Contents
Introduction
Why response-modified radiation treatment?
How do radiation-induced responses modify
radiotherapy?
Perspective on response-modified radiotherapy
Conclusion
Introduction
Radiation oncology has undergone 100 years of
development and has now entered the ‘precision radiotherapy’ era.
New radiotherapy modalities, such as intensity modulated radiation
therapy (IMRT), image guided radiotherapy (IGRT), volumetric
modulated arc therapy (VMAT), biologically guided radiation therapy
(BGRT), adaptive radiation therapy (ART) and hadron radiotherapy
are emerging, each with unique characteristics (1). However, a discrepancy between supply
and demand still exists; the current outcomes of radiation therapy
are still far from the high demand of cancer patients for therapy
efficacy and quality of life. Great advancements in radiation
biology (2), radiation physics
(3) and imaging technology
(4) are bringing about new
opportunities to further improve the outcomes of radiation
treatment. In recent years, radiation-induced reponses of tumor and
normal tissues have increasingly been used as feedback to modify
radiotherapy in order to get the highest therapeutic gain. In this
review, we briefly discuss how and why this new treatment strategy
has come into being, as well as its current status and
characteristics. Also, the future developments of this treatment
modality are discussed.
Why response-modified radiation
treatment?
Demand for individualized radiotherapy as
a driving force
Accurate delivery of the ionizing radiation dose has
greatly improved over the past 2–3 decades, allowing more precise
deposition of therapeutic agents to the tumor while progressively
reducing any unwanted dose to surrounding normal tissues. Such
techniques allow the dose to the tumor to be increased to levels
that would be unachievable without precise targeting (5,6).
With the improvement of tumor control and survival, the
requirements of improving quality of life have also increased.
However, the challenge is that not only do the same types of tumor
tissue from different patients show differences in sensitivity to
radiation, but the sensitivity of tumor tissues of the same patient
during radiotherapy also shows dynamic changes. All of these
disparities in radiation sensitivity necessitate the use of
different radiation doses. Thus, to achieve the greatest efficacy
with minimal side effects, individualized response-guided radiation
therapy is required (7).
Advances in understanding radiobiological
responses have made ‘response-modified radiation therapy’
possible
Biology is a fast-growing branch of science. With
the development of molecular biotechnologies, we gain deeper
understanding of biological phenomena and mechanisms. This has led
to advances in radiation biology, such as radiation-induced early
responses, cell proliferation, hypoxia and inherent
radiosensitivity, as well as screening of a series of specific
molecular markers. To date, ATM, ADR, γ-H2AX, MDM2 and Bcl/Bax have
been verified in preliminary clinical trials (8). We currently have a fairly
comprehensive understanding of the biological responses of
radiotherapy-induced molecules, cells, tissues and systems at all
levels. All these data on radiobiological responses may be used to
modify personalized radiotherapy.
Progression in radiation physics and
bio-imaging technology provides technical support for response
modified radiation treatment
Newly emerging biological/physiological imaging
techniques, such as functional imaging, molecular imaging and
metabolic imaging, differ from traditional anatomical imaging. The
processes of physiology, biochemistry, metabolism and signal
transduction of cells may be visualized through bio-imaging
technology to generate real-time, dynamic biological information of
tumor and normal tissues in vivo (9,10).
Similarly, radiation physics has experienced significant
development in recent years. A number of new localization and
fixing techniques, dose calculation algorithms and treatment
concepts make radiotherapy even more precise. With deeper
understanding of the concepts of factors such as the biologically
effective dose (BED), equivalent uniform dose (EUD), α/β, tumor
control probability (TCP) and normal tissue complication
probability (NTCP), biological responses could be translated into a
means for radiation physics optimization (11–13).
To conclude, there is an urgent clinical need for
this new radiation model, and its feasibility is based on growing
knowledge regarding radiation responses and advances in modern
radiation physics and imaging technology. Therefore, we believe
that response modified radiotherapy will become the mainstream mode
of radiation therapy.
How do radiation-induced responses modify
radiotherapy?
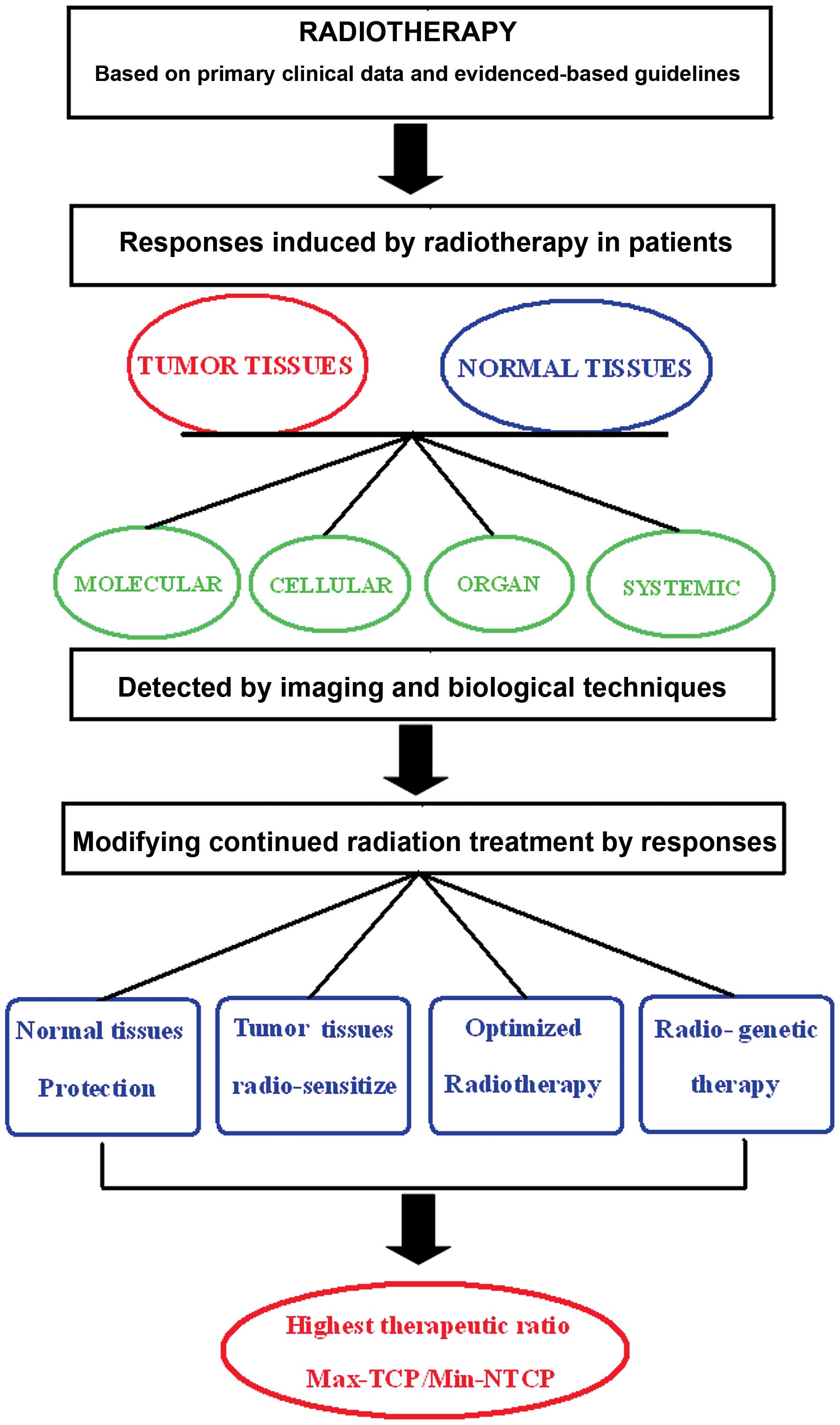
The modality of response modified radiotherapy may
be described as follows. Based on the biological responses to
radiation of tumor and normal human tissues, the optimization of
radiation treatment planning and modification of tissue sensitivity
are carried out dynamically by adjusting to changes in responses to
radiation, which will lead to the best therapeutic ratio (Fig. 1). Specifically, radiobiological
responses include: i) molecular reactions such as DSB (14–18),
ATM (19,20), ATR (21), NBS1 (22), BRCA1 (23), DNA-PK (24,25),
HIF-1a (26,27), γ-H2AX (28,29),
as well as the early response molecules such as Egr-1 (30) and c-fos (31); ii) cellular responses such as
apoptosis (32), autophagy
(16,33–35),
cell proliferation rate (36) and
changes in cell cycle (37–43);
iii) tissue and organ level responses, including volume changes
(44), inflammation, edema and
fibrosis (8,45,46);
and iv) the overall level of responses, including changes in
expression of cytokines such as IL-1 (47), IL-6 (48), TNFα (49) and TGFβ (45). Radiation responses should be
considered on multiple, comprehensive levels in the tumor and
normal tissue, as well as in the early stage of acute response and
the late stage tissue responses. Based on the above indicators of
radiation response, the optimization of radiation therapy includes
(Table I) i) the optimization of
the radiation treatment planning, including the dose-painting
techniques on regions with different sensitivity in the same target
volume and the dose-fractionation model and ii) the tumor tissue
radiosensitizer (37) and
radiation protection of the normal tissue (50). For example, based on the close
relationship between TGFβ and pulmonary fibrosis, if high levels of
TGFβ in peripheral blood of patients undergoing chest radiotherapy
are detected, appropriate measures should be taken to block
radiation-induced pulmonary fibrosis. Thus, radiotherapy may be
modified along the entire process.
 | Table I.Responses of tumor and normal tissues
in patients treated by radiotherapy and techniques to modify the
radiotherapy. |
Table I.
Responses of tumor and normal tissues
in patients treated by radiotherapy and techniques to modify the
radiotherapy.
| Level | Response | Detection
methods | How to modify
radiotherapy |
|---|
| Molecular | DSB, ATM, ATR,
NBS1, BRCA1, DNA-PK, HIF-1a, γ-H2AX, Egr-1, c-fos | Molecular imaging,
molecular biological methods | Enhance
radiosensitivity, radio-genetic therapy |
| Cellular | Apoptosis,
autophagy, cell cycle arrest, proliferation rate | Biological
imaging
Functional MRI | Biological target
definition, dose painting, optimal fractionation |
| Organ | Volume changes,
inflammation, fibrosis | CT, Functional
MRI | Target
modification, normal tissue protection |
| Systemic | IL-1, IL-6, FGF2,
TNFα, TGFβ | Biological assays,
biochemistry assays | Normal tissue
protection, NTCP modelling |
Response-modified radiotherapy is not only a new
modality of treatment, but also a novel radiotherapy philosophy. A
variety of optimization methods can be inserted at specific stages
of radiotherapy. Every fractionation in the whole process of
radiotherapy should be unique and should be dynamically optimized
according to specific tumor and normal tissue responses.
Response-modified radiotherapy is a new treatment
modality developed from adaptive radiation therapy (ART) (51,52),
however, it places more emphasis on the comprehensive information
from multiple stages and across disciplines, such as molecular
biology, physiology and biochemistry. Thus, in addition to the
features of ART in terms of physics, it also has the following
characteristics:
Information integration. This model makes full use
of multiple levels of information from the human body, that is,
different levels of response caused by radiation. The main
biological characteristics of tumor and normal tissue, including
physiological and biochemical features, are obtained noninvasively
from the whole body and the local site, at static and dynamic
levels. Biological and physical approaches are then used to
translate this information into radiotherapy optimizing
strategies.
Evidence-based modification. Generally speaking, the
use of molecular markers that ‘predict’ the radiosensitivity of the
tumor is hard to achieve with the desired sensitivity and
specificity. Response-modified radiotherapy avoids this problem; it
only considers the final outcomes directly caused by radiation
therapy, regardless of its mechanisms and biological process. For
the treatment modality of response-modified radiotherapy,
radiotherapy is optimized based on the integrated information of
the final actual outcomes of tumor and normal tissues caused by
radiation. For instance, cell apoptosis in tumors is detected using
molecular imaging after a fraction of radiotherapy, then the
acquired apoptosis information is used to modify further treatment
planning.
Technology integration. It is worth noting that the
biggest advantage of the modality is that its optimization
integrates all aspects of treatment throughout the course of
radiotherapy, which include radiotherapy planning optimization, the
implementation of radiotherapy quality assurance, sensitivity
modification of tumor and surrounding normal tissues to radiation,
the use of various physical and biological measures.
This radiotherapy model combines a variety of
radiation responses in all the normal tissues and tumor tissues and
truly achieves individualized technologies. At the same time, it
maximizes the use of the most systematic and comprehensive
optimization tools, which bring the greatest benefits to patients
with minimal side effects and maximal efficacy.
In recent years, the response-guided radiotherapy
modality has gradually been incorporated into daily radiotherapy
practice. For instance, for nasopharyngeal carcinoma treatment, in
the course of radiotherapy, we dynamically observe the changes in
tumor volume to re-delineate the target volume and modify the
radiation plan to maximize the protection of at-risk organs without
missing the tumor target. Following years of extensive work and
systematic research, our centre has reviewed the alterations of
nasopharyngeal carcinoma volume in radiation treatment and used
this knowledge as a basis to explore the best timeline for
replanning. Our results revealed that the revised target volume had
100% coverage, doses on the normal tissue were reduced by 15%; side
effects was reduced by 40%, with a local control rate of 92% and
5-year survival rate of 87% (53).
Similarly, this treatment model has also been implemented at
certain other radiation oncology centres. Wang et al
investigated the target volume and dose distribution changes during
nasopharyngeal carcinoma radiotherapy. CT scans were performed
following 18 fractionations of radiotherapy, and doses and target
size of the former and new plans were compared. The results
revealed that the bilateral parotid gland volume had reduced by 6
cc, and the new plan decreased the parotid gland dose by 2.57–2.97
Gy; similar dose changes were also achieved for other at-risk
organs. When compared with the former plan, the new plan decreased
the dose in the brain stem from 6.51 Gy to 0.08 Gy and the dose in
the spinal cord from 7.8 Gy to 0.05 Gy. Accordingly, they concluded
that in the case of nasopharyngeal carcinoma radiotherapy,
replanning based on the changes in tumor size may better protect
at-risk organs such as the parotid gland, spinal cord and brain
stem (54). Wang et al
reported that in 28 cases of replanning of nasopharyngeal carcinoma
IMRT, the dose in high-risk targets increased by 4.9–10.8%, the
maximum dose in the spinal cord decreased by 5–9.23 Gy and the
average dose in the parotid gland decreased by 4.23–10.03 Gy with
replanning following 25 fractionations of radiotherapy followed by
CT scan (55).
Hansen et al analyzed the replanned target
regions and dose changes on the organs at-risk of 13 patients with
head and neck cancer by IMRT. The results suggest that replanning
could increase the dose for lesions and high-risk clinical volume
by 0.8–6.3 Gy dose and 0.2–7.4 Gy, respectively, while decreasing
the doses in the spinal cord and brain stem by 0.2–15.4 Gy and
0.6–8.1 Gy, respectively (56).
Mechalakos et al used weekly CBCT to observe the effect of
volume reduction in a neck mass on the dose to the spinal cord in a
case of recurrent nasopharyngeal carcinoma. The results
demonstrated that volume reduction of the lesion had little impact
on the dose distribution in the spinal cord (57). Zhao et al reported that with
replanning following 15 fractionations of radiotherapy in 33
patients with nasopharyngeal carcinoma, the 3-year disease-free
survival rate was 72.71%, which was higher than that when a single
plan was used over the entire course (68.16%) (P<0.05). In
particular, the advantage was most pronounced in patients with
local advanced disease (58).
Zhang and Li studied the effects of radiotherapy replanning in
non-small-cell lung cancer radiotherapy. The preliminary results
indicated that multiple-planning radiation treatment not only
greatly increased the dose for the lesions but also maximized the
protection of the healthy lung tissue (59).
The above replanning treatments of nasopharyngeal
carcinoma and lung cancer essentially used the radiation responses
of tumor to modify the radiotherapy. The changes in tumor size
during radiotherapy is the main response to radiation. Using this
response to re-delineate the tumor target, revise the radiation
treatment planning and modify the IMRT of nasopharyngeal carcinoma
reflects the core spirit of response-modified radiotherapy. It may
be inferred that further advancements in molecular imaging
technology would result in biological responses increasingly being
used to guide radiotherapy optimization.
Another example of response-modified radiotherapy in
practice is that we usually use different doses and fractionations
in several lesions of the same patient, observe the changes in
lesions and normal tissue response following several fractionations
of radiotherapy to identify the best therapeutic dose and then
apply this fractionation model in the next step of the treatment.
According to our preliminary statistical results, this treatment
model not only greatly increases the tumor control rate but also
significantly reduces the side effects of radiotherapy and achieves
the maximum benefit to patients. These results once again
demonstrate the scientific rationale of response-modified
radiotherapy.
Perspective on response-modified
radiotherapy
This new radiotherapy modality considers the
individual differences in radiation-induced biological responses
and feeds this difference back to the radiation treatment planning
and implementation process through repeated revisions to achieve
tailor-made radiotherapy. Given the complexity of biological
phenomena, it is difficult to find ideal, consistent predictive
factors. Radiotherapy response involves complex molecular networks,
therefore a single or a small number of molecular markers are
hard-pressed to anticipate the actual results of radiotherapy
(60–62). However, response-modified
radiotherapy is based on the actual responses from the tumor and
normal tissue and uses this information to amend and optimize
radiotherapy planning. Sidestepping the complexities of biological
mechanisms of radiation and using actual response feedback to guide
radiation therapy is an efficient and pragmatic way of working and
eliminates the need for detailed considerations of complex
intermediate mechanisms in treatment.
This real-time response guided radiation therapy
approach not only delivers the most effective radiation doses to
tumors but also takes into account the dose tolerance of normal
tissues. The current standard normal tissue tolerance doses are
empirical, rather than the actual tolerance levels of the
individual tissues. Perhaps a specific patient is able to tolerate
a higher dose than the current standard, and thus we are able to
safely increase the therapeutic dose without serious side effects.
This radiotherapy model, taking the individual tumor and normal
tissue response into account, thus guarantees the most effective
dose of radiation to the tumor and achieves the ultimate goal of
safety.
Although certain cancer centers have begun to
explore response-modified radiotherapy and have achieved
encouraging results in cancer treatment, much research is required
before we are able to achieve true personalized treatment in
clinical practice. As such, a number of important issues still need
to be addressed:
Identification of a series of reliable
molecular, cellular and systemic markers for radiation
responses
This would not only reliably represent the control
rate of the tumor and clinical efficacy, but also reflect the
sensitivity and dose tolerance of normal tissue. Although a
considerable number of molecules have been studied, specific and
sensitive markers are rare.
Establishment of a set of technologies
for detecting radiation response
To carry out this modality, we must acquire
non-invasive, real-time and dynamic information regarding in
vivo radiobiological responses in tumor and normal tissues.
Existing molecular imaging techniques, such as PET, SPECT and fMRI,
yet cannot fully satisfy the actual needs of response-modified
radiotherapy (63,64).
Exploration of how to integrate the
information of radiation responses into radiotherapy
optimization
Taking full advantage of the available physical and
mathematical tools, it also require interdisciplinary work to
integrate a variety of radiation responses into the radiation
therapy feedback process.
Conclusion
Modern radiotherapy requires advanced equipment and
a reasonable treatment strategy to gain the best clinical outcome.
Making full use of biological information to generate reliable
radiotherapy models needs to be addressed in the near future.
Response-modified radiotherapy perhaps may not be the most optimal
radiotherapy modality, nevertheless, it will shed new light on the
way to personalized radiotherapy.
References
|
1.
|
Jaffray D, Kupelian P, Djemil T and
Macklis RM: Review of image-guided radiation therapy. Expert Rev
Anticancer Ther. 7:89–103. 2007. View Article : Google Scholar
|
|
2.
|
Eke I and Cordes N: Radiobiology goes 3D:
how ECM and cell morphology impact on cell survival after
irradiation. Radiother Oncol. 99:271–278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Bortfeld T and Jeraj R: The physical basis
and future of radiation therapy. Br J Radiol. 84:485–498. 2011.
View Article : Google Scholar
|
|
4.
|
Hunter KU and Eisbruch A: Advances in
imaging: target delineation. Cancer J. 17:151–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Begg AC, Stewart FA and Vens C: Strategies
to improve radiotherapy with targeted drugs. Nat Rev Cancer.
11:239–253. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Bhide SA and Nutting CM: Recent advances
in radiotherapy. BMC Med. 8:252010. View Article : Google Scholar
|
|
7.
|
Chen GT, Sharp GC and Mori S: A review of
image-guided radiotherapy. Radiol Phys Technol. 2:1–12. 2009.
View Article : Google Scholar
|
|
8.
|
Okunieff P, Chen Y, Maguire DJ and Huser
AK: Molecular markers of radiation-related normal tissue toxicity.
Cancer Metastasis Rev. 27:363–374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Galbán S, Brisset JC, Rehemtulla A,
Chenevert TL, Ross BD and Galbán CJ: Diffusion-weighted MRI for
assessment of early cancer treatment response. Curr Pharm
Biotechnol. 11:701–708. 2010.PubMed/NCBI
|
|
10.
|
Thoeny HC and Ross BD: Predicting and
monitoring cancer treatment response with diffusion-weighted MRI. J
Magn Reson Imaging. 32:2–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Verellen D, Depuydt T, Gevaert T, Linthout
N, Tournel K, Duchateau M, Reynders T, Storme G and De Ridder M:
Gating and tracking, 4D in thoracic tumours. Cancer Radiother.
14:446–454. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Zaider M and Hanin L: Tumor control
probability in radiation treatment. Med Phys. 38:574–583. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Bentzen SM and Gregoire V: Molecular
imaging-based dose painting: a novel paradigm for radiation therapy
prescription. Semin Radiat Oncol. 21:101–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Mladenov E and Iliakis G: Induction and
repair of DNA double strand breaks: The increasing spectrum of
non-homologous end joining pathways. Mutat Res. 711:61–72. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Bekker-Jensen S and Mailand N: Assembly
and function of DNA double-strand break repair foci in mammalian
cells. DNA Repair. 9:1219–1228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Thoms J and Bristow RG: DNA repair
targeting and radiotherapy: a focus on the therapeutic ratio. Semin
Radiat Oncol. 20:217–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Orlowski C, Mah LJ, Vasireddy RS, El-Osta
A and Karagiannis TC: Double-strand breaks and the concept of
short-and long-term epigenetic memory. Chromosoma. 120:129–149.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Eccles LJ, O’Neill P and Lomax ME: Delayed
repair of radiation induced clustered DNA damage: friend or foe?
Mutat Res. 711:134–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Smith J, Tho LM, Xu N and Gillespie DA:
The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and
cancer. Adv Cancer Res. 108:73–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Olcina M, Lecane PS and Hammond EM:
Targeting hypoxic cells through the DNA damage response. Clin
Cancer Res. 16:5624–5629. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Dobbs TA, Tainer JA and Lees-Miller SP: A
structural model for regulation of NHEJ by DNA-PKcs
autophosphorylation. DNA Repair. 9:1307–1314. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Sun Y, Jiang X and Price BD: Tip60:
connecting chromatin to DNA damage signaling. Cell Cycle. 99:30–36.
2010.PubMed/NCBI
|
|
23.
|
Xu Y and Price BD: Chromatin dynamics and
the repair of DNA double strand breaks. Cell Cycle. 10:261–267.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Tomita M: Involvement of DNA-PK and ATM in
radiation- and heat-induced DNA damage recognition and apoptotic
cell death. J Radiat Res (Tokyo). 51:493–501. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Pawelczak KS, Bennett SM and Turchi JJ:
Coordination of DNA-PK activation and nuclease processing of DNA
termini in NHEJ. Antioxid Redox Signal. 14:2531–2543. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Bischoff P, Altmeyer A and Dumont F:
Radiosensitising agents for the radiotherapy of cancer: advances in
traditional and hypoxia targeted radiosensitisers. Expert Opin Ther
Pat. 19:643–662. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Bussink J, Kaanders JH and van der Kogel
AJ: Tumor hypoxia at the micro-regional level: clinical relevance
and predictive value of exogenous and endogenous hypoxic cell
markers. Radiother Oncol. 67:3–15. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Rothkamm K and Horn S: gamma-H2AX as
protein biomarker for radiation exposure. Ann Ist Super Sanita.
45:265–271. 2009.PubMed/NCBI
|
|
29.
|
Sak A and Stuschke M: Use of γH2AX and
other biomarkers of double-strand breaks during radiotherapy. Semin
Radiat Oncol. 20:223–231. 2010.
|
|
30.
|
Weichselbaum RR and Kufe D: Translation of
the radio- and chemo-inducible TNFerade vector to the treatment of
human cancers. Cancer Gene Ther. 16:609–619. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Wyrobek AJ, Manohar CF, Krishnan VV,
Nelson DO, Furtado MR, Bhattacharya MS, Marchetti F and Coleman MA:
Low dose radiation response curves, networks and pathways in human
lymphoblastoid cells exposed from 1 to 10 cGy of acute gamma
radiation. Mutat Res. 722:119–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Blankenberg FG and Norfray JF:
Multimodality molecular imaging of apoptosis in oncology. Am J
Roentgenol. 197:308–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Rodriguez-Rocha H, Garcia-Garcia A,
Panayiotidis MI and Franco R: DNA damage and autophagy. Mutat Res.
711:158–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Chen S, Rehman SK, Zhang W, Wen A, Yao L
and Zhang J: Autophagy is a therapeutic target in anticancer drug
resistance. Biochim Biophys Acta. 1806:220–229. 2010.PubMed/NCBI
|
|
35.
|
Yoon JH, Ahn SG, Lee H, Jung SH and Oh SH:
Role of autophagy in chemoresistance: regulation of the
ATM-mediated DNA-damage signaling pathway through activation of
DNA-PKcs and PARP-1. Biochem Pharmacol. 83:747–757. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Hennequin C, Quero L and Favaudon V:
Determinants and predictive factors of tumour radiosensitivity.
Cancer Radiother. 12:3–13. 2008.
|
|
37.
|
Gravina GL, Festuccia C, Marampon F, Popov
VM, Pestell RG, Zani BM and Tombolini V: Biological rationale for
the use of DNA methyltransferase inhibitors as new strategy for
modulation of tumor response to chemotherapy and radiation. Mol
Cancer. 9:3052010. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Pauwels B, Wouters A, Peeters M, Vermorken
JB and Lardon F: Role of cell cycle perturbations in the
combination therapy of chemotherapeutic agents and radiation.
Future Oncol. 6:1485–1496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Takekawa M, Kubota Y, Nakamura T and
Ichikawa K: Regulation of stress-activated MAP kinase pathways
during cell fate decisions. Nagoya J Med Sci. 73:1–14.
2011.PubMed/NCBI
|
|
40.
|
Kim J, Meyer JL and Dawson LA: Image
guidance and the new practice of radiotherapy: what to know and use
from a decade of investigation. Front Radiat Ther Oncol.
43:196–216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Vral A, Fenech M and Thierens H: The
micronucleus assay as a biological dosimeter of in vivo ionising
radiation exposure. Mutagenesis. 26:11–17. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Parliament MB and Murray D: Single
nucleotide polymorphisms of DNA repair genes as predictors of
radioresponse. Semin Radiat Oncol. 20:232–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Baskar R: Emerging role of radiation
induced bystander effects: Cell communications and carcinogenesis.
Genome Integr. 1:132010. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Cao Y: The promise of dynamic
contrast-enhanced imaging in radiation therapy. Semin Radiat Oncol.
21:147–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Yarnold J and Brotons MC: Pathogenetic
mechanisms in radiation fibrosis. Radiother Oncol. 97:149–161.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Heneweer C and Grimm J: Clinical
applications in molecular imaging. Pediatr Radiol. 41:199–207.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Müller K and Meineke V: Radiation-induced
alterations in cytokine production by skin cells. Exp Hematol.
35(Suppl 1): 96–104. 2007.PubMed/NCBI
|
|
48.
|
Kong FM, Ao X, Wang L and Lawrence TS: The
use of blood biomarkers to predict radiation lung toxicity: a
potential strategy to individualize thoracic radiation therapy.
Cancer Control. 15:140–150. 2008.PubMed/NCBI
|
|
49.
|
Deorukhkar A and Krishnan S: Targeting
inflammatory pathways for tumor radiosensitization. Biochem
Pharmacol. 80:1904–1914. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Fritz G, Henninger C and Huelsenbeck J:
Potential use of HMG-CoA reductase inhibitors (statins) as
radioprotective agents. Br Med Bull. 97:17–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Bussink J, van Herpen CM, Kaanders JH and
Oyen WJ: PET-CT for response assessment and treatment adaptation in
head and neck cancer. Lancet Oncol. 11:661–669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Castadot P, Lee JA, Geets X and Grégoire
V: Adaptive radiotherapy of head and neck cancer. Semin Radiat
Oncol. 20:84–93. 2010. View Article : Google Scholar
|
|
53.
|
Peng Q, Li J, Feng M, Xiao MY and Lang JY:
A study of replanning during the course of IMRT for nasopharyngeal
carcinoma. J Cancer Control Treat. 24:45–47. 2011.(In Chinese).
|
|
54.
|
Wang X, Lu JD, Xu XP, Zhu GP, Ying HM, He
SQ, Hu WG and Hu CS: Anatomic and dosimetric changes during the
treatment course of intensity-modulated radiotherapy for locally
advanced nasopharyngeal carcinoma. Med Dosim. 35:151–157. 2010.
View Article : Google Scholar
|
|
55.
|
Wang W, Yang H, Hu W, Shan G, Ding W, Yu
C, Wang B, Wang X and Xu Q: Clinical study of the necessity of
replanning before the 25th fraction during the course of
intensity-modulated radiotherapy for patients with nasopharyngeal
carcinoma. Int J Radiat Oncol Biol Phys. 77:617–621. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Hansen KE, Bucci MK, Quivey JM, Weinberg V
and Xia P: Repeat CT imaging and replanning during the course of
IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys.
64:355–362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Mechalakos J, Lee N, Hunt M, Ling CC and
Amols HI: The effect of significant tumor reduction on the dose
distribution in intensity modulated radiation therapy for head and
neck cancer: a case study. Med Dosim. 34:250–255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Zhao L, Wan Q, Zhou Y, Deng X, Xie C and
Wu S: The role of replanning in fractionated intensity modulated
radiotherapy for nasopharyngeal carcinoma. Radiother Oncol.
98:23–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Zhang Y and Li J: A study on necessity of
radiotherapy replanning for non-small cell lung cancer. Int J
Radiat Oncol Biol Phys. 78:S541–S542. 2010. View Article : Google Scholar
|
|
60.
|
Søvik A, Malinen E and Olsen DR:
Strategies for biologic image-guided dose escalation: a review. Int
J Radiat Oncol Biol Phys. 73:650–658. 2009.PubMed/NCBI
|
|
61.
|
Purdy JA: Dose to normal tissues outside
the radiation therapy patient’s treated volume: a review of
different radiation therapy techniques. Health Phys. 95:666–676.
2008.
|
|
62.
|
Guha C, Alfieri A, Blaufox MD and Kalnicki
S: Tumor biology-guided radiotherapy treatment planning: gross
tumor volume versus functional tumor volume. Semin Nucl Med.
38:105–113. 2008.PubMed/NCBI
|
|
63.
|
Thorwarth D and Schaefer A: Functional
target volume delineation for radiation therapy on the basis of
positron emission tomography and the correlation with
histopathology. Q J Nucl Med Mol Imaging. 54:490–499.
2010.PubMed/NCBI
|
|
64.
|
Thorwarth D, Geets X and Paiusco M:
Physical radiotherapy treatment planning based on functional PET/CT
data. Radiother Oncol. 96:317–324. 2010. View Article : Google Scholar : PubMed/NCBI
|