Introduction
Breast cancer (BC) is the most common malignancy
(28% of all cancers in females) and the second most common cause of
cancer mortality in women (15% of female cancer mortality) in the
United States (1). The incidence
of BC is also increasing in Taiwan. The number of new cases per
100,000 individuals increased from 34.3 in 1994 to 53.1 in 2007.
The median age at diagnosis was 51 years, and stage IV disease
accounted for approximately 1-5% of new cases. Although there are
numerous adjuvant therapies for early stage BC, the recurrence rate
remains 20–30%. Treatments for BC rely on the availability of
robust clinical and pathological prognostic and predictive factors
to guide decision making and the selection of treatment options.
Pathological characteristics, including the size of the primary
tumor, regional lymph node (LN) metastasis, estrogen receptor (ER),
progesterone receptor (PR), Her-2 expression, lymphovascular
invasion (LVI) and Ki-67 expression, are all established prognostic
markers. In addition to histological prognostic factors, there are
increasing numbers of molecular classification systems for BC,
including the 21-gene RT-PCR assay (Oncotype DX), which can also be
used to predict the benefits of adjuvant chemotherapy (2).
Angiogenesis, the formation of new vessels, is an
essential process in the progression of malignant tumors since
solid tumors cannot grow beyond 1-2 mm in diameter without
angiogenesis (3). As in other
malignancies, angiogenesis is also critical for the growth,
invasion and metastasis of BC. In BC, extensive neovascularization
and tumor thrombus in vessels have been reported to be signs of
poor prognosis (4). Also, the
tumor microvasculature may constitute a target for anti-angiogenic
therapy. Clinical studies have demonstrated that anti-angiogenic
agents such as bevacizumab are able to improve the progression-free
survival (PFS) of patients with advanced BC when combined with
chemotherapy. However, to date there are few predictive markers for
anti-angiogenic agents.
Microvascular density (MVD) has become the
morphological gold-standard for assessing the neovascularization in
human tumors. Several studies have shown that the angiogenic
potential of BC as assessed by MVD correlates with progression and
metastasis; it therefore predicts clinical outcome (5). The evaluation of MVD requires the use
of specific markers for the vascular endothelium and
immunohistochemical (IHC) procedures to visualize microvessels
(6). Traditionally, histological
assessments of MVD in tumors have used pan-endothelial markers,
including CD31, CD34 and von Willebrand factor (vWF) (7–9).
Although CD34 has been reported to be more sensitive than CD31 or
vWF for MVD in BC (10), these
markers are specific for all endothelia and do not target only the
vascular endothelium from tumor-induced neovascularization. In
non-small-cell lung cancer, CD105 (endoglin) has been shown to be
superior to CD34 and CD31 in the evaluation of neovascularization
since it has a greater affinity for the endothelial cells in
tumor-related angiogenic tissue, whereas CD34 and CD31 react
nonspecifically with normal and pathological vessels (11). In solid tumors, CD105 has been
revealed to be upregulated in the endothelial cells of peri- and
intra-tumoral blood vessels and in the stromal components of
several types of cancer (12).
Studies have shown that increased MVD, as assessed by a CD105
antibody, is associated with worse overall and disease-free
survival (DFS) for various types of cancer (13–15).
CD105 is expressed on the cell surface as a 180-kDa
homodimeric transmembrane protein. It is a co-receptor of TGF-β1
and TGF-β2. TGF-β has a complex role in carcino-genesis, as it has
tumor-suppressor and oncogenic activities. In the early stages of
epithelial tumorigenesis, TGF-β has strong anti-proliferative,
pro-apoptotic and tumor growth-inhibiting effects; however, it also
acts as a tumor-promoting factor by stimulating the
epithelial-to-mesenchymal transition and the invasiveness of cancer
cells, inhibiting immune surveillance and stimulating angiogenesis
by inducing vascular endothelial growth factor (VEGF) expression
(16,17). It is expressed almost exclusively
in the endothelial cells of peri- and intra-tumoral blood vessels
and in tumor stromal components. CD105 antagonizes the inhibitory
effects of TGF-β on proliferation and migration, thus promoting the
growth and migration of tumor cells (18).
The use of an antisense agent to inhibit CD105
protein translation in cultured human endothelial cells has been
reported to markedly enhance the ability of TGF-β1 to inhibit in
vitro angiogenesis, suggesting that CD105 is a pro-angiogenic
component in the endothelial cells (18). Monoclonal antibodies and their
immunoconjugates (immunotoxins and radioimmunoconjugates) against
CD105 have been reported to have anticancer effects (19).
In our current study, we analyzed the correlation
between CD105 expression and prognostic markers of BC. We sought to
determine whether CD105 has the potential to be a therapeutic
target for further anti-angiogenic therapies in the future.
Materials and methods
Breast cancer tissue
This study was approved by the Ethics Committee of
Kaohsiung Chang Gung Memorial Hospital. With permission from the
Institutional Review Board of Chang Gung Memorial Hospital, we
collected clinical data and pathological specimens from patients
with BC who were diagnosed between 2000 and 2002 and treated in our
hospital. Patients provided written consent for their tissue
samples to be used for research purposes. In addition, all data
were analyzed anonymously. Clinical data, including age at
diagnosis, clinical stage and pathological stage, date of
recurrence and mortality, and pathological features, including ER,
PR and Her-2 status, were obtained from a combination of clinical
and pathological record reviews and reports of external medical
records.
Tissue microarray
Tissue microarray (TMA) blocks were constructed
using the Manual Tissue Arrayer (MTA-1; Beecher Instruments, Sun
Prairie, WI, USA). Targets for arraying (areas with BC) were
identified by marking the areas on hematoxylin and eosin-stained
sections from each paraffin-embedded block. Three tissue cores with
a diameter of 2 mm were transferred from each donor block to the
recipient TMA block. Liver and skeletal muscle tissue was placed in
the first lane core of the three upper left and two lower left
cores of the TMA block to ensure correct orientation.
Immunohistochemical staining
TMA blocks constructed from formalin-fixed
paraffin-embedded human BC tissue were sectioned at 3-μm thickness
on adhesive-coated glass slides and dried overnight at 37°C. The
slides were deparaffinized in xylene and rehydrated through graded
alcohols to water. For antigen retrieval, the slides were heated in
10 mM citrate buffer (pH 6.0) for 17 min using a pressure cooker.
The slides were subsequently washed using TBS buffer with 0.1%
Tween 20 for this and subsequent washes. Endogenous peroxidase
activity was quenched by treatment with 3%
H2O2. After washing, the slides were
incubated with primary antibodies targeting CD105 (NCL-CD105;
Novocastra, Leica, Newcastle, UK) for 3 h at room temperature (RT),
ER (ER, clone SP1, #RM-9101, Neomarkers, Thermo Fisher Scientific,
Suwanee, GA, USA) for 1 h at RT, PR (PR, NCL-L-PGR-312/2;
Novocastra, Leica) for 1 h at RT and Her-2 (clone SP3, #RM-9103;
Neomarkers, Thermo Fisher Scientific) for 1 h at RT. Following the
incubation of the primary antibody and a wash step, the HRP polymer
(87-8963; Invitrogen, Grand Island, NY, USA) was added. The slides
were analyzed using the DAB substrate chromogen system K3468 (Dako,
Glostrup, Denmark). The TMA slides were counterstained with
hematoxylin and then coverslipped using Entellan® new
(Merck KGaA, Darmstadt, Germany) mounting medium. Incubation
without the primary antibody was used as a negative control.
The intensity of these markers was determined by two
independent pathologists and classified into low and high. A score
of low was given if fewer than 10% of the cells were stained either
in the nucleus or cytosol; if more than 10% of the cells were
stained, this was scored as high. The intensity of the protein
expression was tested for correlation with the tumor stage and the
prognosis of the disease. The Fisher's exact test was used to test
the hypothesis of independence between categorical variables.
Evaluation of MVD
The MVD was evaluated by the immunohistochemical
analysis of tumor vessels for CD105 expression in tissue
microarrays. Any immune-positive single cell or cluster of cells
clearly with lumen was considered to be an individual vessel, as
previously recommended (20).
Areas of fibrosis, necrosis and inflammation, as well as vessels
with a muscle wall, were excluded from the count. The sections were
scanned at a magnification level of x100 by two observers
simultaneously to select the most vascularized (hot-spots) of the
three tissue array spots for each patient. The microvessels in the
hot-spots were counted at a magnification level of x200, and their
density was expressed as the mean number of
microvessels/mm2. Mean values of CD105 staining were
calculated for each individual tumor and used for further
analysis.
Statistical analysis
Survival curves in relation to predictive markers
were illustrated by Kaplan-Meier survival plots and the difference
in time to progression was evaluated by the log-rank test.
Spearman's rho correlation was used to test the correlation between
the immunohistochemical findings and conventional clinical
features, including pathological stage and expression of markers.
P<0.05 was considered to indicate a statistically significant
result. Statistics software (SPSS, version 10.0; SPSS, Inc.,
Chicago, IL, USA) was used for all calculations.
The cumulative probability of DFS and overall
survival (OS) was estimated by the product-limit method. The
log-rank test was used to compare the homogeneity of DFS and
survival functions across strata defined by categories of
prognostic variables.
Results
Characteristics of the patients and
pathological findings
In total, 201 patients were enrolled into this
study. The median age at diagnosis was 66 years. The major
histological type was infiltrating ductal carcinoma. Approximately
one-half of the patients were diagnosed at stage II disease. ER was
positive in 132/201 (65.7%) patients, PR was positive in 117/201
(58.2%) patients and Her-2 was positive in only 31/201 (15.4%)
patients (Table I).
 | Table I.Patient and disease characteristics at
baseline (n=201). |
Table I.
Patient and disease characteristics at
baseline (n=201).
| Characteristics | No. of patients | % |
|---|
| Age at diagnosis,
years median (range) | 66 (24–84) | |
| Histology | | |
| Infiltrating ductal
carcinoma | 186 | 92.5 |
| Infiltrating
lobular carcinoma | 6 | 3.0 |
| Medullary
carcinoma | 5 | 2.5 |
| Other | 4 | 2.0 |
| Stage at
diagnosis | | |
| I | 34 | 16.9 |
| II | 106 | 52.7 |
| III | 52 | 25.9 |
| IV | 9 | 4.5 |
| Estrogen receptor
status | | |
| Positive | 132 | 65.7 |
| Negative | 63 | 31.3 |
| Unknown | 6 | 3.0 |
| Progesterone
receptor status | | |
| Positive | 117 | 58.2 |
| Negative | 66 | 32.8 |
| Unknown | 18 | 9.0 |
| Her-2 status | | |
| Negative | 145 | 72.1 |
| Positivea | 31 | 15.4 |
| Unknown | 25 | 12.4 |
|
Triple-negative | 29 | 14.4 |
Evaluation of MVD from the intensity of
CD105 staining
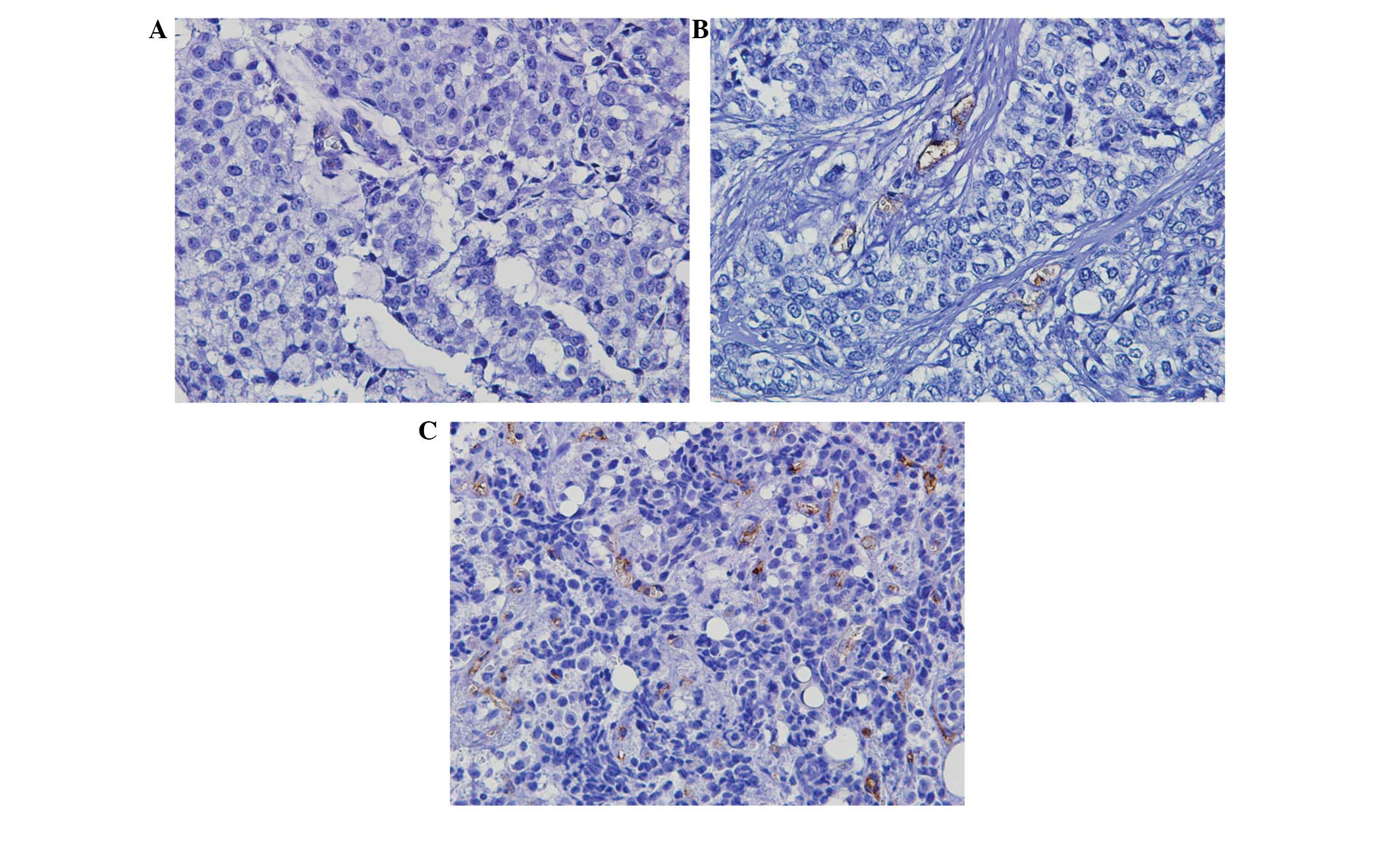
Positive CD105 was observed as thin, linear deposits
in the membranes and cytoplasm of the endothelial cells within
microvessels. Evaluation of MVD from the intensity of the CD105
staining revealed a median of 11 microvessels. The median
microvessel count of 11 was selected as the cut-off value to define
low and high groups (Fig. 1).
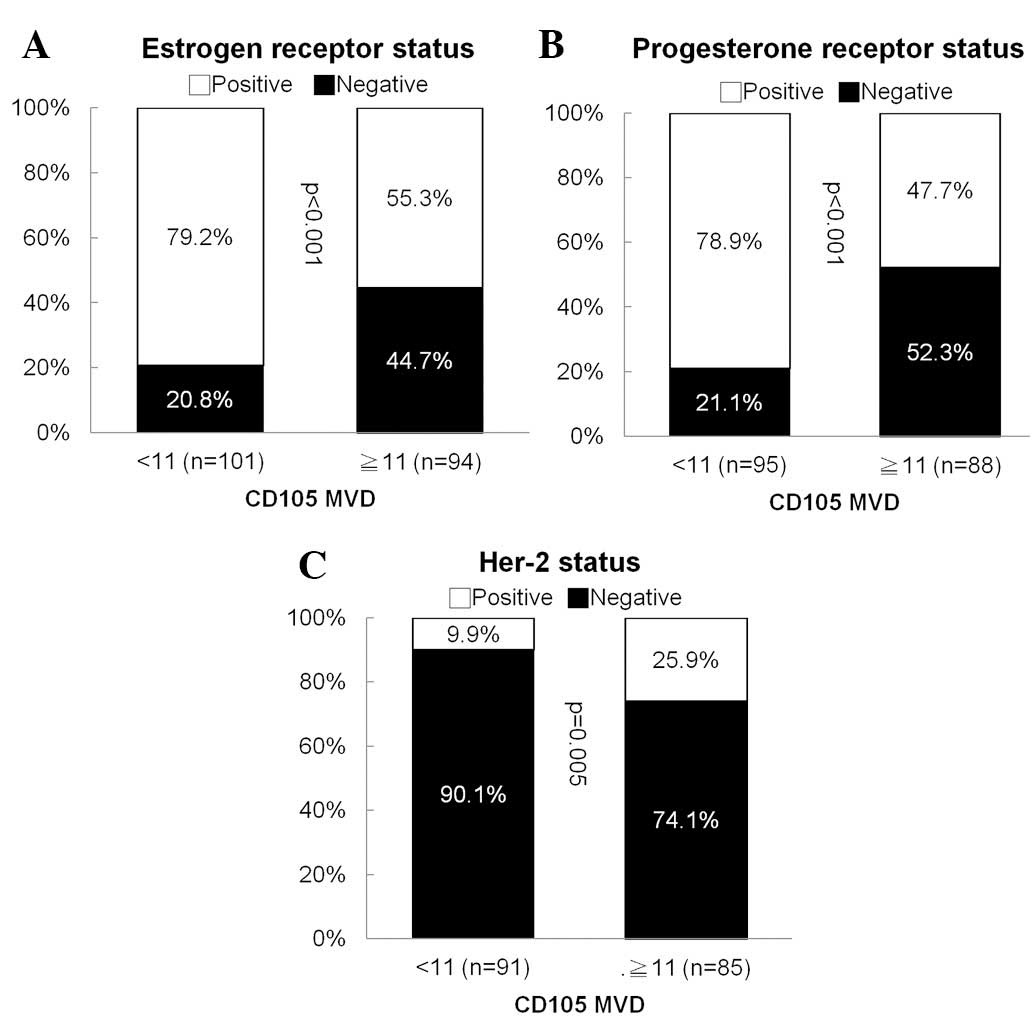
CD105 correlated inversely with hormone
receptor (HR) expression
In a bivariant correlation analysis between
clinical-pathological variables and CD105, no correlation was found
among age of diagnosis, stage and tumor grade. However, we found
that CD105 correlated inversely with ER (p<0.001) and PR
(p<0.001) expression. On the other hand, it correlated
positively with Her-2 (p=0.005) expression (Fig. 2). No correlation between CD105
expression and triple-negative BCs was identified.
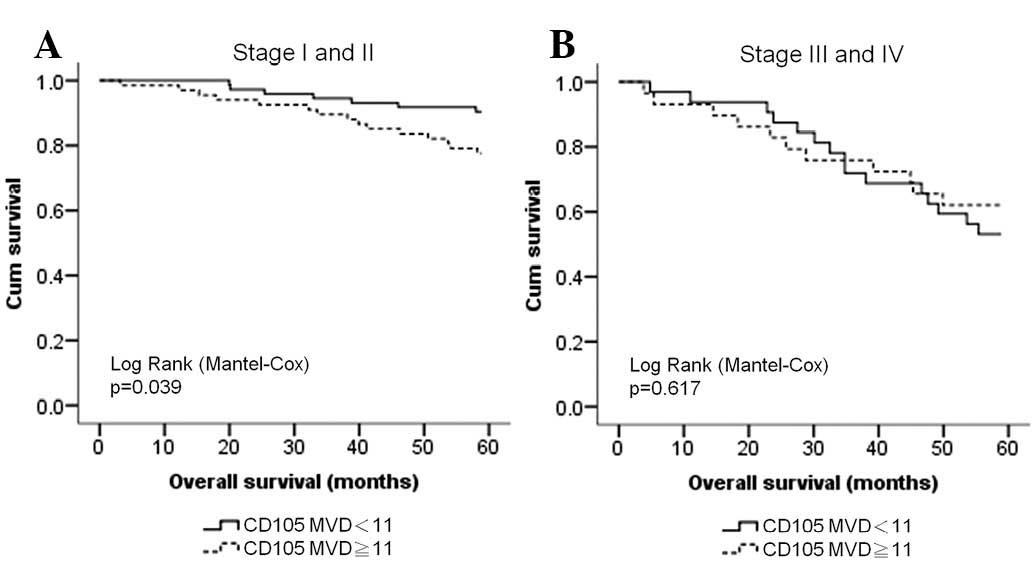
CD105 is a poor prognostic factor for
early stage BCs
Since angiogenesis is a predictive marker for cancer
prognosis, we also analyzed the correlation between CD105
expression and survival for different stages of BC. We found that
although the number of microvessels (cut-off, 11 microvessels) did
not correlate with the DFS of stage I to III BC patients, it had a
stronger correlation with OS in patients with early stage BC
(stages I and II) than in patients with late stage BC (stages III
and IV; Fig. 3).
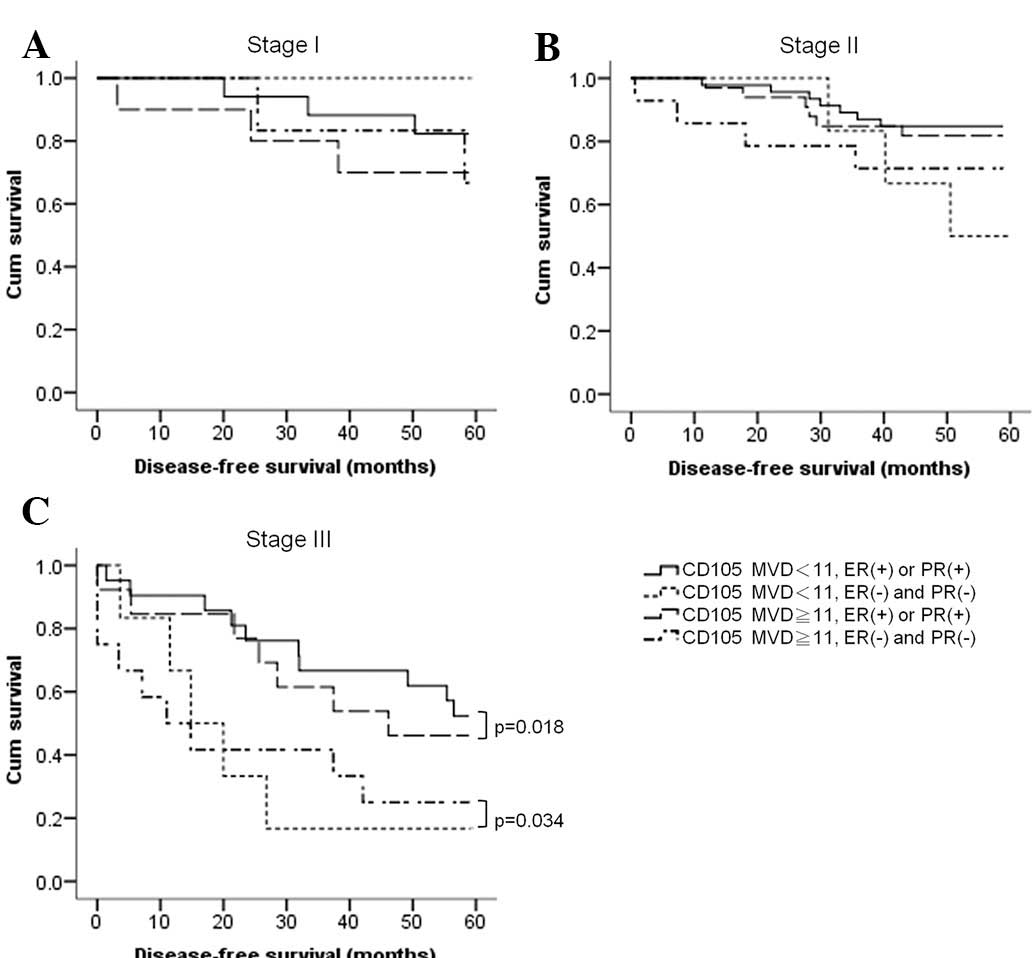
Hormone receptors correlate with the OS
of BC patients of all stages
We also analyzed a combination of CD105 expression
and the expression of HRs and Her-2, using a microvessel count of
11 as a cut-off point. We found that at the early stages (stages I
and II), both HRs and MVD influenced OS; we found no difference
between CD105 and HRs in the prediction of OS. However, at stage
III, only HRs had a predictive value for OS, either in patients
with tumors expressing lower levels of CD105 (p=0.018) or in
patients with tumors expressing higher levels of CD105 (p=0.034;
Fig. 4).
Discussion
Breast cancer is at present the most common cancer
in women in Taiwan and its incidence is growing. Although TNM
status is the first consideration when selecting treatment for BC,
in this era of high-throughput methods, there are numerous novel
biomarkers which have been reported for prognostic and predictive
purposes. Among these, the most commonly used bio markers are HRs
and Her-2, which categorize BCs into luminal A, luminal B, Her-2
type and basal-type classifications (21); such classifications are also
relevant in prognosis. The identification of new markers has led to
a more definitive insight into tumor biology and substantiates the
importance of existing biomarkers.
There are several drugs that target molecules in
various oncogenic pathways. These include trastuzumab and lapatinib
which target Her-2, RAD-001 which targets mTOR and bevacizumab
which targets VEGF. The development of anti-Her-2 agents has
improved the poor prognosis for Her-2-positive BC. In order to
maximize the therapeutic effects of the anti-Her-2 agents, only
Her-2-positive BCs are indicated for these drugs. In contrast,
anti-angiogenic agents have been reported to be more effective in
the treatment of triple-negative BC (22).
Angiogenesis is critical for the proliferation,
growth and metastasis of cancer cells. Therefore, by inhibiting
angiogenesis, these processes are also inhibited. Currently there
are several agents that target the neovascularization pathway, but
none of them has a predictive marker. Even elevated VEGF levels in
the circulations of cancer patients may not be a relevant biomarker
(23). Although several types of
malignancies are hypervascular (24,25),
it is not known how significant the angiogenesis pathway is to
these tumors. High MVD alone is not a predictive marker for
anti-angiogenic agents since anti-angiogenic agents target new
vessels in tumors only. As CD105 expression is more specific for
areas of neovascularization, CD105 has the potential to be a
predictive marker for anti-angiogenic agents.
HR-positive BCs usually have a more favorable
prognosis than HR-negative cases of BC. A primary reason for this
is the development of antihormonal agents which can prevent the
recurrence and delay the progression of HR-positive BC (26). This reverse correlation also
explains the favorable prognosis and slow progression of
HR-positive BC. However, the overexpression of Her-2 has been
reported to upregulate VEGF synthesis and thus may increase
angiogenesis and metastasis in BC (27). In the current study, we found that
the expression of CD105 correlated negatively with HR expression
but positively with Her-2 expression, which is compatible with the
behavior and prognostic significance of these markers.
Tumorigenesis comprises multiple steps. When a tumor
grows to more than 1 mm3 in size, angiogenesis becomes
critical for the tumor to grow and metastasize. However, when the
tumor grows beyond this size, the signal transduction pathways
become more complicated, with a greater number of overactive
pathways which are responsible for tumor growth, survival,
angiogenesis, metastasis and drug resistance (28). At an advanced stage,
neovascularization may not be the most crucial prognostic factor
since there are already several active oncogenic pathways. In
clinical practice and animal models, small tumors are more easily
controlled by anti-angiogenic agents than larger tumors, which also
implies that the role of angiogenesis is less significant when
tumors grow larger. In our study, we found that the prognostic
effect of MVD was even more considerable when tumors were at their
early stages, which might correlate with the aggressiveness of
primary tumors and primary resistance to adjuvant therapies.
The poor outcome for the patients with increased MVD
as measured by CD105 staining is in line with previously published
studies concerning BC and other malignancies (29). Higher MVD correlated with reduced
DFS and OS in BC patients, and decreased survival rates in patients
with increased CD105 and CD31 counts have also been reported
(30).
We report for the first time that MVD measured by
CD105 staining is significantly associated with Her-2 molecular
subtypes, but is inversely associated with HR expression. In
Her-2-overexpressing BC, hormone therapy is less effective than in
Her-2-negative BCs, which may be due to cross-talk between HRs and
the Her-2 pathway (31,32). Trastuzumab has been proved to be
effective on Her-2-overexpressing BC as a monotherapy or as a part
of combination therapy with either chemotherapy or endocrine
therapy (32), but the reported
response rate was less than 70%, and resistance always developed
(33). Methods of improving the
therapeutic effects of anti-Her-2 agents and preventing the
emergence of resistance are necessary.
The targeting of TGF-β signaling leads to the
inhibition of angiogenesis and reverses a stem cell phenotype to a
more differentiated luminal phenotype (34). These effects are potentially
inhibited by a dual blockade of the TGF-β and CD105 pathways
(35). Moreover, the anti-CD105
antibody as a single agent as well as CD105 as a target for an oral
DNA vaccine have also been reported to be effective agents for
suppressing or preventing tumor progression and prolonging survival
in in vitro models (36–38).
Since overactivity of the Her-2 pathway promotes
angiogenesis and neovascularization contributes to the progression
and metastasis of Her-2-overexpressing BC, a combination of
anti-Her-2 and anti-angiogenic treatments may be feasible This
combination may not only increase the therapeutic effect but also
delay the emergence of resistance (39). The targeting of tumor
neovascularization with vaccines against CD105 in a Her-2-driven BC
cell line has been reported (38).
In our study, we found that MVD measured by CD105 staining
correlated with Her-2 expression, which indicates that combination
therapy may be of benefit in the treatment of Her-2-overexpressing
BC which also has high CD105 expression.
In conclusion, we found that MVD measured by CD105
staining correlated positively with Her-2 expression, but
negatively with HR expression. The significance of MVD on OS is
greater for early stage BCs.
Acknowledgements
We thank J.K. Hsiao for his technical
support. This study was supported by a grant from the Chang Gung
Memorial Hospital (CMRPD890111).
References
|
1.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2.
|
Cianfrocca M and Gradishar W: New
molecular classifications of breast cancer. CA Cancer J Clin.
59:303–313. 2009. View Article : Google Scholar
|
|
3.
|
Folkman J, Watson K, Ingber D and Hanahan
D: Induction of angiogenesis during the transition from hyperplasia
to neoplasia. Nature. 339:58–61. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Gasparini G: Clinical significance of the
determination of angiogenesis in human breast cancer: update of the
biological background and overview of the Vicenza studies. Eur J
Cancer. 32A:2485–2493. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Uzzan B, Nicolas P, Cucherat M and Perret
GY: Microvessel density as a prognostic factor in women with breast
cancer: a systematic review of the literature and meta-analysis.
Cancer Res. 64:2941–2955. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Nico B, Benagiano V, Mangieri D, Maruotti
N, Vacca A and Ribatti D: Evaluation of microvascular density in
tumors: pro and contra. Histol Histopathol. 23:601–607.
2008.PubMed/NCBI
|
|
7.
|
Fox SB, Gasparini G and Harris AL:
Angiogenesis: pathological, prognostic, and growth-factor pathways
and their link to trial design and anticancer drugs. Lancet Oncol.
2:278–289. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Woodfin A, Voisin MB and Nourshargh S:
PECAM-1: a multi-functional molecule in inflammation and vascular
biology. Arterioscler Thromb Vasc Biol. 27:2514–2523. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Mineo TC, Ambrogi V, Baldi A, et al:
Prognostic impact of VEGF, CD31, CD34, and CD105 expression and
tumour vessel invasion after radical surgery for IB-IIA non-small
cell lung cancer. J Clin Pathol. 57:591–597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
da Silva BB, Lopes-Costa PV, dos Santos
AR, et al: Comparison of three vascular endothelial markers in the
evaluation of microvessel density in breast cancer. Eur J Gynaecol
Oncol. 30:285–288. 2009.PubMed/NCBI
|
|
11.
|
Tanaka F, Otake Y, Yanagihara K, et al:
Evaluation of angio-genesis in non-small cell lung cancer:
comparison between anti-CD34 antibody and anti-CD105 antibody. Clin
Cancer Res. 7:3410–3415. 2001.PubMed/NCBI
|
|
12.
|
Fonsatti E, Del Vecchio L, Altomonte M, et
al: Endoglin: an accessory component of the TGF-beta-binding
receptor-complex with diagnostic, prognostic, and
bioimmunotherapeutic potential in human malignancies. J Cell
Physiol. 188:1–7. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kumar S, Ghellal A, Li C, et al: Breast
carcinoma: vascular density determined using CD105 antibody
correlates with tumor prognosis. Cancer Res. 59:856–861.
1999.PubMed/NCBI
|
|
14.
|
Dallas NA, Samuel S, Xia L, et al:
Endoglin (CD105): a marker of tumor vasculature and potential
target for therapy. Clin Cancer Res. 14:1931–1937. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yao Y, Kubota T, Takeuchi H and Sato K:
Prognostic significance of microvessel density determined by an
anti-CD105/endoglin monoclonal antibody in astrocytic tumors:
comparison with an anti-CD31 monoclonal antibody. Neuropathology.
25:201–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Elliott RL and Blobe GC: Role of
transforming growth factor beta in human cancer. J Clin Oncol.
23:2078–2093. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Yamamoto T, Kozawa O, Tanabe K, et al:
Involvement of p38 MAP kinase in TGF-beta-stimulated VEGF synthesis
in aortic smooth muscle cells. J Cell Biochem. 82:591–598. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Li C, Hampson IN, Hampson L, Kumar P,
Bernabeu C and Kumar S: CD105 antagonizes the inhibitory signaling
of transforming growth factor beta1 on human vascular endothelial
cells. FASEB J. 14:55–64. 2000.PubMed/NCBI
|
|
19.
|
Seon BK, Haba A, Matsuno F, et al:
Endoglin-targeted cancer therapy. Curr Drug Deliv. 8:135–143. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Marinho A, Soares R, Ferro J, Lacerda M
and Schmitt FC: Angiogenesis in breast cancer is related to age but
not to other prognostic parameters. Pathol Res Pract. 193:267–273.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Sørlie T, Perou CM, Tibshirani R, et al:
Gene expression patterns of breast carcinomas distinguish tumor
subclasses with clinical implications. Proc Natl Acad Sci USA.
98:10869–10874. 2001.PubMed/NCBI
|
|
22.
|
Liedtke C and Kiesel L: Current issues of
targeted therapy in metastatic triple-negative breast cancer.
Breast Care (Basel). 6:234–239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Niers TM, Richel DJ, Meijers JC and
Schlingemann RO: Vascular endothelial growth factor in the
circulation in cancer patients may not be a relevant biomarker.
PLoS One. 6:e198732011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Pang R and Poon RT: Angiogenesis and
antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett.
242:151–167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Webber K, Cooper A, Kleiven H, Yip D and
Goldstein D: Management of metastatic renal cell carcinoma in the
era of targeted therapies. Intern Med J. 41:594–605. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Kesisis G, Kontovinis LF, Gennatas K and
Kortsaris AH: Biological markers in breast cancer prognosis and
treatment. J BUON. 15:447–454. 2010.PubMed/NCBI
|
|
27.
|
Klos KS, Wyszomierski SL, Sun M, et al:
ErbB2 increases vascular endothelial growth factor protein
synthesis via activation of mammalian target of rapamycin/p70S6K
leading to increased angiogenesis and spontaneous metastasis of
human breast cancer cells. Cancer Res. 66:2028–2037. 2006.
View Article : Google Scholar
|
|
28.
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Chien CY, Su CY, Hwang CF, et al:
Clinicopathologic significance of CD105 expression in squamous cell
carcinoma of the hypopharynx. Head Neck. 28:441–446. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Dales JP, Garcia S, Andrac L, et al:
Prognostic significance of angiogenesis evaluated by CD105
expression compared to CD31 in 905 breast carcinomas: Correlation
with long-term patient outcome. Int J Oncol. 24:1197–1204.
2004.
|
|
31.
|
Rau KM, Kang HY, Cha TL, Miller SA and
Hung MC: The mechanisms and managements of hormone-therapy
resistance in breast and prostate cancers. Endocr Relat Cancer.
12:511–532. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Davoli A, Hocevar BA and Brown TL:
Progression and treatment of HER2-positive breast cancer. Cancer
Chemother Pharmacol. 65:611–623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Roy V and Perez EA: Beyond trastuzumab:
small molecule tyrosine kinase inhibitors in HER-2-positive breast
cancer. Oncologist. 14:1061–1069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Nam JS, Terabe M, Mamura M, et al: An
anti-transforming growth factor beta antibody suppresses metastasis
via cooperative effects on multiple cell compartments. Cancer Res.
68:3835–3843. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
She X, Matsuno F, Harada N, Tsai H and
Seon BK: Synergy between anti-endoglin (CD105) monoclonal
antibodies and TGF-beta in suppression of growth of human
endothelial cells. Int J Cancer. 108:251–257. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Uneda S, Toi H, Tsujie T, et al:
Anti-endoglin monoclonal antibodies are effective for suppressing
metastasis and the primary tumors by targeting tumor vasculature.
Int J Cancer. 125:1446–1453. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Lee SH, Mizutani N, Mizutani M, et al:
Endoglin (CD105) is a target for an oral DNA vaccine against breast
cancer. Cancer Immunol Immunother. 55:1565–1574. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Wood LM, Pan ZK, Guirnalda P, Tsai P,
Seavey M and Paterson Y: Targeting tumor vasculature with novel
Listeria-based vaccines directed against CD105. Cancer Immunol
Immunother. 60:931–942. 2011. View Article : Google Scholar
|
|
39.
|
Foy KC, Liu Z, Phillips G, Miller M and
Kaumaya PT: Combination treatment with HER-2 and VEGF peptide
mimics induces potent anti-tumor and anti-angiogenic responses in
vitro and in vivo. J Biol Chem. 286:13626–13637. 2011. View Article : Google Scholar : PubMed/NCBI
|