPNANs with respect to their mean dose, maximum dose and
irradiated volume exceeding different dose levels.
Statistical analysis
Independent-samples t-test was used to test the mean
differences in each parameter (i.e. mean volume of the brachial
plexus between the right and left sides, dose to the brachial
plexus, mean and maximum doses to the BPANs and BPNANs and
irradiated volumes of the brachial plexus at different dose
levels). All statistical tests were 2-sided, and p≤0.05 was
considered statistically significant. Analyses were performed using
Microsoft Office Excel (Version 2007) and SPSS software (SPSS 17.0,
SPSS, Inc., Chicago, IL, USA).
Results
The mean volume of, and the dose to the
brachial plexus
The mean volumes were 6.02±2.78 cm3 for
the left brachial plexus and 6.17±1.90 cm3 for the right
(p=0.837). The maximum dose to the left brachial plexus ranged from
59.12 to 78.47 Gy. The percentage of patients receiving the maximum
dose to the left brachial plexus exceeding 60, 66 and 70 Gy was
96.4 (27/28), 57.1 (16/28) and 25.0% (7/28), respectively, whereas
the maximum dose to the right brachial plexus ranged from 59.74 to
80.31 Gy. The percentage of patients receiving the maximum dose to
the right brachial plexus exceeding 60, 66 and 70 Gy was 96.4
(27/28), 64.3 (18/28) and 39.3 (11/28), respectively. The minimum
and mean doses to the brachial plexus for the left side exhibited
no significant differences compared with the corresponding doses to
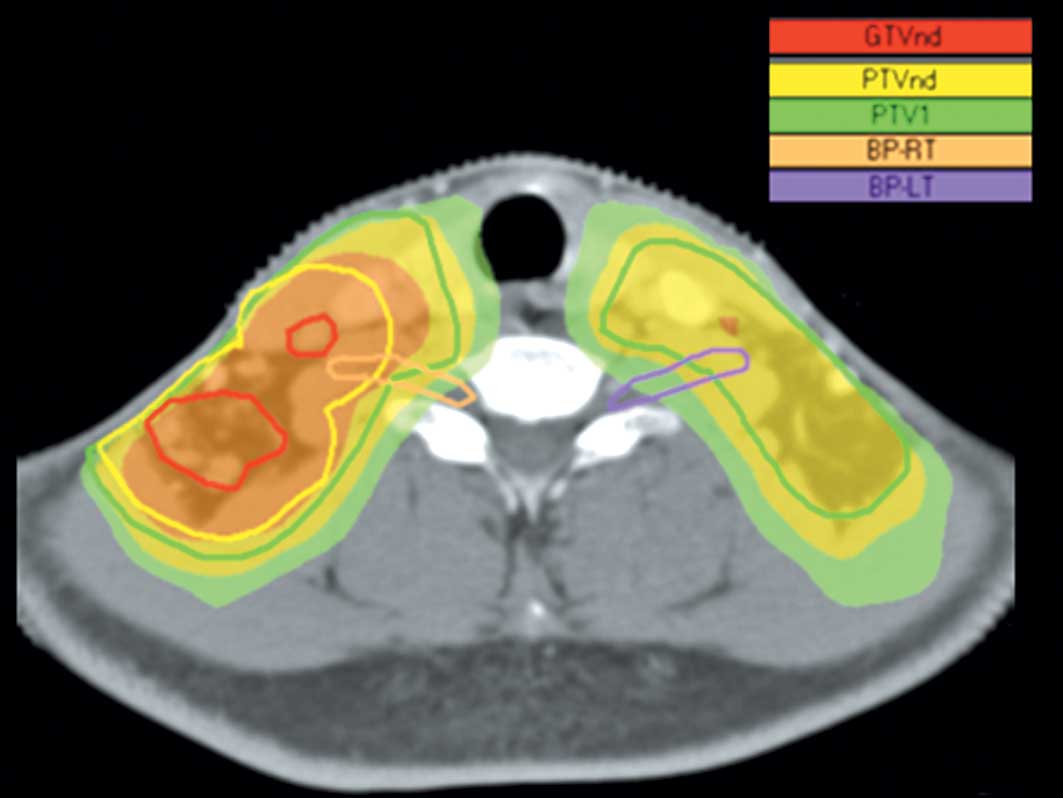
the brachial plexus for the right side (Table II). A typical example of the
dose-distribution to the brachial plexus in a patient with T2bN3M0
disease is shown in Fig. 1.
 | Table II.Mean volume of, and dose to the
brachial plexus. |
Table II.
Mean volume of, and dose to the
brachial plexus.
| Left side (mean ±
SD) | Right side (mean ±
SD) | Difference in the
mean (95% CI) | P-value |
|---|
| Mean volume of BP
(cm3) | 6.02±2.78 | 6.17±1.90 | −0.15
(−1.63–1.32) | 0.837 |
| Dose to BP (Gy) | | | | |
| Max | 66.82±4.99 | 67.77±5.53 | −0.95
(−3.77–1.87) | 0.502 |
| Min | 22.43±4.87 | 22.36±15.35 | 0.07
(−8.03–8.16) | 0.067 |
| Mean | 50.56±15.10 | 51.70±15.07 | −1.14
(−9.23–6.94) | 0.778 |
Mean and maximum doses to the BPANs and
BPNANs
We identified 16 BPANs, which consisted of 7 BPANs
on the left side and 9 BPANs on the right side. We also identified
40 BPNANs, including 21 BPNANs on the left side and 19 BPNANs on
the right side. For the left brachial plexus, the maximum doses to
the BPANs and the BPNANs were 72.84±3.91 and 64.81±3.47 Gy,
respectively (p<0.001). For the right brachial plexus, the
maximum doses to the BPANs and the BPNANs were 72.91±4.74 and
64.91±3.52 Gy, respectively (p<0.001). No significant
differences were found in the mean doses to the BPANs and the
BPNANs for either side (Table
III).
 | Table III.Mean and maximum doses to the BPANs
and BPNANs. |
Table III.
Mean and maximum doses to the BPANs
and BPNANs.
| BPAN (mean ± SD) | BPNAN (mean ±
SD) | Difference in mean
(95% CI) | P-value |
|---|
| Dose to left-sided BP
(Gy) | | | | |
| Mean | 54.62±17.48 | 49.20±14.44 | 5.42
(−8.21–19.05) | 0.421 |
| Max | 72.84±3.91 | 64.81±3.47 | 8.03
(4.82–11.24) | <0.001 |
| Dose to right-sided
BP (Gy) | | | | |
| Mean | 57.34±11.75 | 48.57±16.07 | 8.77
(−3.16–20.71) | 0.143 |
| Max | 72.91±4.74 | 64.91±3.52 | 8.01
(4.77–11.24) | <0.001 |
Irradiated volume of the brachial plexus
at different dose levels
V40, V50, V60 and V66 represent the percentage of a
specific structure exceeding 40, 50, 60 and 66 Gy, respectively. In
the present study, absolute volumes were used for analyzing the
irradiated volume of the brachial plexus at different dose levels.
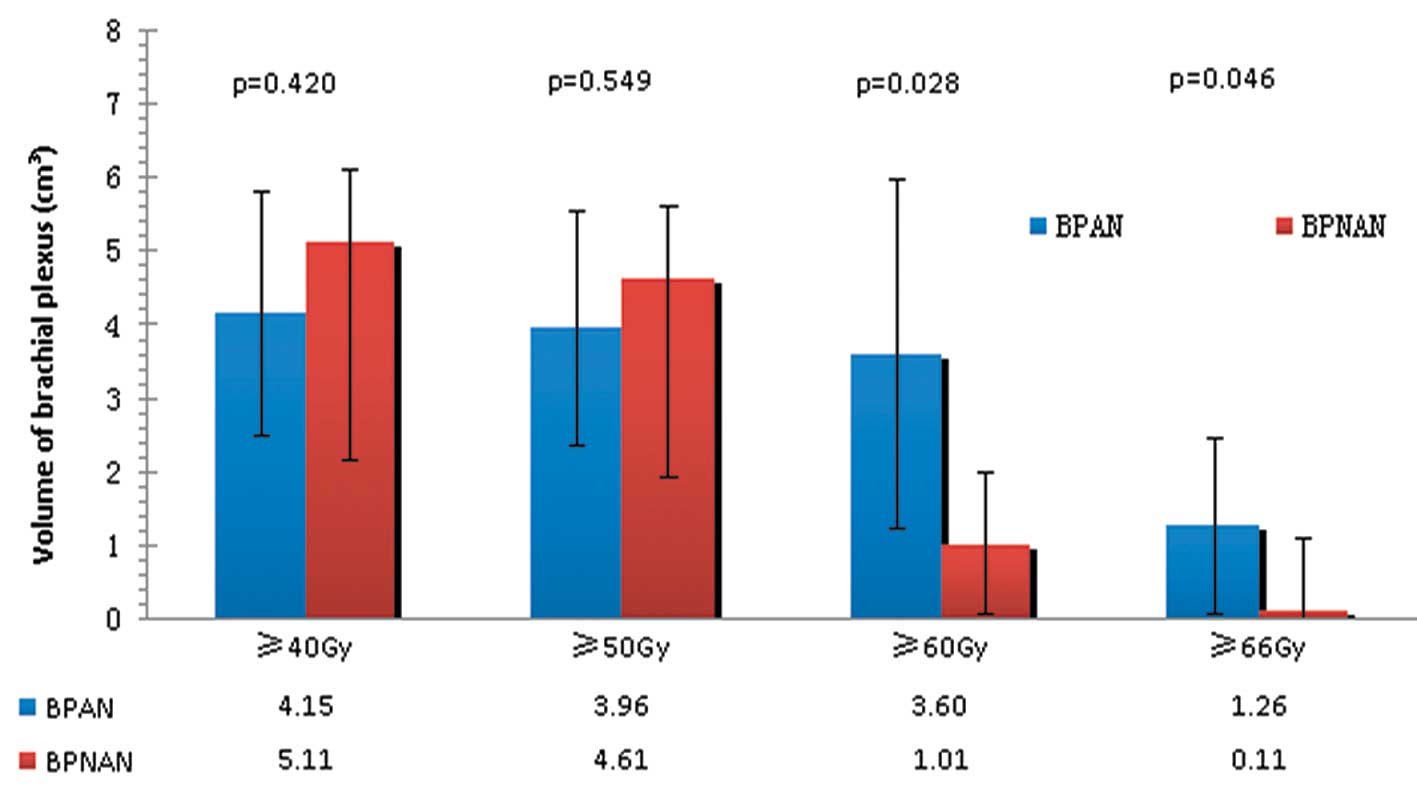
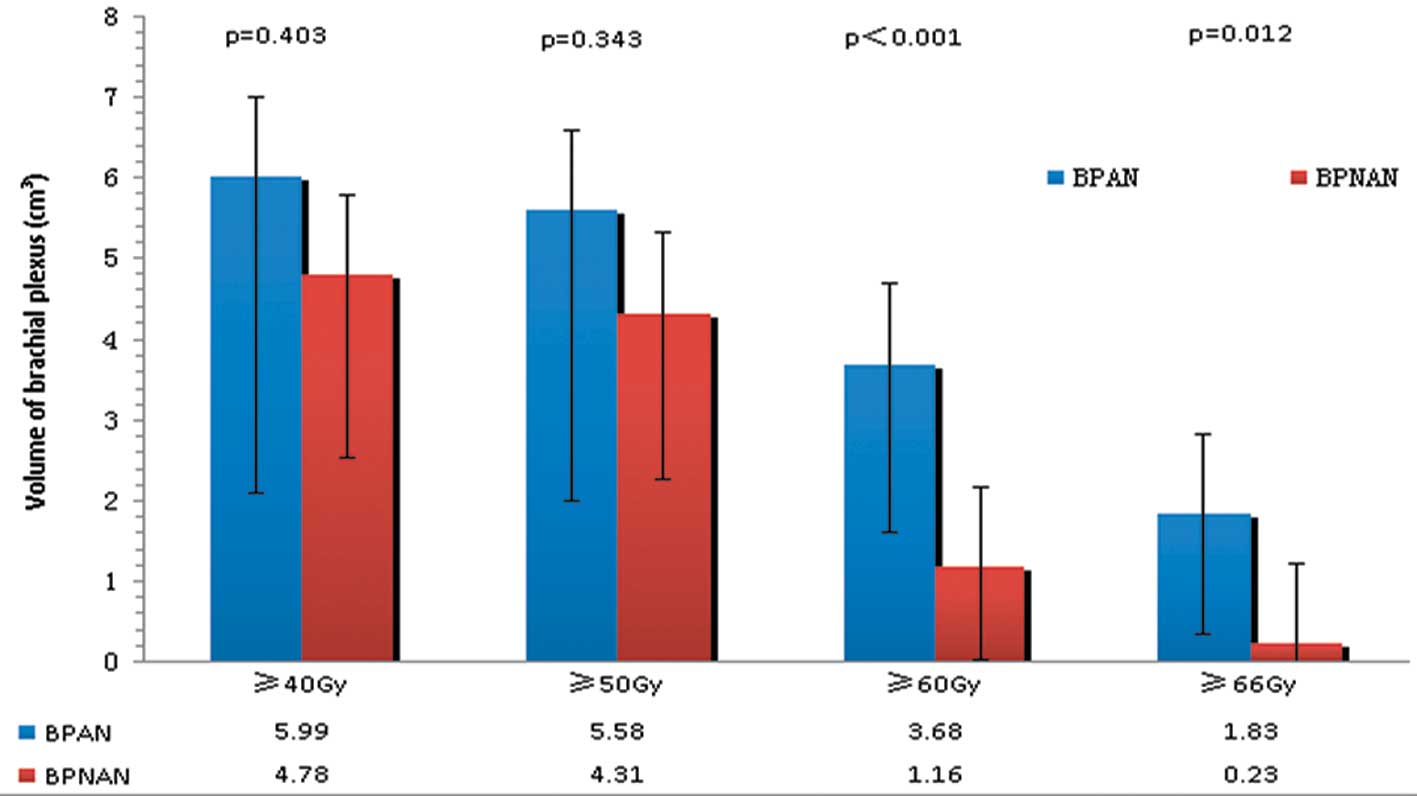
As shown in Figs. 2 and 3, there were no significant differences
in V40 and V50 between the BPANs and BPNANs. V40 for the left BPANs
was 4.15 cm3, whereas for the left BPNANs, it was 5.11
cm3 (p=0.420). V50 for the left BPANs was 3.96
cm3, whereas for the left BPNANs it was 4.61
cm3 (p=0.549). V40 was 5.99 cm3 for the right
BPANs and 4.78 cm3 for the right BPNANs (p=0.403). V50
was 5.58 cm3 for the right BPANs and 4.31 cm3
for the right BPNANs (p=0.343). By contrast, the difference between
the left BPANs and BPNANs was statistically significant not only
for V60 (3.60 vs. 1.01 cm3, p=0.028) but also for V66
(1.26 vs. 0.11 cm3, p=0.046). Similar results were found
on the right side. There were significant differences in V60 (3.68
vs. 1.16 cm3, p<0.001) and V66 (1.83 vs. 1.23
cm3, p=0.012) between the right BPANs and BPNANs.
Discussion
Injuries to the brachial plexus associated with RT
have been documented in various cancer types, including breast
cancer (7), lung cancer (8) and head-and-neck cancer (9). The brachial plexopathy is often
gradually deteriorated and no effective treatment is available.
Thus, the major aim of RT is to diminish or, at best, to avoid an
excessive radiation dose to the brachial plexus. With the rapid
evolvement of radiation therapy techniques during the last decade,
IMRT has been widely accepted as the preferential technique for
treating head-and-neck cancer. It is generally believed that IMRT
is superior to conventional RT with respect to local and/or
regional tumor control and critical organ sparing. However, in a
recent study, Chen et al (10) found that the dose to the brachial
plexus was significantly increased among patients with
head-and-neck cancer undergoing IMRT compared with conventional RT
when no brachial plexus constraint was used. Contouring the
brachial plexus as an avoidance structure during treatment planning
may minimize the radiation dose to this region.
The brachial plexus starts in the posterior triangle
of the neck and travels distally into the upper extremity, where it
divides into rami, trunks, divisions, cords, and terminal nerve
branches. The anatomic location of the brachial plexus is the area
of the thoracic outlet, between the first rib and the clavicle. The
brachial plexus extends from the lateral border of the scalene
anterior muscle to the caudal border of the pectoralis minor
(11). An accurate delineation of
the brachial plexus is a prerequisite for achieving brachial
plexus-sparing during the planning phase. Hall et al
(6) proposed a standard method for
contouring the brachial plexus on a treatment planning CT scan.
Although the method used anatomic landmarks on axial CT images as a
surrogate for identifying the location of the brachial plexus, it
did provide a reliable set of guidelines for the consistent
contouring of the brachial plexus (12). Magnetic resonance (MR) imaging can
help identify the anatomy of the brachial plexus. Fusion CT-MR
imaging facilitates the delineation, even though its routine
application is restricted due to unavailability at most radiation
oncology facilities and other reasons, such as difference in
patient positioning when performing CT imaging and MR imaging
(13). Therefore, CT imaging alone
remains the widely accepted tool for contouring the brachial plexus
to date. By following the instructions, we delineated the brachial
plexus in 28 NPC patients. Unlike others, we separated the left-
and the right-sided brachial plexus when delineating. The mean
volumes between the left and right brachial plexus had no
significant difference, indicating that this structure has
bilateral symmetry. In addition, no significant differences were
noted in the maximum, minimum and the mean dose to the brachial
plexus between the two sides.
Due to the high incidence of cervical nodal
metastasis, the entire neck - including the retropharyngeal nodes
and levels I-V lymph nodes - is considered to be at risk for
involvement. Radical radiation delivered to the gross nodal disease
may increase the risk of an excessive dose to the brachial plexus.
In the current study, we found that the BPANs received a
significantly higher maximum dose than the BPNANs (72.84±3.91 vs.
64.81±3.47 Gy on the left side, p<0.001; 72.91±4.74 vs.
64.91±3.52 Gy on the right side, p<0.001), reflecting the close
proximity of nodal regions to the brachial plexus. In fact,
although the brachial plexus did not abut the lymph nodes, its
maximum dose was close to the target dose (Table III). In addition, for the brachial
plexus on both sides, the BPANs had significant differences in V60
and V66, compared with the BPNANs. The findings are similar to the
results of other researchers. Millender et al (14) retrospectively evaluated the
radiation dose to the brachial plexus in 16 patients with
head-and-neck cancer treated with extended field IMRT technique and
found that the median maximum-point dose was higher when the plexus
was adjacent to grossly positive nodes (71.9 Gy) than when it was
adjacent to node-negative regions (65.7 Gy). The median volumes
receiving a dose greater than 60 Gy were 2.95 and 0.84 cc,
respectively.
Dose constraints to the brachial plexus suggested by
the Radiation Therapy Oncology Group (RTOG) in several protocols
(RTOG 0435, RTOG 0522, and RTOG 0615) range from 60 to 66 Gy at 2
Gy per fraction. McGary et al (3) found, that doses to the brachial
plexus approximated the target dose when the brachial plexus was
not defined as an avoidance structure and the target dose was set
to 66 or 70 Gy. A hot spot could be seen in the brachial plexus
that exceeded 70 Gy. In our study, 96.4% of the brachial plexus on
both sides had a maximum dose exceeding 60 Gy, and the percentages
of patients receiving the maximum dose exceeding 66 Gy were 57.1
and 64.3% for the left and right plexus, respectively. These
findings indicate that for the majority of NPC patients the maximum
dose to the brachial plexus will be beyond the recommended
constraints, when no brachial plexus is delineated as a restricted
volume.
There were no data available on the incidence of
brachial plexopathy for our patients since the study was conducted
retrospectively and patients had no regular follow-up.
Nevertheless, to our knowledge, the present report represents the
first attempt to analyze the radiation dose to the brachial plexus
among patients treated solely for nasopharyngeal carcinoma.
In conclusion, a large proportion of patients are
exposed to the maximum dose to the brachial plexus exceeding the
RTOG recommended restraints, when the brachial plexus is not
outlined. For the brachial plexus on both sides, the BPANs had
higher volumes in V60 and V66 than the BPNANs. The BPANs received a
significantly higher maximum dose than that of the BPNANs. A
further study is underway to assess whether brachial plexus
contouring assists in dose reduction to the brachial plexus for
IMRT optimization.
Acknowledgements
The present study was financed by
grants from the Sci-Tech Office of the Guangxi Zhuang Autonomous
Region, China (nos. 0816004-40 and 099300B-6).
References
|
1.
|
Lu H, Peng L, Yuan X, Hao Y, Lu Z, Chen J,
Cheng J, Deng S, Gu J, Pang Q and Qin J: Concurrent
chemoradiotherapy in locally advanced nasopharyngeal carcinoma: a
treatment paradigm also applicable to patients in Southeast Asia.
Cancer Treat Rev. 35:345–353. 2009. View Article : Google Scholar
|
|
2.
|
Gao Y, Zhu G, Lu J, Ying H, Kong L, Wu Y
and Hu C: Is elective irradiation to the lower neck necessary for
N0 nasopharngeal carcinoma ? Int J Radiat Oncol Biol Phys.
77:1397–1402. 2010. View Article : Google Scholar
|
|
3.
|
McGary JE, Grant WH, Teh BS, Paulino AC
and Butler E: Dosimetric evaluation of the brachial plexus in the
treatment of head and neck cancer. Int J Radiat Oncol Biol Phys.
69(Suppl 3): S464–S465. 2007. View Article : Google Scholar
|
|
4.
|
Lo SS and Lu JJ: Natural history,
presenting symptom, and diagnosis of nasopharyngeal carcinoma.
Nasopharyngeal Cancer-Multidisciplinary Management. Lu JJ, Cooper
JS and Lee AWM: Springer-Verlag; Berlin, Heidelberg: pp. 462009
|
|
5.
|
Lu H, Chen J, Huang B, Cheng J, Peng L,
Hao Y, Liao C, Mo Y, Wu D and Qin J: Feasibility and efficacy study
of weekly cisplatin with concurrent intensity-modulated radiation
therapy for nasopharyngeal carcinoma – preliminary results. Oral
Oncol. 46:743–747. 2010.PubMed/NCBI
|
|
6.
|
Hall W, Guiou M, Lee N, Dublin A, Narayan
S, Vijayakumar S, Purdy JA and Chen AM: Development and validation
of a stan-darlized method for contouring the brachial plexus:
preliminary dosimetric analysis among patients treated with IMRT
for head-and-neck cancer. Int J Radiat Oncol Biol Phys.
72:1362–1367. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Johansson S, Svensson H and Denekamp J:
Timescale of evolution of late radiation injury after postoperative
radiotherapy of breast cancer patients. Int J Radiat Oncol Biol
Phys. 48:745–750. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Chamberlain DD, Rowe BP and Decker RH:
Simultaneous treatment of synchronous primary lung cancers with
stereotactic body radiotherapy (SBRT). Int J Radiat Oncol Biol
Phys. 75(Suppl 1): S4702009. View Article : Google Scholar
|
|
9.
|
Chen AM, Hall W, Guiou M, Mathai M,
Vijayakumar S and Purdy JA: Brachial plexopathy after radiation
therapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys.
75(Suppl 1): S31–S32. 2009. View Article : Google Scholar
|
|
10.
|
Chen AM, Hall WH, Li BQ, Guiou M, Wright
C, Mathai M, Dublin A and Purdy JA: Intensity-modulated
radiotherapy increases dose to the brachial plexus compared with
conventional radiotherapy for head-and-neck cancer. Br J Radiol.
84:58–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Leinberry CF and Wehbé MA: Brachial plexus
anatomy. Hand Clin. 20:1–5. 2004. View Article : Google Scholar
|
|
12.
|
Yi SK, Hall WM, Mathai M, Dublin AB, Gupta
V, Purdy JA and Chen AM: Validating the RTOG-endorsed brachial
plexus contouring atlas: an evaluation of reproducibility among
patients treated by intensity-modulated radiotherapy for
head-and-neck cancer. Int J Radiat Oncol Biol Phys. 82:1060–1064.
2011.
|
|
13.
|
Truong MT, Nadgir RN, Hirsch AE,
Subramaniam RM, Wang JW, Wu R, Khandekar M, Nawaz AO and Sakai O:
Brachial plexus contouring with CT and MR imaging in radiation
therapy planning for head and neck cancer. Radiographics.
30:1095–1103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Millender LE, Bucci MK, Quivey JM, Chin CT
and Xia P: Evaluation of dose to the brachial plexus using
intensity-modulated radiation therapy for treatment of head and
neck cancer. Int J Radiat Oncol Biol Phys. 60(Suppl 1): S5052004.
View Article : Google Scholar
|