Introduction
Peritoneal metastasis is a common type of
metastasis, and occurs in 34% of gastric cancer patients with
recurrence, even following curative resection of the primary tumor
(1,2). Peritoneal recurrence develops from
micrometastasis, which is already seeded in the peritoneum prior to
surgery and it is thought that there are also intraperitoneal free
cancer cells exfoliated from the serosal surface of the primary
tumor. A better understanding of the molecular mechanism underlying
the systemic spread of gastric cancer, including its peritoneal
dissemination, is essential to determine optimal therapeutic
strategies.
Hypoxia is a hallmark of solid tumor formation, and
is an independent prognostic factor in a diverse range of malignant
tumors (3). Hypoxia is associated
with local invasion, meta-static spread, resistance to radiotherapy
and chemotherapy, and a poor prognosis in a number of human
carcinomas. Hypoxia-inducible factor-1 α (HIF-1α) is a key
transcription factor involved in the cellular response to hypoxic
conditions, and is involved in angiogenesis, glycolysis, the
control of vascular tone and erythropoiesis (3). HIF-1 is a heterodimeric protein
consisting of a constitutively expressed β subunit and the HIF-1α
subunit. Previous reports have demonstrated that upregulation of
HIF-1α occurs in cancer cells subjected to hypoxia, that there is a
hypoxia/HIF-1α cascade that is activated in solid cancer, and that
there are relationships between the upregulation of HIF-1α and a
poor prognosis and chemo-resistance in gastric cancer (3,4).
However, little is known concerning the mechanism and contributory
roles of HIF-1α expression and the development of peritoneal
dissemination in gastric cancer.
We previously established two gastric cancer cell
lines with HIF-1α knockdown (KD), MKN45-KD and MKN74-KD (4), and demonstrated the involvement of
HIF-1α expression in chemoresistance using nude mouse xenograft
models. In the present study, we first compared the development of
peritoneal dissemination in the peritoneal cavity of nude mice
between HIF-1α KD and control (SC) gastric cancer cell lines. Then,
we elucidated the molecular mechanism underlying this process by
investigating changes in the expression of metastasis-related genes
between the KD and SC cells. This study was performed to clarify
whether or not HIF-1α expression affects the development of
peritoneal dissemination and to isolate candidate genes regulated
by HIF-1α that may be involved in peritoneal dissemination.
Materials and methods
Cell lines and culture
Two gastric cancer cell lines, MKN45 and MKN74, were
purchased from Riken Cell Bank (Ibaraki, Japan). MKN45 was derived
from a poorly differentiated adenocarcinoma and MKN74 was derived
from a moderately differentiated adenocarcinoma. To analyze the
role of HIF-1α in peritoneal dissemination, we established two
HIF-1α knockdown sublines (designated MKN45-KD and MKN74-KD,
respectively) and control sublines (MKN45-SC and MKN74-SC,
respectively) as reported in a previous study (4). These four gastric cancer cell lines,
MKN45-SC, MKN45-KD, MKN74-SC and MKN74-KD, were used for this
study. The cell lines were cultured as described in a previous
study (4).
Animal experiments
The animal protocols were approved by the Animal
Care Committee of Saga University. Female athymic BALB/cA Jcl mice
(nu/nu, 5-weeks old) were obtained from Nihon Crea Co. (Osaka,
Japan). They were maintained under specific pathogen-free
conditions and were given γ-irradiated food and autoclaved water.
We examined the peritoneal dissemination using the four gastric
cancer cell lines, MKN45-SC and -KD, and MKN74-SC and -KD. To
generate the xenograft model, cancer cells (5x106) were
suspended in 100 μl of PBS, and injected on day 0 into the
abdominal cavity. Five mice per group were injected with the
various cell lines. All of the mice were sacrificed on day 28, and
then the number of disseminated nodules and their total weight were
measured.
Western blot analysis
The western blot analysis was performed as
previously described (4). In
brief, the whole cell lysate was prepared using a lysis buffer
composed of 150 mM NaCl, 50 mM Tris-HCl (pH 7.6), 0.5% Triton X-100
and a protease inhibitor cocktail mix (Roche, Mannheim, Germany).
The samples were dissolved in NuPage™ LDS sample buffer
(Invitrogen, Carlsbad, CA, USA) and 1 M dithiothreitol. A total of
20 μg of protein was subjected to NuPage 4–12% Bis-Tris Gel
(Invitrogen) electrophoresis and electrophoretically transferred
onto an Amersham™ Hybond™-ECL membrane (GE Healthcare,
Buckinghamshire, UK) in transfer buffer. The primary antibodies
used in this analysis were anti-HIF-1α (BD Biosciences, San Jose,
CA, USA) and anti-β-actin (Sigma). Following incubation with the
corresponding secondary antibodies, the signals were developed
using an Amersham™ ECL Plus Western Blotting Detection System (GE
Healthcare).
Total RNA extraction and real-time
quantitative reverse transcription-polymerase chain reaction
Total RNA was extracted using an Isogen®
RNA extraction kit (Nippon Gene, Osaka, Japan), converted to cDNA
using a ReverTra Ace reverse transcription reaction kit (Toyobo,
Osaka, Japan) and subjected to real-time quantitative reverse
transcription (RT)-PCR using Light Cycler FastStart DNA Master™
SYBR Green I kit and a Light Cycler™ instrument system (Roche)
(4). The mRNA expression levels of
HIF-1α, CA9 and metastasis-related genes, such as integrin α2, α3,
α5, α6, αv, β1, β3, β4, β5, β6, matrix metallopeptidase (MMP)-1,
MMP-7, MMP-11, α-catenin, β-catenin, CD44 and E-cadherin, were
examined. The primer sequences used are shown in Table I.
 | Table IPrimer sequences used in quantitative
real-time PCR. |
Table I
Primer sequences used in quantitative
real-time PCR.
| Gene symbol | Forward primer | Reverse primer |
|---|
| CA9 |
CCGAGCGACGCAGCCTTTGA |
GGCTCCAGTCTCGGCTACCT |
| CD44 |
CAGCACCATTTCAACCACAC |
AGCACTTCCGGATTTGAATG |
| CDH1 |
CTGAAAGCGGCTGATACTGAC |
GGAGTTCAGGGAGCTCAGACT |
| CTNNA1 |
CCCAAGTTTTCCGTGAACAT |
GCTTGCAGACATTCGAACAA |
| CTNNB1 |
ACCTTTCCCATCATCGTGAG |
AATCCACTGGTGAACCAAGC |
| HIF-1α |
CTCATCAGTTGCCACTTCCA |
CCTCACACGCAAATAGCTGA |
| ITGA2 |
TGTCCTGTTGACCTATCCACTG |
AGGCTCATGTTGGTTTTCATCT |
| ITGA3 |
CTACCACAACGAGATGTGCAATA |
ATCATGTAGCTGTTTCCTTTCCA |
| ITGA5 |
TGTTGGTGAATTCAGTGGTGA |
GAGCCATTAAGGATGGTGACA |
| ITGA6 |
GCGAGCAAGCTATGAAATCTG |
CTGTGCCGAGGTTTGTAAGAG |
| ITGAV |
ATCTGTGAGGTCGAAACAGGAT |
ATCCGAAATAAGCTGACGTGAT |
| ITGB1 |
TACTTGTGAAGCCAGCAACG |
CACGTTTGCCCTTGAAACTT |
| ITGB3 |
TCAATGAGGAAGTGAAGAAGCA |
GTCTTGGCATCAGTGGTAAACA |
| ITGB4 |
ACCCAGTACAGGACACAGGACTA |
AGGAGTAGTTGGTGACAGCAAAG |
| ITGB5 |
TGCAGCACCAAGAGAGATTG |
CTCATCCCTGCATAGGCTGT |
| ITGB6 |
AATGACTCCCTCCACCTCCT |
TGCTGTCCAAGTGACAGAGC |
| MMP1 |
AGGTCTCTGAGGGTCAAGCA |
TCCTCCAGGTCCATCAAAAG |
| MMP7 |
AGCTCATGGGGACTCCTACC |
GTGAGCATCTCCTCCGAGAC |
| MMP11 |
CCGCCTCTACTGGAAGTTTG |
GCACAGCCAAAGAAGTCAGG |
| ACTB |
CGAGCGCGGCTACAGCTT |
TCCTTAATGTCACGCACGATTT |
Adhesion assay
To quantify the tumor cell adhesion to the
extracellular matrix and the monolayer mesothelium, an adhesion
assay was performed. The in vitro adhesion assay was carried
out using the CytoSelect 48-well cell adhesion assay extracellular
matrix (ECM) array (Cell Biolabs, Inc, San Diego, USA) according to
the manufacturer’s instructions. Another adhesion assay using the
monolayer mesothelium was performed as described below. The
mesothelial cell line, MeT-5A (CRL-9444), was purchased from the
American Type Culture Collection (Manassas, VA, USA). A total of
2x104 MeT-5A cells were added in 100 μl of medium to
each well of a 96-well plate. The plate was incubated at 37°C under
normoxic conditions overnight. Then a mesothelial monolayer was
established. Each well was washed with PBS to remove non-adherent
cells, then 2x104 cells of each gastric cancer cell line
were added to 100 μl of medium to each well. The plate was then
incubated at 37°C under normoxic conditions for 5 h. Each well was
washed with PBS to remove non-adherent cells, and then the MTT
assay was performed using a Cell-Titer 96™ nonradioactive cell
proliferation assay kit (Promega, Madison, WI, USA). The adhesion
index of the mesothelium was calculated using the following
formula: Adhesion index = (Adherent cells in well)/(Seeded cells in
well).
Immunohistochemistry
The immunohistochemical staining of MMP-1 and MMP-11
was performed according to a previous report (4). The anti-MMP-1 antibody (Thermo
Scientific, Fremont, CA, USA) and anti-MMP-11 antibody (Abcam,
Tokyo) were used as the primary antibodies.
Statistical analysis
The statistical analysis was carried out using the
SPSS 1.5 statistical software package for Windows (SPSS Japan Inc.)
A P-value <0.05 was considered to be indicative of statistical
significance.
Results
Characteristics of the development of
peritoneal dissemination dependent on HIF-1α in xenograft tumors in
nude mice
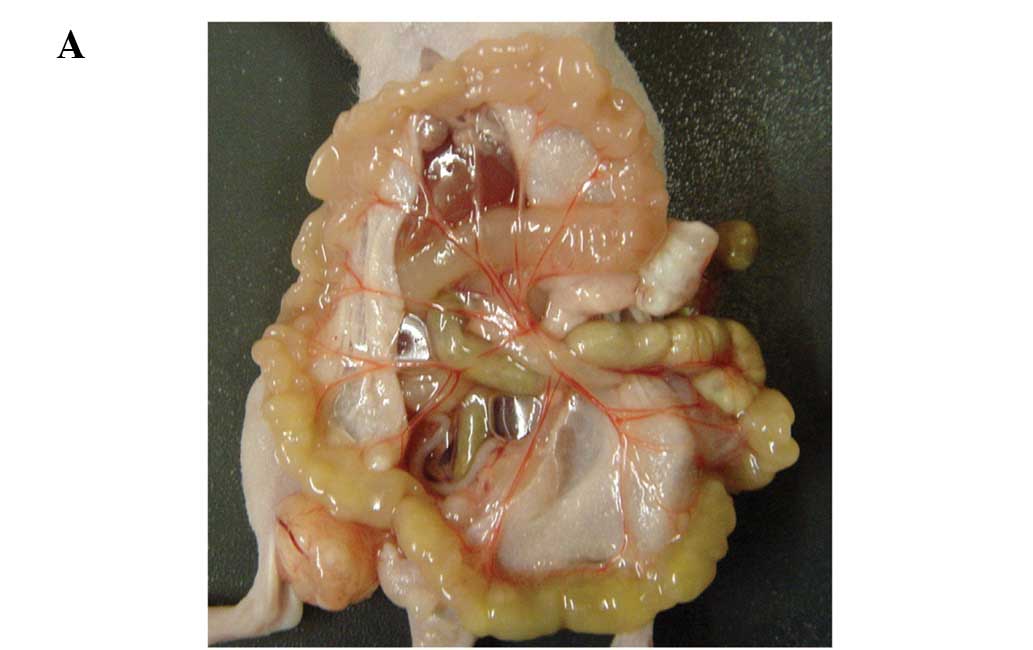
All four gastric cancer cell lines, MKN45-SC, -KD,
MKN74-SC, -KD, resulted in peritoneal dissemination in nude mice (5
of 5; 100%, respectively). The differences in the development of
peritoneal dissemination between the SC and KD cell lines were
compared using the MKN45 and MKN74 cells. Regarding the MKN45 cell
lines, the HIF-1α KD cell line resulted in significantly greater
numbers of disseminated nodules and a heavier weight of nodules
than the SC cell lines (p<0.05, p<0.05, respectively,
Fig. 1A–D). In the MKN74 cell
lines, HIF-1α KD also tended to result in greater numbers of
disseminated nodules and a heavier weight of nodules than the SC
cells, although there were no significant differences (p=0.175,
p=0.251, respectively, for the number and weight; Fig. 1C and D). Microscopic observation
revealed that the disseminated nodules in the KD cells tended to
have a larger necrotic area than the SC cells (Fig. 1E). The area of necrosis in the
largest disseminated nodule in each mouse was estimated, and the
area of dissemination of the two KD cell lines was found to be
significantly larger than that of the SC lines (Fig. 1E and F, p<0.01).
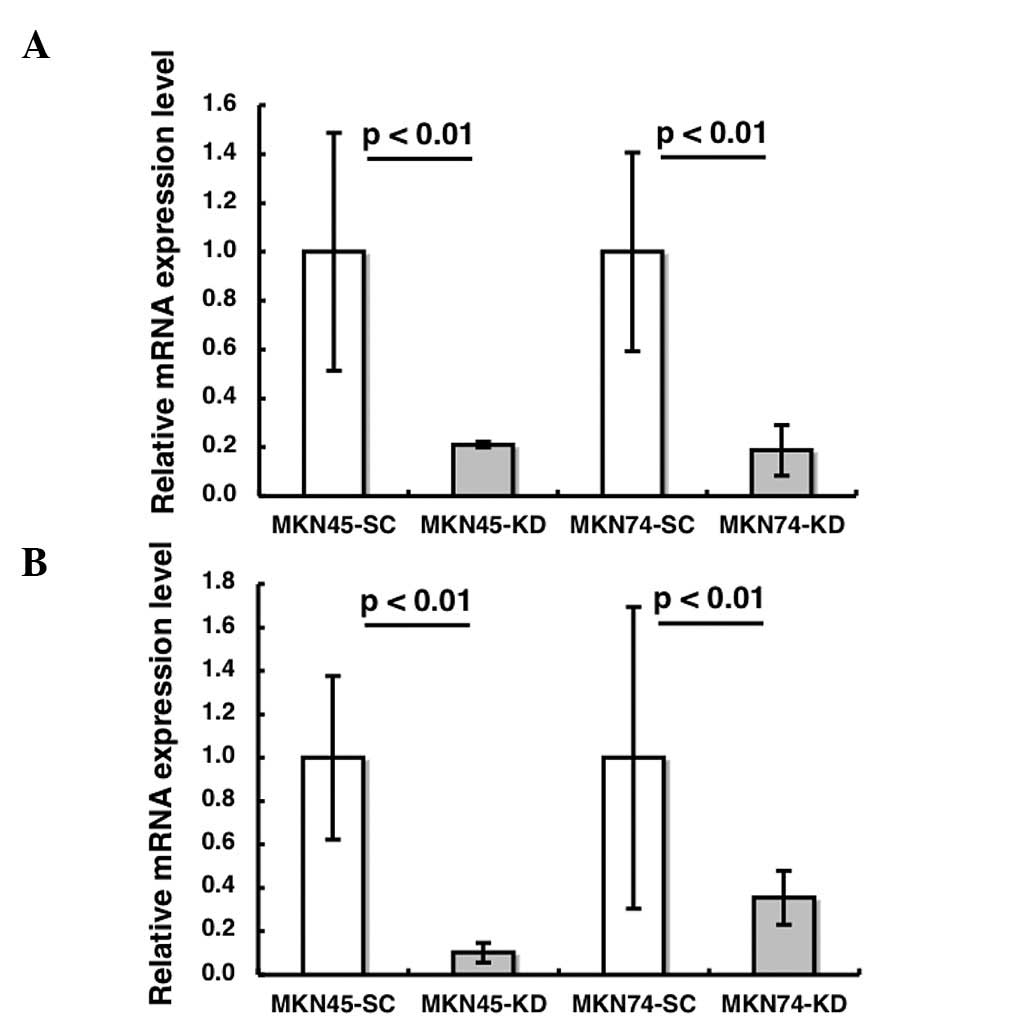
HIF-1α siRNA significantly decreases the
expression of HIF-1α and its target gene in xenograft tissues
The induction of HIF-1α and its target gene, CAIX,
were validated by RT-PCR and/or immunoblotting in nude mouse
tissues. The mRNA expression levels of HIF-1α and CAIX were
significantly reduced in the HIF-1α KD tissues compared to the SC
tissues (p<0.01, p<0.01, respectively Fig. 2A and B). The immunoblotting
analysis demonstrated that HIF-1α was undetectable in HIF-1 KD
tumors, whereas HIF-1α expression was observed in SC tumors
(Fig. 2C).
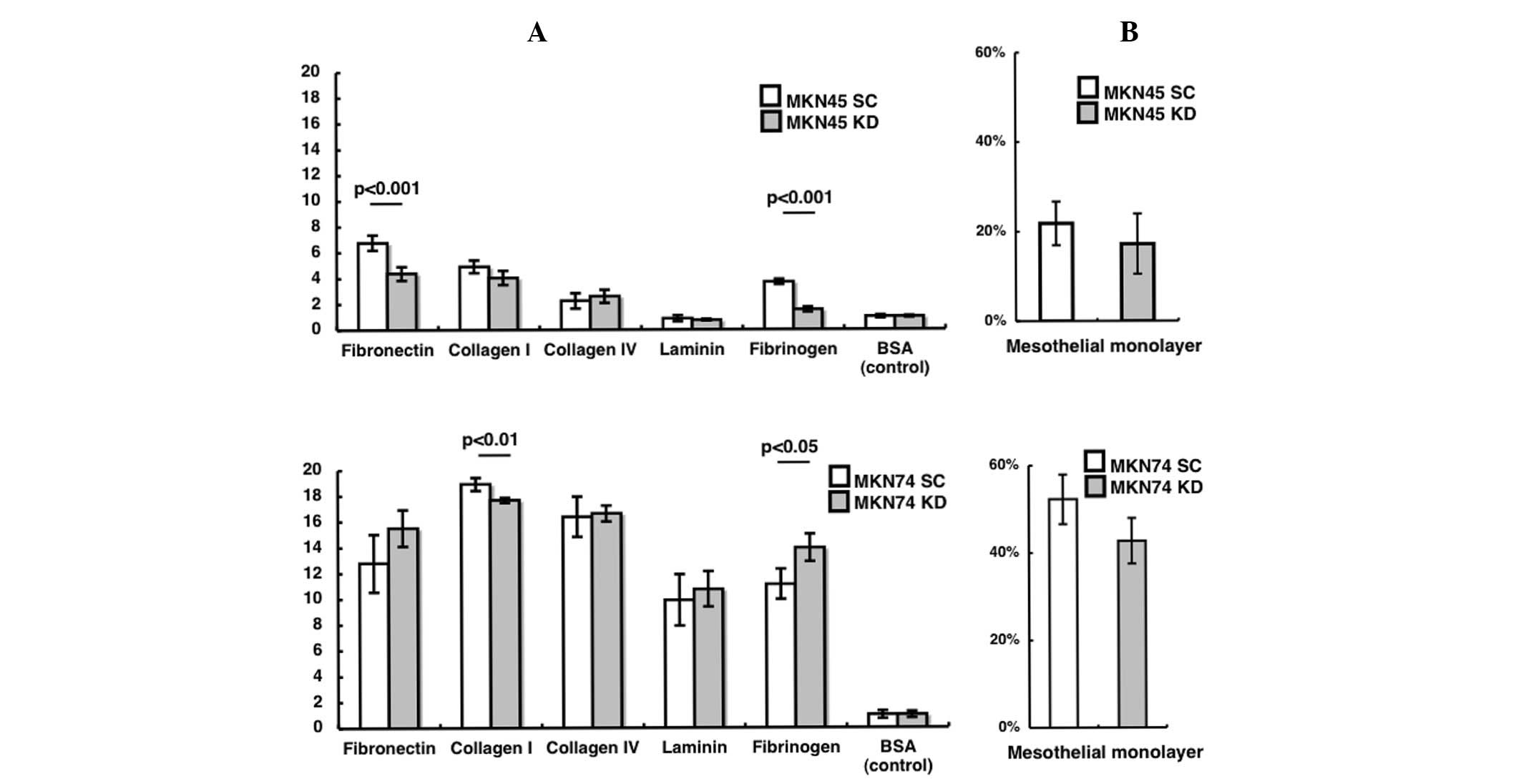
Adhesion to the extracellular matrix or a
monolayer mesothelium
To investigate why the HIF-1α KD cell lines develop
a greater number of peritoneal nodules than the SC cell lines, an
adhesion assay was performed. In the MKN45 cells, attachment to
fibronectin and fibrinogen was significantly decreased in the KD
compared to the SC cell line (Fig.
3A; p<0.001, P<0.001, respectively). In the MKN74 cells,
attachment to collagen I was significantly decreased and that to
fibrinogen was significantly increased in the KD compared to the SC
cell line (Fig. 3A; P<0.01,
P<0.05, respectively). In terms of adhesion to the mesothelial
monolayer, no significant difference was found between the KD and
SC sublines for both the MKN45 and MNK74 cell lines (Fig. 3B).
mRNA expression of metastasis-related
genes in the various cell lines
To elucidate why HIF-1α KD cells demonstrated an
increased number of disseminated nodules compared to the SC cells,
the mRNA expression levels of metastasis-related genes were
analyzed in each cell line. We investigated the mRNA levels in the
two paired SC and KD cell lines under normoxic and hypoxic
conditions and subsequently estimated the expression ratios of the
KD/SC cells. This analysis isolated a candidate gene, which was
commonly upregulated in the KD cells of the MKN74 and MKN45 cell
lines, compared with the SC cells. In the MKN45 cells, the
expression level of MMP-1 was higher in the KD than SC cells under
both normoxic (KD/SC ratio: 2.88) and hypoxic (KD/SC ratio: 1.96)
conditions (Table II). In the
MKN74 cells, the higher ratio was also observed under both normoxic
(KD/SC ratio: 3.86) and hypoxic conditions (KD/SC ratio: 3.23).
MMP-11 was more highly expressed in KD than in SC MKN45 cells
(KD/SC ratio: 1.58 under normoxia, 1.70 under hypoxia) and MKN74
cells (KD/SC ratio: 10.70 under normoxia, 4.32 under hypoxia). On
the other hand, CD44, catenin β1, integrin α5 and integrin β4
demonstrated higher mRNA expression under both conditions of
normoxia and hypoxia in the MKN74 cells. However, higher expression
of these genes was not observed in the MKN45 cells. Taken together,
the results suggest that MMP-1 and MMP-11 are commonly upregulated
in both KD cell lines under normoxic and hypoxic conditions.
 | Table IIRelative differences in gene
expression in the cell lines under conditions of normoxia and
hypoxia. |
Table II
Relative differences in gene
expression in the cell lines under conditions of normoxia and
hypoxia.
| | MKN45-KD/MKN45-SC
| MKN74-KD/MKN74-SC
|
|---|
| Gene symbol | Gene description | Nx | Hx | Nx | Hx |
|---|
| CD44 | CD44 molecule | 1.34 | 0.72 | 25.74 | 5.59 |
| CDH1 | E-cadherin | 0.98 | 0.75 | 0.52 | 1.00 |
| CTNNA1 | Catenin α1 | 1.06 | 0.78 | 1.00 | 1.08 |
| CTNNB1 | Catenin β1 | 0.70 | 0.75 | 1.67 | 2.40 |
| ITGA2 | Integrin α2 | 1.38 | 0.48 | 0.51 | 0.52 |
| ITGA3 | Integrin α3 | 0.56 | 0.40 | 1.07 | 0.68 |
| ITGA5 | Integrin α5 | 0.84 | 0.55 | 1.75 | 1.64 |
| ITGA6 | Integrin α6 | 1.65 | 0.83 | 1.40 | 3.09 |
| ITGAV | Integrin αV | 1.04 | 1.21 | 0.75 | 2.22 |
| ITGB1 | Integrin β1 | 1.35 | 1.49 | 1.09 | 1.58 |
| ITGB3 | Integrin β3 | 0.79 | 1.01 | 7.62 | 0.89 |
| ITGB4 | Integrin β4 | 1.54 | 0.65 | 1.59 | 1.84 |
| ITGB5 | Integrin β5 | 1.67 | 1.29 | 0.91 | 1.06 |
| ITGB6 | Integrin β6 | 0.81 | 0.68 | 0.93 | 1.49 |
| MMP1 | Matrix
metallopeptidase 1 | 2.88 | 1.96 | 3.86 | 3.23 |
| MMP7 | Matrix
metallopeptidase 7 | 0.09 | 0.13 | 1.56 | 1.01 |
| MMP11 | Matrix
metallopeptidase 11 | 1.58 | 1.70 | 10.70 | 4.32 |
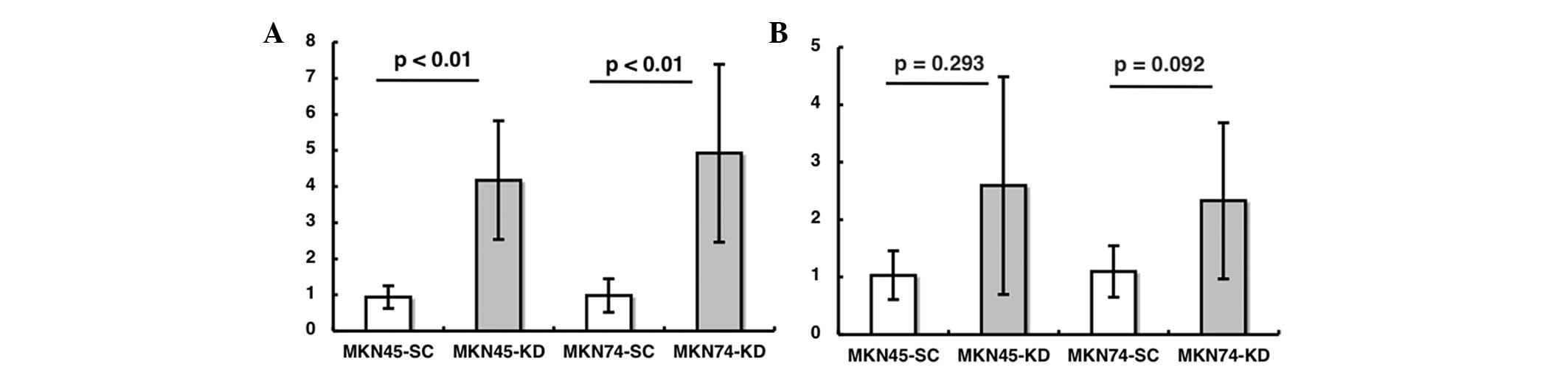
mRNA and protein expression of MMP-1 and
-11 in disseminated nodules in nude mice
The mRNA expression level of MMP-1 in the
disseminated nodules in nude mice was analyzed. The expression was
significantly increased in the nodules derived from the KD sublines
compared to those from the SC sublines for the MKN45 and MKN74 cell
lines (Fig. 4A; p<0.01,
p<0.01, respectively). The mRNA expression of MMP-11 in nude
mouse tissues tended to be increased in the KD compared to the SC
sublines, although the difference was not significant (Fig. 4B; p=0.293, p=0.092). An
immunohistochemical study of MMP-1 in nude mouse tissues
demonstrated that there was significantly stronger staining in
MKN74-KD than in MKN74-SC cells (Fig.
4C and D). However, the MMP-11 staining in the MKN74 cells did
not demonstrate any significant difference between the SC and KD
cells (data not shown).
Discussion
Peritoneal metastasis is the most common cause of
tumor progression in gastric cancer. When the dissemination of
multiple peritoneal nodules are obvious, curative surgery with a
standard gastrectomy cannot be achieved. In addition, peritoneal
metastasis exhibits resistance against various chemotherapeutic
drugs, and causes massive ascites and intestinal obstruction. A
previous study reported that 50–60% of gastric cancer patients with
serosal invasion following curative resection eventually developed
peritoneal metastasis (5), and the
average survival following peritoneal recurrence was only 4.9
months (6). Therefore, clarifying
the mechanism underlying the peritoneal metastasis is essential not
only for preventing the formation of gross metastatic nodules in
the peritoneum, but also for considering the treatment
strategy.
As a key regulator of the cellular adaptive response
to hypoxia, HIF-1α plays a critical role in tumorigenesis (3). Hypoxic cancer cells may undergo a
series of genetic and metabolic changes that allow them not only to
survive and proliferate, but also to become more resistant to
radiation therapy and chemical agents. The formation of metastases
is a major factor in disease progression. In the present study, we
silenced the function of HIF-1α to evaluate its role in the
peritoneal dissemination of gastric cancer cell lines. The
molecular mechanism underlying the peritoneal dissemination of
cancer cells remains poorly understood and, so far, there have been
no prognostic markers indicating which primary gastric tumors are
likely to develop peritoneal dissemination.
As previously reported, the protein expression of
HIF-1α in MKN45-KD and MKN74-KD cells was undetectable even
following hypoxic stimulation, and the mRNA expression of its
target genes, GLUT1 and CA9, was significantly suppressed in
comparison to the SC cells (4). We
also confirmed that the HIF-1α and CA9 mRNA expression levels were
significantly reduced in the disseminated nodules derived from the
two KD cell lines (Fig. 2A and B),
and the HIF-1α protein was not detectable in the disseminated
nodules of either of the KD cell lines (Fig. 2C).
We made the unexpected observation that the loss of
HIF-1α accelerates the peritoneal dissemination of MKN45 and MKN74
cell lines. This was contrary to our expectation, since several
previous studies had demonstrated that HIF-1α is involved in cell
proliferation and/or tumorigenesis in gastric cancer cell lines
(7), colorectal cancer cell lines
(8), endothelial cell lines
(9) and chondrocytes (10). However, a few studies have
described the tumor-suppressor functions of HIF-1α. For example,
Carmeliet et al (11)
reported that the loss of HIF-1α led to more aggressive growth of
embryonic stem cell-derived teratocarcinomas. Blouw et al
(12) reported that astrocytomas
deficient in HIF-1α grow faster, and penetrate the brain more
rapidly and extensively than their normal counterparts. We also
previously reported that HIF-1α KD MKN45 tumors in xenografts grown
in nude mice demonstrated more rapid growth, in comparison to the
control MKN45 tumors (4). Our
results and previous reports suggest that HIF-1α expression may act
as a negative factor in the formation of metastasis under certain
conditions, such as subcutaneous and peritoneal cavity growth.
The mechanism of peritoneal dissemination of gastric
cancer has been described to occur through several steps;
detachment of cancer cells from the primary tumor, attachment to
the distant peritoneum, invasion into the subperitoneal space and
proliferation with vascular neogenesis (13). Regarding attachment to the
peritoneum, the adhesion assay for the extracellular matrix and
mesothelial cells was performed. We found that the attachment to
fibronectin and fibrinogen in the MKN45 cells and collagen I in the
MKN74 cells were significantly decreased in KD cells in comparison
to SC cells. On the other hand, in the MKN74 cells, only fibrinogen
was significantly increased in the KD cells compared to the SC
cells. These results indicated that cell attachment molecules have
little impact on the development of dissemination related to HIF-1α
expression.
In order to further clarify why the KD cell lines
demonstrated more peritoneal dissemination than the SC cell lines,
we next compared the mRNA expression level of metastasis-related
genes, such as integrin family member MMP, α and β catenin, CD44
and E-cadherin, between the KD and SC cell lines. In this analysis,
the mRNA expression levels of MMP-1 and MMP-11 were significantly
upregulated in the HIF-1α KD cell lines under both normoxic and
hypoxic conditions, compared with the SC cells (Table II). In addition, the protein
expression of MMP-1, but not MMP-11, was dramatically increased in
the MKN74-KD cells in comparison to the MKN74-SC cells (Fig. 4C). These results indicated that the
effects on MMP-1 may be the most critical effect mediated by the
loss of HIF-1α expression.
Matrix metalloproteinases (MMPs) are believed to
mediate a number of physiological and pathological processes, such
as the degradation of the extracellular matrix, tissue remodeling,
inflammation, tumor invasion and metastasis. The present study
focused on the expression of MMP 1, 7 and 11, since several studies
previously reported the relationships between the MMP members and
invasion or dissemination in gastric cancer (14–21).
For example, Yanagihara et al (22) reported that a cDNA microarray
analysis demonstrated dramatic upregulation of MMP-1 (ratio: 29.63)
in the 44As3 gastric cancer cell subline which shows frequent
metastasis to the peritoneum, in comparison to parental HSC-44PE
cells. In addition, previous studies reported that an MMP inhibitor
reduced the peritoneal dissemination of human gastric cancer cell
lines in mice (18,23). Taken together, our results and the
previous findings indicate that the upregulation of MMP-1
expression following the loss of HIF-1α might be an important step
in the development of peritoneal dissemination from MKN45 and MKN74
gastric cancer cells.
Knocking down HIF-1α, which leads to MMP-1
upregulation, might contribute to the degradation of the
extracellular matrix of the peritoneum, allowing the invasion of
cancer cells and the formation of peritoneal metastasis. However,
contrary to this result, Miyoshi et al reported that hypoxic
stress accelerated cancer invasion by upregulating the expression
levels of MMP-7 and -14 by a HIF-1α-independent pathway (24). Fujiwara et al demonstrated
that treatment with HIF-1α siRNA resulted in the downregulation of
MMP-2 mRNA under hypoxic conditions in all of the glioma cell lines
examined (25). Considering these
controversial findings, it is possible that each member of the MMP
family might play a different role in cancer progression, such as
cancer invasion by the primary tumor, distant metastasis and
peritoneal dissemination.
In conclusion, the present study demonstrates for
the first time that the loss of HIF-1α contributes to the
development of aggressive peritoneal dissemination by upregulating
MMP-1 in gastric cancer cell lines. Therefore, HIF-1α expression
might be suppressed during the development of peritoneal
dissemination from primary gastric cancer.
References
|
1.
|
Yoo CH, Noh SH, Shin DW, Choi SH and Min
JS: Recurrence following curative resection for gastric carcinoma.
Br J Surg. 87:236–242. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Fujiwara Y, Doki Y, Taniguchi H, et al:
Genetic detection of free cancer cells in the peritoneal cavity of
the patient with gastric cancer: present status and future
perspectives. Gastric Cancer. 10:197–204. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Semenza GL: Defining the role of
hypoxia-inducible factor 1 in cancer biology and therapeutics.
Oncogene. 29:625–634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Nakamura J, Kitajima Y, Kai K, et al:
HIF-1alpha is an unfavorable determinant of relapse in gastric
cancer patients who underwent curative surgery followed by adjuvant
5-FU chemotherapy. Int J Cancer. 127:1158–1171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Broll R, Weschta M, Windhoevel U, et al:
Prognostic significance of free gastrointestinal tumor cells in
peritoneal lavage detected by immunocytochemistry and polymerase
chain reaction. Langenbecks Arch Surg. 386:285–292. 2001.
View Article : Google Scholar
|
|
6.
|
Lee CC, Lo SS, Wu CW, et al: Peritoneal
recurrence of gastric adenocarcinoma after curative resection.
Hepatogastroenterology. 50:1720–1722. 2003.PubMed/NCBI
|
|
7.
|
Stoeltzing O, McCarty MF, Wey JS, et al:
Role of hypoxiainducible factor 1alpha in gastric cancer cell
growth, angiogenesis, and vessel maturation. J Natl Cancer Inst.
96:946–956. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Dang DT, Chen F, Gardner LB, et al:
Hypoxia-inducible factor-1alpha promotes nonhypoxia-mediated
proliferation in colon cancer cells and xenografts. Cancer Res.
66:1684–1693. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Tang N, Wang L, Esko J, et al: Loss of
HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF
autocrine loop necessary for tumorigenesis. Cancer Cell. 6:485–495.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Pfander D, Cramer T, Schipani E and
Johnson RS: HIF-1alpha controls extracellular matrix synthesis by
epiphyseal chondrocytes. J Cell Sci. 116:1819–1826. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Carmeliet P, Dor Y, Herbert JM, et al:
Role of HIF-1alpha in hypoxia-mediated apoptosis, cell
proliferation and tumour angiogenesis. Nature. 394:485–490. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Blouw B, Song H, Tihan T, et al: The
hypoxic response of tumors is dependent on their microenvironment.
Cancer Cell. 4:133–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Liotta LA: Tumor invasion and metastases –
role of the extra-cellular matrix: Rhoads Memorial Award lecture.
Cancer Res. 46:1–7. 1986.
|
|
14.
|
Fujimoto D, Hirono Y, Goi T, Katayama K
and Yamaguchi A: Prognostic value of protease-activated receptor-1
(PAR-1) and matrix metalloproteinase-1 (MMP-1) in gastric cancer.
Anticancer Res. 28:847–854. 2008.PubMed/NCBI
|
|
15.
|
Yoshikawa T, Yanoma S, Tsuburaya A, et al:
Expression of MMP-7 and MT1-MMP in peritoneal dissemination of
gastric cancer. Hepatogastroenterology. 53:964–967. 2006.PubMed/NCBI
|
|
16.
|
Mizutani K, Kofuji K and Shirouzu K: The
significance of MMP-1 and MMP-2 in peritoneal disseminated
metastasis of gastric cancer. Surg Today. 30:614–621. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Yonemura Y, Endou Y, Fujita H, et al: Role
of MMP-7 in the formation of peritoneal dissemination in gastric
cancer. Gastric Cancer. 3:63–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Yonemura Y, Endo Y, Fujita H, et al:
Inhibition of peritoneal dissemination in human gastric cancer by
MMP-7-specific anti-sense oligonucleotide. J Exp Clin Cancer Res.
20:205–212. 2001.PubMed/NCBI
|
|
19.
|
Deng H, Guo RF, Li WM, Zhao M and Lu YY:
Matrix metal-loproteinase 11 depletion inhibits cell proliferation
in gastric cancer cells. Biochem Biophys Res Commun. 326:274–281.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Zhao ZS, Chu YQ, Ye ZY, Wang YY and Tao
HQ: Overexpression of matrix metalloproteinase 11 in human gastric
carcinoma and its clinicopathologic significance. Hum Pathol.
41:686–696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Yonemura Y, Fujimura T, Ninomiya I, et al:
Prediction of peritoneal micrometastasis by peritoneal lavaged
cytology and reverse transcriptase-polymerase chain reaction for
matrix metalloproteinase-7 mRNA. Clin Cancer Res. 7:1647–1653.
2001.
|
|
22.
|
Yanagihara K, Takigahira M, Tanaka H, et
al: Development and biological analysis of peritoneal metastasis
mouse models for human scirrhous stomach cancer. Cancer Sci.
96:323–332. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Wada N, Otani Y, Kubota T, et al: Reduced
angiogenesis in peritoneal dissemination of gastric cancer through
gelatinase inhibition. Clin Exp Metastasis. 20:431–435. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Miyoshi A, Kitajima Y, Ide T, et al:
Hypoxia accelerates cancer invasion of hepatoma cells by
upregulating MMP expression in an HIF-1alpha-independent manner.
Int J Oncol. 29:1533–1539. 2006.PubMed/NCBI
|
|
25.
|
Fujiwara S, Nakagawa K, Harada H, et al:
Silencing hypoxiainducible factor-1alpha inhibits cell migration
and invasion under hypoxic environment in malignant gliomas. Int J
Oncol. 30:793–802. 2007.PubMed/NCBI
|