Introduction
Gene therapy for cancer treatment is now gaining an
increasing interest from across the globe (1,2). A
variety of strategies have been used to date, including the
inhibition of oncogene expression, tumor angiogenesis and
multidrug-resistant gene expression, and the induction of
tumor-suppressor and suicide gene expression or the induction of
antitumor immunity. Combining these strategies in order to improve
antitumor effects is plausible as carcinogenesis involves a
multitude of factors, as well as multi-step and multi-stage
processes (3).
Inducing a suicide gene to tumor cells holds broad
applications in tumor treatment. This therapy involves introducing
genes of non-mammalian enzymes into cancer cells, so that they can
convert non-toxic prodrugs into highly toxic substances. This will,
in turn, kill not only the infected cells but also the adjacent
ones. Thymidine kinase (TK), which converts the prodrug ganciclovir
(GCV) into phosphorylated GCV (p-GCV), and cytosine deaminase (CD),
which converts the prodrug 5-fluorocytosine (5-FC) into
5-fluorouracil (5-FU), are two popular prodrug-converting
non-mammalian enzymes and are encoded by the TK and CD genes,
respectively. In the tumor cell, p-GVC inhibits cellular DNA
synthesis, leading to tumor cell death via apoptotic/non-apoptotic
mechanisms, whereas 5-FU, a chemotherapy drug, interferes with
nucleoside metabolism by being incorporated into RNA and DNA,
leading to cytotoxicity and cell death (4,5).
Both have been incorporated into the two most common suicide
gene/prodrug systems: the herpes simplex virus thymus kinase
(HSV-TK) gene/GCV system (HSV-TK/GCV) and the E. coli
cytosine deaminase (Ec-CD) gene/5-fluorocytosine (5-FC) system
(Ec-CD/5-FC) (6,7). A combination of two or more suicide
genes, or the combination of the suicide gene with other genes to
make a new fusion gene, would theoretically confer various
synergistic effects, as a single suicide gene or a single gene
interfering therapy easily leads to drug resistance, and its
treatment effects vary according to tumor cell type.
Previous studies have shown that a suicide gene,
combined with chemotherapy drugs or other genes, is able to enhance
antitumor action (8–10). The fusion gene that connects the CD
gene with the TK gene is a widely used one. With the
prodrug-converting enzyme activities of CD and TK genes, it breaks
the dependence of tumor cell types, eliminates drug resistance and
expands the application of the therapy.
Hamstra et al (11) found that the CD gene from yeast
(yCD) in comparison with the E. coli CD gene (bCD) can more
effectively alter catalytic 5-FC into 5-FU. In our previous study,
we constructed the fusion suicide gene yCDglyTK containing yeast
CD, using enhanced the CEA promoter to drive its expression in
carcinoembryonic antigen (CEA)-positive cells. We found that this
fusion suicide gene was more effective on the SGC7901 human gastric
adenocarcinoma cell line when used with the prodrug 5-FC alone
(12).
Telomerase, a ribonucleoprotein enzyme responsible
for adding the telomeric repeats onto a chromosome, plays an
important role in the development of cellular immortality and
oncogenesis (13,14). Previous studies have shown that
telomerase activity is found in 85–90% of all human tumors, but not
in their adjacent normal cells (15,16).
This makes telomerase a good target not only for cancer diagnosis,
but also for the development of novel therapeutic agents (17,18).
Human telomerase is composed of three components:
human telomerase RNA (hTR), telomerase reverse transcriptase
(hTERT) and telomerase associated protein 1 (hTEP1). hTERT is the
catalytic subunit of telomerase. It is expressed in cells with
telomerase activity and its expression level is positively
correlated with telomerase activity (19). The SGC7901 human gastric
adenocarcinoma cell line is the major subtype of gastric cancer
cell lines with high hTERT gene expression (20). RNA interference (RNAi) targeting
hTERT reduces the expression of the mRNA and protein of hTERT,
exerting antitumor effects.
In our previous studies, a plasmid carrying the
fusion suicide gene yCDglyTK was constructed (12,21).
In order to enhance the antitumor effect of the system, in the
present study, this fusion suicide gene was combined with
hTERT-targeted shRNA, and a new combined plasmid pcDNA3.1(-)
hTERT-shRNA/yCDglyTK was constructed. Its bioactivities and
antitumor effect were investigated in the SGC7901 human gastric
cancer cell line.
Materials and methods
Cell line
The SGC7901 human gastric cancer cell line was
obtained from the Central Laboratory of Xiangya Hospital, Central
South University (Changsha, China). SGC7901 cells were grown in
RPMI-1640 containing 10% calf serum at 37°C in a 5% CO2
humidified incubator.
Reagents
Restriction enzymes XhoI, NheI,
EcoRI, XbaI, HindIII were purchased from MBI
Fermentas. T4-DNA ligase (New England Biolabs), rTaq DNA polymerase
(Takara), SYBR-Green Real Master mix, DNA Marker IV and DNA Marker
DL2000 (Tiangen Biotech Co.) were used. Calf serum, RPMI-1640
(Thermo Scientific), trypsin (Beyotime). G418 (Sigma), calcium
phosphate nanoparticles (from our laboratory, Xiangya Hospital,
Changsha, China) were obtained. Reverse transcriptase kit (MBI
Fermentas), goat anti-CD antibody (Abcam), mouse anti-human
telomerase reverse transcriptase antibody (Santa Cruz
Biotechnology, Inc.), the cell cycle detection kit (KeyGen Biotech
Co., Ltd.) and 5-fluorocytosine (5-FC; Sigma) were also used in the
study.
Plasmids
Plasmids used in this study are listed in Table I. The cells were set according to
the following groups: i) SGC7901 (group A; control), ii)
SGC7901/Null (group B; sham), iii) SGC7901/TERT-siRNA (group C;
pTERT), iv) SGC7901/yCDglyTK (group D; pCD/TK) and v)
SGC7901/TERT-siRNA-yCDglyTK (group E; pTERT/CD/TK).
 | Table IPlasmids used in this study. |
Table I
Plasmids used in this study.
| Plasmids | Antibiotic
resistance | Characteristics of
the insert | Source |
|---|
| pYr1.1 | Kan/Neo | hU6 promoter,
blank | Yrbio, Changsha,
China |
|
pcDNA3.1(-)CV-yCDglyTK | Amp/Neo | CEA promoter,
yCDglyTK gene | Constructed in our
laboratory |
|
pYr1.1-hTERT-shRNA | Kan/Neo | hU6 promoter,
hTERT-shRNA | Constructed in our
laboratory |
|
pcDNA3.1(-)hTERT-yCDglyTK | Amp/Neo | hU6 promoter,
hTERT-shRNA, CEA promoter, yCDglyTK gene | Constructed in our
laboratory |
Construction of shRNA-directed
hTERT-expressing plasmid
Following a searching for the hTERT mRNA sequence in
GenBank, according to RNAi design software (Integrated DNA
Technologies, Inc., Coralville, IA, USA) and Qian et al
(22), we selected
5′-TGGTGGATGATTTCTTGTT-3′ as the target sequence. We designed a
pair of complementary short hairpin RNA (shRNA). Oligonucleotides
were chemically synthesized by Shanghai Health Industry. The
sequences were as follows: hTERT-shRNA, F,
5′-CACCTGGTGGATGATTTCTTGTTTTCAAGACGAACAAGAAATCATCCACCATTTTTTG-3′
and R,
5′-AGCTCAAAAAATGGTGGATGATTTCTTGTTCGTCTTGAAAACAAGAAATCATCCACCA-3′.
The shRNA template oligonucleotides were cloned to pYr1.1 between
the XhoI and EcoRI restriction sites. The expression
of shRNA was regulated by the U6 promoter. Then we sequenced the
interfering plasmid pYr1.1-hTERT-shRNA for confirmation of the
target sequence.
Construction of a new plasmid
pcDNA3.1(-)hTERT-shRNA-yCDglyTK
shRNA expression cassette of pYr1.1-hTERT-shRNA was
amplified by PCR (containing the U6 promoter). Primer sequences
were as follows: P1, 5′-GCTAGCATCCAAGGTCGGGCAGGA-3′ and P2,
5′-TCTAGAGGTCTCGAGCTCAAAAAATGGT-3′, product was 356 bp. The
conditions of PCR were P1 (10 μM) 0.25 μl, P2 (10 μM) 0.25 μl,
ddH2O 19.75 μl, 10X LA PCR buffer (Mg2+ Plus) 2.5 μl,
DNTPs (2.5 mM) 1 μl, LA Taq polymerase 0.25 μl and template 1 μl.
The thermal cycle profile for PCR was 94°C for 5 min, followed by
30 cycles of 20 sec at 94°C, 25 sec at an annealing temperature of
53°C, 25 sec at 72°C, and an additional 3 min of incubation at 72°C
after completion of the last cycle for extension.
PCR products were analyzed using agarose gel
electrophoresis and stored at 4°C. The plasmid
pcDNA3.1(-)CV-yCDglyTK was digested by NheI and XbaI.
The digestion products were analyzed in agarose gel
electrophoresis. Then, the hTERT-shRNA expression cassette and the
target plasmid pcDNA3.1(-) CV-yCDglyTK linear fragments were
recovered, and the two fragments were connected to form the plasmid
pcDNA3.1(-) hTERT-shRNA-yCDglyTK. The new plasmid was transformed
into competent E. coli DH 5α, then colonies were picked and
plasmids were extracted. We subsequently sequenced the new plasmid
and confirmed that the sequence was correct.
Establishment of stably transfected cell
lines
SGC7901 cells were plated in four 6-well plates at a
density estimated to reach 80% confluence after 24 h. Transfection
was performed using calcium phosphate nanoparticles. Calcium
phosphate nanoparticles were respectively added to the plasmid
pYr1.1 blank, pYr1.1-hTERT-shRNA, pcDNA3.1(-)CV-yCDglyTK and
pcDNA3.1(-)hTERT-shRNA/yCDglyTK. SGC7901 cells were then
transfected with each of the plasmid transfection mixtures. To
select the SGC7901 cells which stably expressed the plasmids, the
cells were treated with 400 μg/ml G418 for 14 days until all the
non-transfected control cells were killed. The cells continued to
be cultured with 200 μg/ml G418, and the medium was replaced every
3 days during the course. At the end of the culture period, the
cells of the different colonies were picked and cultured for 3
weeks. The stably transfected cell lines were then established for
the subsequent studies.
Immunofluorescence assay
All of the SGC7901 cells (transfected and
non-transfected) were plated in 6-well plates until they reached
60% confluence. The plates were washed with phosphate-buffered
saline (PBS), treated with 4% paraformaldehyde for 20 min,
permeabilized with 0.3% Triton for 15 min, and blocked with 1% BSA
for 30 min, and the primary antibodies (mouse anti-human telomerase
reverse transcriptase antibody and goat anti-CD antibody) were
added. Incubation was carried out in a wet box at 4°C overnight.
The plates were washed in PBS again, adding secondary antibodies
(respectively Cy3-labeled anti-mouse and FITC-labeled anti-goat),
and incubation was carried out at 37°C for 1 h, followed by washing
with PBS. Cells were dehydrated and mounted with antifade mounting
media, and observed and photographed under a fluorescence
microscope against time.
Real-time quantitative PCR
The gene mRNA level was analyzed using real-time
quantitative PCR (RT-qPCR). First, the four SGC7901 cell groups
were collected. Total RNA from the cells was extracted using a
TRIzol reagent. The concentration of RNA was determined by
measuring the absorbance at 260 nm using a RNA spectrophotometer
(PerkinElmer, Fremont, USA); the cDNA was synthesized using a
reverse transcriptase kit (MBI Fermentas, Hanover, MD, USA). The
reaction conditions were as follows: cDNA 1 μl, sense 2 μl,
antisense 2 μl, 2X SYBR-Green qPCR mix 25 μl and ultra-pure water
to make up 50 μl. The thermal cycle profile for PCR was 95°C for 3
min, followed by 40 cycles of 30 sec at 95°C, and 40 sec at a
annealing temperature of 60°C. The PCR instrument detected the
fluorescence signal. After the reaction, 2 μl of product were used
in 1.2% agarose gel electrophoresis.
The following specific primers were used: hTERT
primers (expected fragment size 195 bp), sense,
5′-ACACCTGCCGTCTTCACTTC-3′ and antisense,
5′-TAGGGTCCTTCTCAGGGTCT-3′; yCDglyTK primers (expected fragment
size 240 bp), sense, 5′-GGTGTTCCTATTGGCGGATGTCT-3′ and antisense,
5′-ACCGACAACACAGCGTGGAAT-3′; β-actin as internal reference (size
254 bp), sense, 5′-CTGTCTGGCGGCACCACCAT-3′ and antisense,
5′-GCAACTAAGTCATACTCCGC-3′.
Western blot analysis
SGC7901 cells of the different groups were
harvested, washed with PBS, and the cell lysate was cracked for 30
min on ice. The product was centrifuged at 12,000 rpm for 15 min;
the supernatant was extracted for determination of the total
protein. The protein concentration was detected by the BCA method;
40 μg protein and the corresponding volume of 6X loading buffer
were diluted, and the mixture was boiled at 100°C for 5 min. The
protein samples were electrophoresed in 15% SDS-PAGE. The gel
contents were electrotransferred to PVDF membranes, and the
membranes were blocked in 5% skimmed milk for 2 h at room
temperature. Subsequently, the membranes were incubated with
primary antibodies (mouse anti-human telomerase reverse
transcriptase antibody and goat anti-CD antibody) at 4°C overnight,
and with secondary antibodies (anti-mouse and anti-goat) at room
temperature for 2 h, with three washes after incubations. The
immunolabeled proteins were detected by enhanced chemiluminescence
(ECL). An endogenous housekeeping gene and β-actin were also
quantified and used to normalize hTERT and CD.
Detection of 5-FC sensitivity of the
cells
SGC7901 cells of all the groups were plated in
96-well plates, and cultured at 37°C in a 5% CO2 cell
incubator, with the medium replaced daily. Untransfected SGC7901
cells were set as the control group; the other 4 groups were
cultured with 200 μg/ml 5-FC. After a 96-h culture, 20 μl of MTT (5
mg/ml) was added and incubated for 4 h. The supernatant was
absorbed carefully; 150 μl DMSO was added to each well. The cells
were kept away from light for 10 min at room temperature. The
absorbance was measured at 570 nm wavelength (OD570) with a model
680 microtiter plate reader (Bio-Rad, USA). Experiments were
repeated 3 times and each group was set with 5 parallel wells.
Detection of the apoptosis rate in the
cells
SGC7901 cells in each group were collected, washed
with phosphate-buffered saline (PBS), and then mixed with 70%
ethanol at 4°C overnight. After being washed with PBS, 100 μl of
RNase A was added to the cells at 37°C for 30 min, then lucifuged
with 400 μl PI at 4°C for 30 min. Flow cytometry (Beckman Coulter,
Inc., USA) was used for analyzing the cells. The assays were
repeated three times.
Statistical analysis
All data are expressed as the mean ± standard
deviation (SD). Statistical analysis was performed by SPSS 13.0 and
significance was defined as P<0.05.
Results
Construction and confirmation of the
plasmid pcDNA3.1(-) hTERT-shRNA/yCDglyTK
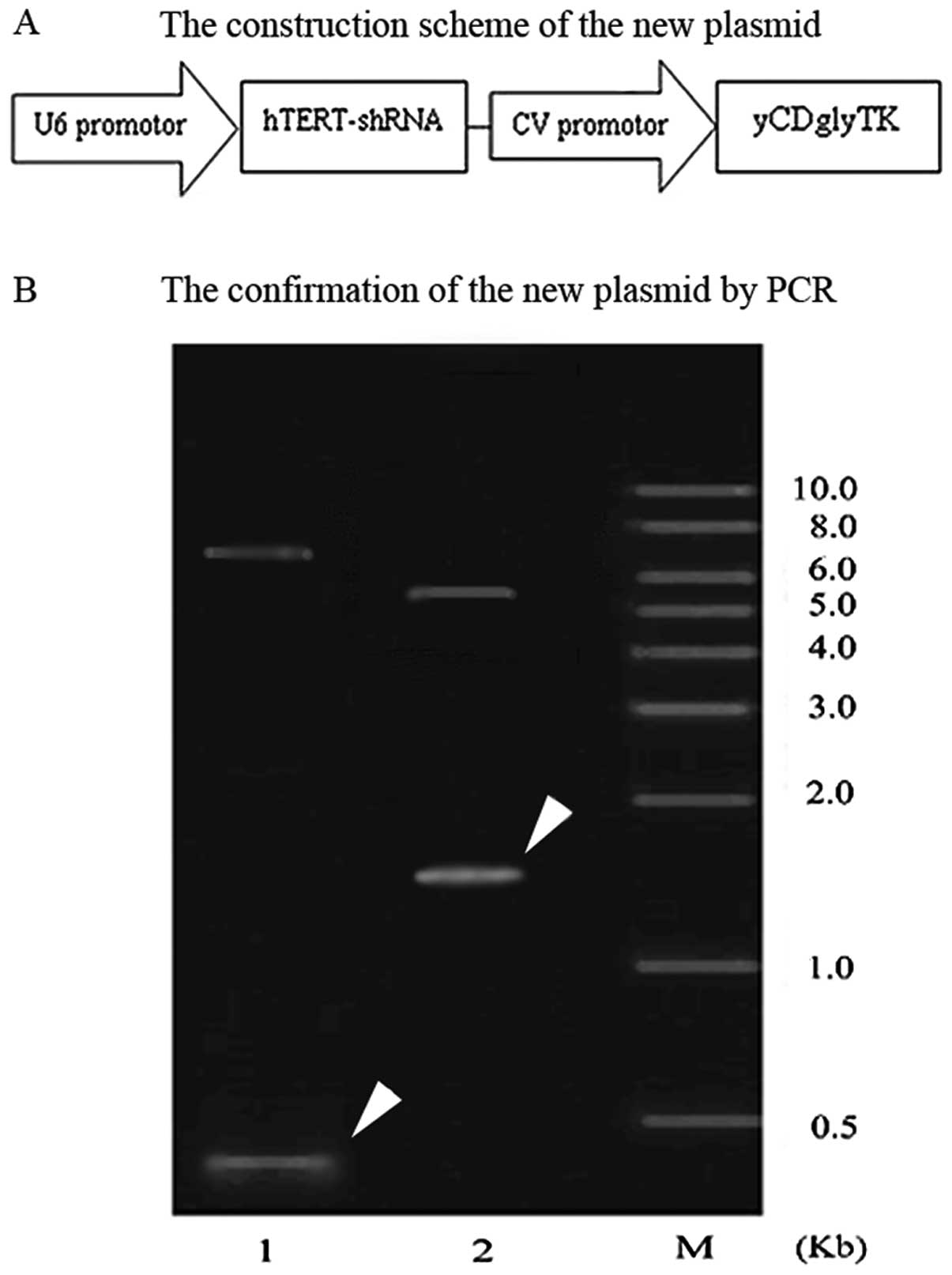
The construction scheme of the expression plasmid
pcDNA3.1(-)hTERT-shRNA/yCDglyTK is shown in Fig. 1A. The shRNA expression cassette was
amplified by PCR using pYr1.1-hTERT-shRNA as a template.
hTERT-shRNA expression cassettes were subcloned to
pcDNA3.1(-)-yCDglyTK, thus constructing the new plasmid
pcDNA3.1(-)hTERT-shRNA/yCDglyTK. The new plasmid was extracted and
confirmed by PCR (Fig. 1B). The
shRNA is shown as a band of ∼356 bp in lane 1, and yCDglyTK as a
1654-bp band in lane 2, confirming that both the exogenous hTERT
interfering shRNA and the fusion suicide gene yCDglyTK were
successfully inserted into the plasmid.
Establishment of stably transfected cell
lines
SGC7901 cells were cultured with G418 following the
procedure stated above. After 7 days, the cells in the control
groups were killed. Three weeks later, clones were formed in the
transfected groups. The cells were continually cultured with G418
culture, and at the end of the culture, only the cells that had
been successfully transfected with the plasmids survived. As a
result, stably transfected cell lines were established and were
further analyzed in the following experiments.
Immunofluorescence assay detecting gene
expression
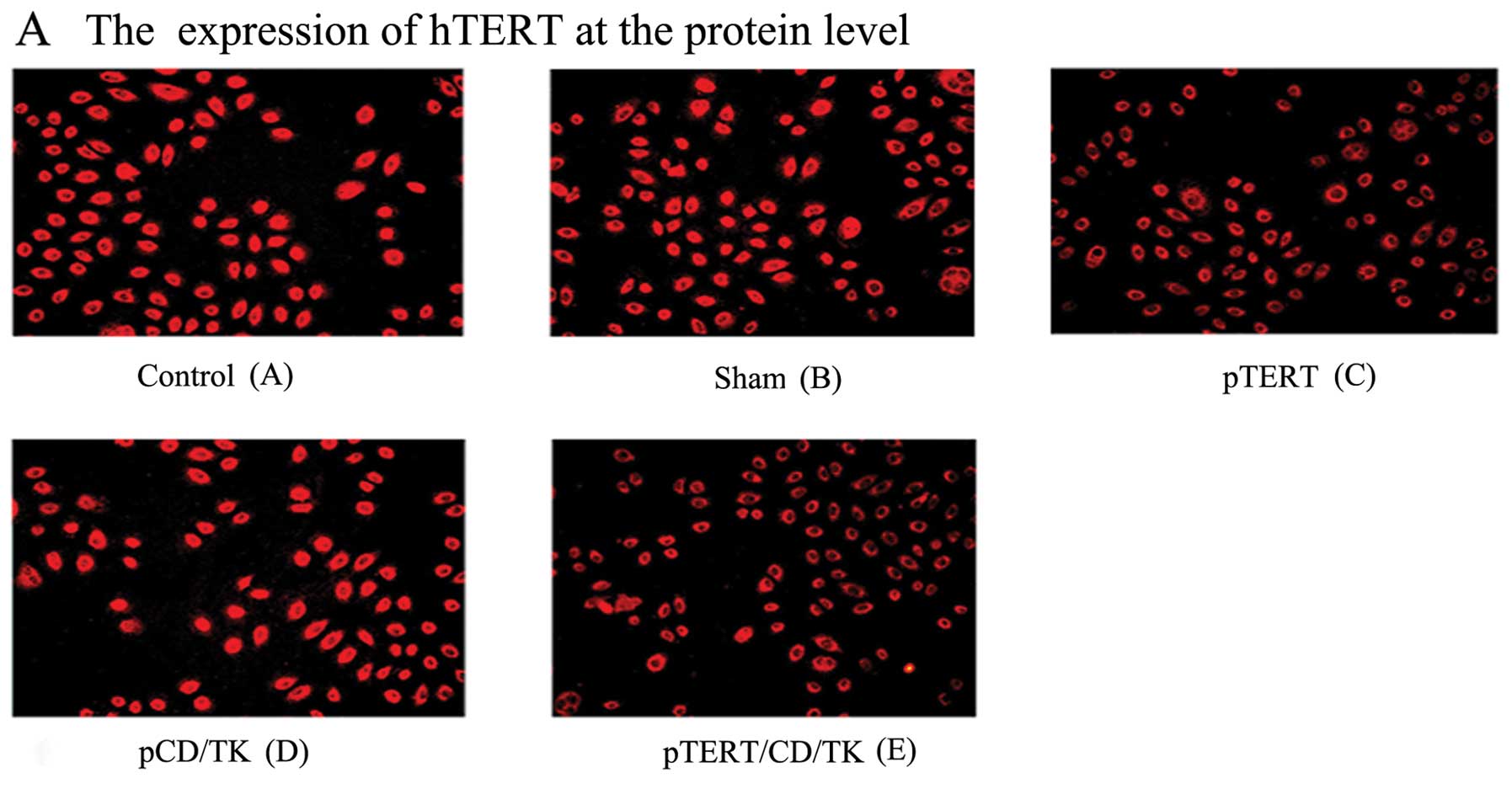
Immunofluorescence detection showed that the
expression of hTERT in the cells of groups C (pTERT) and E
(pTERT/CD/TK) was significantly weaker than in groups A (control),
B (sham) and D (pCD/TK) (Fig. 2A).
Fusion suicide gene CD/TK protein was detected in the cells of
groups D and E; there was no CD/TK expression in the other groups
(Fig. 2B). This demonstrated that
the expression of hTERT in groups C and E was inhibited, and that
CD/TK protein was expressed in groups D and E. The plasmid
pcDNA3.1(-)hTERT-shRNA/yCDglyTK regulated the shRNA hTERT and CD/TK
expression.
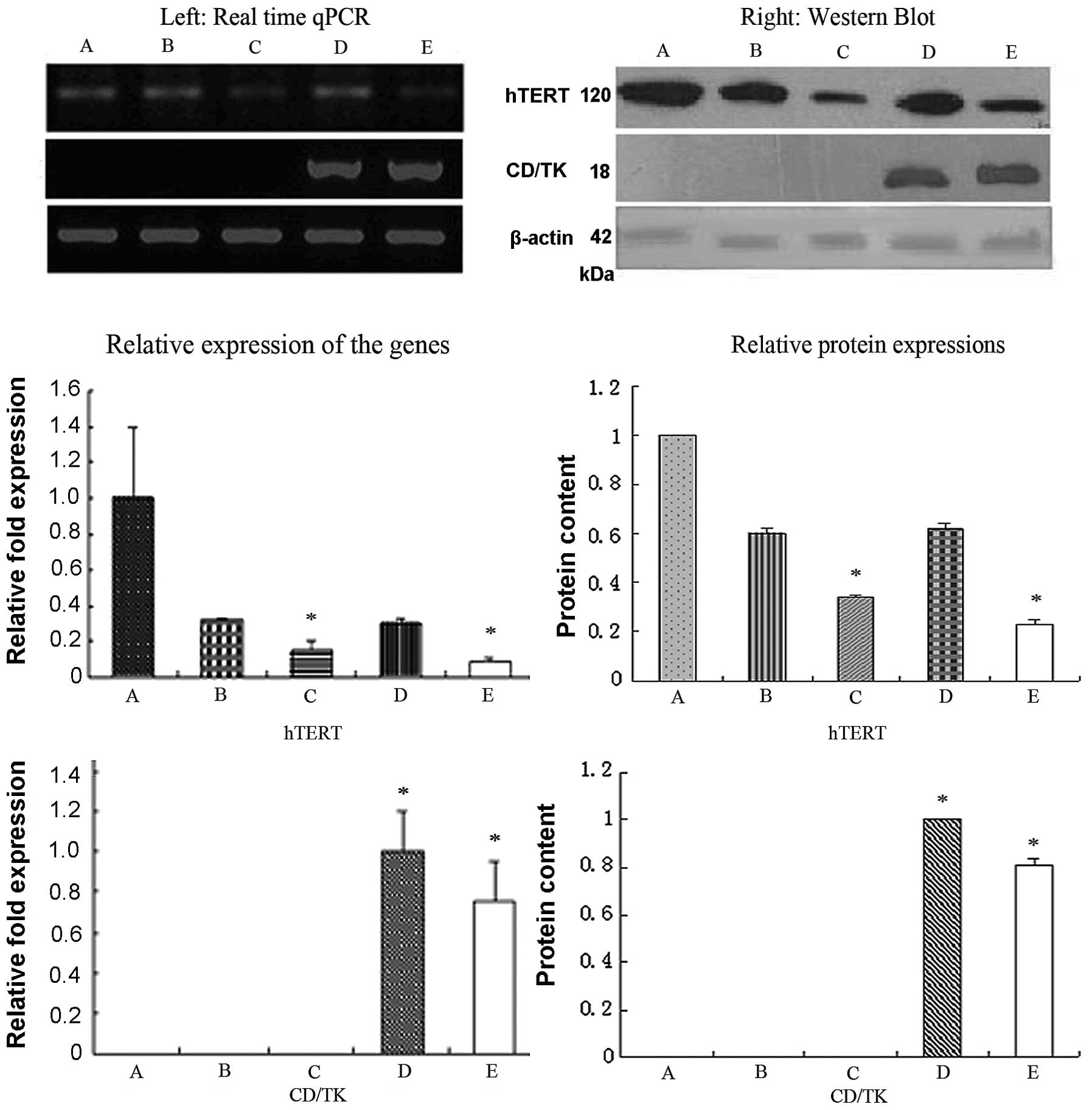
RT-qPCR and western blot analysis
detecting gene expression
RT-qPCR and western blot analysis (Fig. 3) showed that the hTERT expression
at both the mRNA and protein levels was significantly lower in
groups C and E (P<0.01), compared with groups A, B and D. There
were no significant differences between groups C and E (P<0.01).
These results suggest that pYr1.1-hTERT-shRNA and
pcDNA3.1(-)hTERT-shRNA-yCDglyTK inhibited the expression of hTERT.
The expression of fusion suicide gene-CD/TK was also detected by
RT-qPCR and western blot analysis; in groups E and D, the CD/TK
gene was expressed at both the mRNA and protein level, whereas no
expression was observed in the other groups. This suggests that in
the new combined gene plasmid pcDNA3.1(-)hTERT-shRNA/yCDglyTK, the
U6 promoter regulates the shRNA hTERT expression and enhances the
CEA promoter in regulating CD/TK expression.
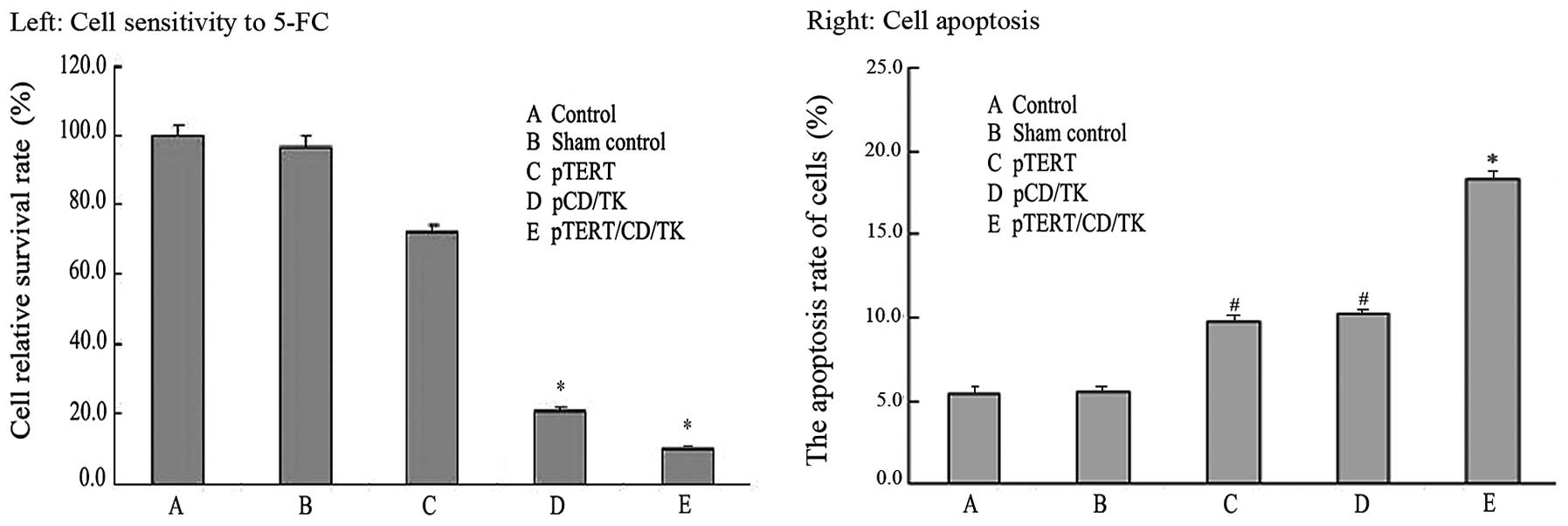
5-FC sensitivity in the cells
The survival rate of the control group was 100%.
With prodrug 5-FC treatment for 96 h, the relative survival rate of
the control group was not significantly decreased, suggesting that
the cell toxicity of 5-FC is limited. The relative survival rate of
group C slightly decreased, suggesting that hTERT expression
inhibited cell proliferation. The cells of groups D and E were
sensitive to 5-FC; their relative survival rates were significantly
lower than that in the control groups (A and B) and group C, with
group E experiencing the greatest decline in survival rate
(P<0.05) (Fig. 4). This
suggests that the newly constructed plasmid pcDNA3.1(-)
hTERT-shRNA/yCDglyTK inhibits cell proliferation more
effectively.
Detection of the apoptosis rate of the
cells in each group
The apoptosis rate of each cell group was measured
by flow cytometry (Fig. 4). The
results showed that the apoptosis rates of groups A and B were
5.45±0.45 and 5.58±0.38%, respectively. The apoptosis rates of
groups C, D and E were 9.76±1.54, 10.34±1.27 and 18.38±1.29%
respectively, which were statistically higher in comparison with
the control groups (P<0.01), indicating that the new plasmid
pcDNA3.1(-)hTERT-shRNA/yCDglyTK induced cell apoptosis more
effectively.
Discussion
In gene therapy for cancer treatment, the key
factors for success lay in finding effective combinations of genes
and efficiently delivering them into tumor cells to regulate
multiple gene expression, since carcinogenesis is a multi-step and
multi-stage process (3). In this
study, a novel plasmid, pcDNA3.1(-)hTERT-shRNA/yCDglyTK, was
constructed successfully and delivered into SGC7901 cells by
calcium phosphate nanoparticles. This new plasmid consists of a
fusion suicide gene yCDglyTK and a hTERT/shRNA gene. Through
comparisons of all the cell groups (including normal and sham
control, single and multiple gene groups), we found that SGC7901
cells, which had been transfected with the plasmid carrying both
hTERT/shRNA and the fusion suicide gene, were the more sensitive to
the prodrug 5-FC. In these cells, the apoptosis rate was increased
and the cell survival rate was decreased as a result of successful
transfection of the new plasmid. The cell survival rate was
slightly decreased in the sham control group, suggesting that the
prodrug 5-FC cytotoxicity in normal gastric cancer was limited.
The fusion suicide gene, yCDglyTK, was constructed
in our previous study (21), where
it was expected to work with two prodrugs, 5-FC and GCV. However,
in that study, we found that this fusion suicide gene demonstrated
a stronger antitumor effect only when used with 5-FC. One
explanation is that the TK/GCV system may reduce the cytotoxicity
of the CD/5-FC system (23), and
this was the reason why we used 5-FC alone in this study.
As hTERT plays an important role in the development
of cellular immortality and oncogenesis, the inhibition of hTERT
expression would be an ideal technique for gene therapy. Many
studies have shown that hTERT gene expression in gastric cancer is
increased, the tumor cell growth is suppressed, and tumor cell
apoptosis is induced by inhibiting hTERT expression using the RNAi
technique (19,20).
In the present study, we combined hTERT-specific
RNAi with the fusion suicide gene to obtain a more effective
antitumor effect. We first built the interference plasmid
pYr1.1-hTERT-shRNA targeting the hTERT gene. Then, the shRNA
expression cassette (including the U6 promoter) was subcloned into
pcDNA3.1(-) CV-yCDglyTK to construct the combined gene plasmid. In
this plasmid, there were two promoters regulating three different
genes: U6 promoter regulating hTERT-shRNA expression, and the
enhanced CEA promoter regulating the expression of the fusion
suicide gene yCDglyTK. This new plasmid was delivered into SGC7901
cells and was verified by the establishment of the stably
transfected cell line in G418 culture, by immunofluorescence assay,
RT-qPCR and western blot analysis. Strong yCDglyTK expression in
SGC7901 cells transfected with this new plasmid was noted, whereas
hTERT expression was significantly downregulated in those
cells.
In conclusion, the plasmid
pcDNA3.1(-)hTERT-shRNA/yCDglyTK, which consists of an hTERT
gene-specific shRNA and a fusion suicide gene yCDglyTK, was
successfully constructed in this study. It was confirmed to have a
synergistic antitumor effect on the SGC7901 human gastric cancer
cell line.
Acknowledgements
This study was partly supported by the
Nature Science Foundation in China.
References
|
1.
|
Szlosarek PW and Dalgleish AG: Potential
applications of gene therapy in the patient with cancer. Drugs
Aging. 17:121–132. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Xu CT and Pan BR: Current status of gene
therapy in gastroenterology. World J Gastroenterol. 4:85–89.
1998.
|
|
3.
|
Deng DJ: Progress of gastric cancer
etiology: N-nitrosamides 1999s. World J Gastroenterol. 6:613–618.
2000.PubMed/NCBI
|
|
4.
|
Robe PA, Princen F, Martin D, Malgrange B,
Stevenaert A, Moonen G, et al: Pharmacological modulation of the
bystander effect in the herpes simplex virus thymidine
kinase/ganciclovir gene therapy system: effects of dibutyryl
adenosine 3′, 5′-cyclic monophosphate, alpha-glycyrrhetinic acid,
and cytosine arabino-side. Biochem Pharmacol. 60:241–249.
2000.PubMed/NCBI
|
|
5.
|
Noordhuis P, Holwerda U, Van der Wilt CL,
Van Groeningen CJ, Smid K, Meijer S, et al: 5-Fluorouracil
incorporation into RNA and DNA in relation to thymidylate synthase
inhibition of human colorectal cancers. Ann Oncol. 15:1025–1032.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
DeFatta RJ, Li Y and De Benedetti A:
Selective killing of cancer cells based on translational control of
suicide gene. Cancer Gene Ther. 9:573–578. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Binley K, Askham Z, Martin L, Spearman H,
Day D, Kingsman S and Naylor S: Hypoxia-mediated tumour targeting.
Gene Ther. 10:540–549. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Fumoto S, Nishi J and Nakamura J: Gene
therapy for gastric diseases. Curr Gene Ther. 8:187–200. 2008.
View Article : Google Scholar
|
|
9.
|
Nakaya H, Ishizu A, Ikeda H, et al: In
vitro model of suicide gene therapy for alpha-fetoprotein-producing
gastric cancer. Anticancer Res. 23:3795–3800. 2003.PubMed/NCBI
|
|
10.
|
Isomoto H, Ohtsuru A, Braiden V, et al:
Heat-directed suicide gene therapy mediated by heat shock protein
promoter for gastric cancer. Oncol Rep. 15:629–635. 2006.PubMed/NCBI
|
|
11.
|
Hamstra DA, Rice DJ, Fahmy S, et al:
Enzyme/prodrug therapy for head and neck cancer using a
catalytically superior cytosine deaminase. Hum Gene Ther.
10:1993–2003. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Liu T, Zhang GY, Chen YH, et al:
Tissue-specific expression of suicide genes delivered by
nanoparticles inhibits gastric carcinoma growth. Cancer Biol Ther.
5:3379–3387. 2006.PubMed/NCBI
|
|
13.
|
Holt SE and Shay JW: Role of telomerase in
cellular proliferation and cancer. J Cell Physiol. 180:10–18. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Granger MP, Wright WE and Shay JW:
Telomerase in cancer and aging. Crit Rev Oncol Hematol. 41:29–40.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Shay JW and Bacchetti S: A survey of
telomerase activity in human cancer. Eur J Cancer. 33:787–791.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kim NW: Clinical implications of
telomerase in cancer. Eur J Cancer. 33:781–786. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Helder MN, Jong S, Vries EG and Zee AG:
Telomerase targeting in cancer treatment: new developments. Drug
Resist Updat. 2:104–115. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Stewart SA and Weinberg RA: Telomerase and
human tumorigenesis. Semin Cancer Biol. 10:399–406. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Henson JD, Neumann AA, Yeager TR and
Reddel RR: Alternative lengthening of telomeres in mammalian cells.
Oncogene. 21:598–610. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Kakeji Y, Machara Y, Koga T, et al:
Gastric cancer with high telomerase activity shows rapid
development and invasiveness. Oncol Rep. 8:107–110. 2001.PubMed/NCBI
|
|
21.
|
Zhang GY, Liu T, Chen YX, et al: Tissue
specific cytotoxicity of colon cancer cells mediated by
nanoparticle-delivered suicide gene in vitro and in vivo. Clin
Cancer Res. 15:201–207. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Qian XB, Cheng J, Chen A, et al: Long-term
effects of short hairpin RNA-targeted human telomerase reverse
transcriptase on suppression of SGC7901 cell proliferation by
inhibition of telomerase activity. Oncol Rep. 19:575–581.
2008.PubMed/NCBI
|
|
23.
|
Rogulski KR, Kim JH, Kim SH and Freytag
SO: Glioma cells transduced with an Escherichia coli
CD/HSV-1 TK fusion gene exhibit enhanced metabolic suicide and
radiosensitivity. Hum Gene Ther. 8:73–85. 1997.PubMed/NCBI
|