Introduction
In 1992, Reynolds and Weiss (1) for the first time separated neural
stem cells (NSCs) from the subventricular zone (SVZ) of mice. Since
then, NSCs with the potential for self-renew and differentiation
into neurons, astrocytes and oligodendrocytes have been identified
in the subgranular zone (SGZ) of adult rats, non-human primates and
humans. These residual NSCs live in a special microenvironment and
are crucial for the maintenance of neurogenesis in the brain of
adults (2). The physiological
functions of adult NSCs are affected by genes, growth factors,
neurotransmitters, stress, steroids, age and environment.
Regulation of intracranial adult NSCs may promote the proliferation
and differentiation of NSCs and the migration of NSCs into target
sites, which may be beneficial for the repair of damaged neurons
and the subsequent improvement of neurological diseases. At
present, studies are increasing their focus on the role of genes in
the regulation of NSCs. To obtain a promoter that can regulate the
expression of exogenous genes in NSCs is critical for gene
regulation. Nestin is specifically expressed in NSCs (3). However, the promoter has the
potential of only non-specific regulation (4). The intron-2 of the nestin gene is a
promoter specific to neural precursor cells in the nervous system
(5). In the present study, the
promoter sequence of the nestin gene was fused with intron-2 and
the regulatory potential of the fusion gene in NSCs was
investigated.
Materials and methods
Main reagents and instruments
Saturated chloroform, proteinase K, primers,
restriction enzymes (HindIII, NotI, BamHI,
MluI), T4 DNA ligase reaction mixture (Shanghai Sangon
Biological Engineering Technology and Services Co, Ltd., Shanghai,
China), PrimeSTAR™HS DNA polymerase (Takara Bio, Inc.),
EndoFree Plasmid Maxi kit, QIAquick gel extraction kit (Qiagen), SV
Minipreps DNA purification system (Promega),
Lipofectamine™ 2000 reagent (Invitrogen), DMEM,
RPMI-1640 (Gibco-BRL), calf serum (Hangzhou Sijiqing Biological
Engineering Materials Co., Ltd.), COS-7 cells, C6 cells (Cell Bank
of Chinese Academy of Sciences, Shanghai Institute of Cell Biology,
Shanghai, China), MEF cells, plasmids (pEGFP-N1 and pcDNA3) and
DH5a were used in the present study. TC2323 CO2
incubator (Sheldon Manufacturing, Inc., USA), IX70-S8F23 inverted
phase contrast microscope (Olympus, Japan), PTC-100 PCR instrument
(MJ Research, Inc., USA), BH2-RFL-3T fluorescence microscope
(Olympus, Japan) and BD FACalibur flow cytometer (BD Biosciences,
USA) were also used in the present study.
Primer design
The primers were designed according to the promoter
sequence of the nestin gene in GenBank. NF1 and NR were primers for
the full sequence of the nestin promoter (4,000 bp), and NF2 and NR
for the core sequence of the nestin promoter (390 bp): NF1,
5′-CATACGCGTGAGCTCCCT AAACCTATCCCC-3′ (MluI); NR,
5′-AGCAAGCTTAA GCGGACGTGGAGCACTAG-3′ (HindIII); NF2,
5′-CATACG CGTTCCCTGAGACCTGCCTGATC-3′ (MluI). The primers for
intron-2 of the nestin gene were designed according to the sequence
of nestin in the Genbank. The anticipated length of intron-2
following amplification was 1680 bp: HF, CTAGGA
TCCGTACACAGTACTGACTGTCTCCTTG (BamHl); HR,
CATACGCGTGTTGCATGTCCTGCCACTGCAGGATC (MluI).
Amplification, retrieval and sequencing
of the target genes
DNA was extracted as template DNA from the tails of
specific pathogen-free C57BL/6J mice (SCXK[Jiangzu]2010-0003) by
using the phenol-chloroform extraction method. The PrimeSTAR HS DNA
polymerase was used in the PCR assay for the amplification of the
full sequence and core sequence of the nestin promoter and intron-2
of the nestin gene. The PCR products were subjected to 1% agarose
(containing 0.1 μg/ml ethidium bromide) electrophoresis, and
the bands were visualized under a gel analysis system. Qiagen gel
retrieval kit was used for the retrieval of products. The products
were sequenced by Shanghai Sangong Pharmaceutical Co., Ltd.
Construction of the recombinant
expression vector
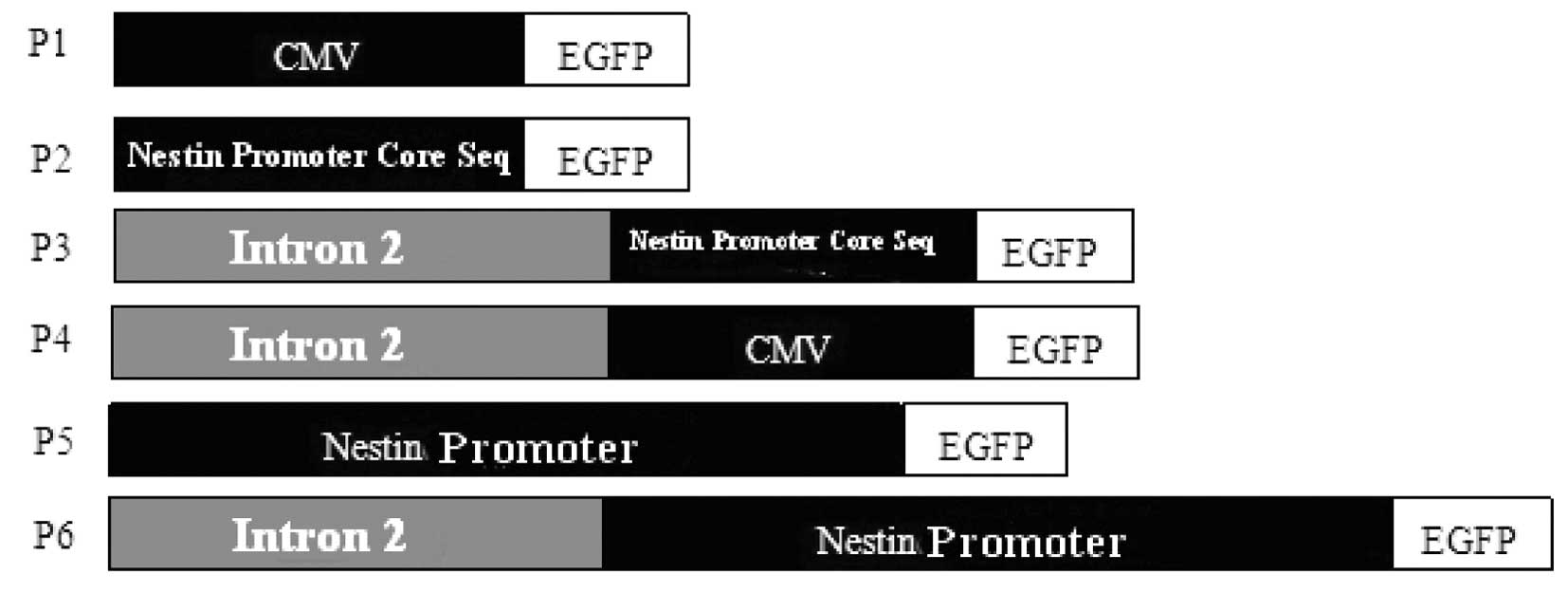
pcDNA3.1 was used as a template for the construction
of recombinant plasmids which are shown in Fig. 1.
P1 plasmid. The EGFP fragment was collected
from the pEGFP-N1 plasmid after digestion with HindIII and
NotI. At the same time, the pcDNA3.1 plasmid was digested
with HindIII and NotI. The EGFP was ligated to the
fragments from pcDNA3.1 by using T4 DNA ligase forming pcDNA3-EGFP
which was used for genetic transformation. Plasmid extraction was
performed with the Minipreps DNA purification system and the
products were identified by HindIII and NotI.
P2 plasmid. The P1 plasmid was digested with
HindIII and MluI and the band at 5,500 bp was
retrieved. At the same time, the core sequence of the nestin
promoter was collected following digestion with HindIII and
MluI. The collected fragments were ligated followed by
transformation. Plasmid extraction was performed with the Minipreps
DNA purification system and the products were identified by
HindIII and MluI.
P3 plasmid. The P1 plasmid was digested with
BglII and MluI and the band at 5,700 bp was
retrieved. At the same time, the intron-2 of the nestin gene was
collected following digestion with BamHl and MluI.
The collected fragments were ligated followed by transformation.
Plasmid extraction was performed with the Minipreps DNA
purification system and PCR was performed for the amplification of
intron-2. The products were identified by MluI.
P4 plasmid. The P2 plasmid was digested with
BglII and MluI and the band at 6,000 bp was
retrieved. At the same time, the intron-2 of the nestin gene was
collected following digestion with BamHl and MluI.
The collected fragments were ligated followed by transformation.
Plasmid extraction was performed with the Minipreps DNA
purification system and PCR was performed for the amplification of
intron-2. The products were identified by MluI.
P5 plasmid. The nestin gene was digested with
HindIII and MluI and then collected. The P1 plasmid
was digested with two restriction enzymes and the band at 5,800 bp
was retrieved. The collected fragments were ligated followed by
transformation. Plasmid extraction was performed with the Minipreps
DNA purification system and the products were identified by
HindIII and MluI.
P6 plasmid. The nestin gene was digested with
HindIII and MluI and then collected. The P4 plasmid
was digested with two restriction enzymes and the band at 5,800 bp
was retrieved. The collected fragments were ligated followed by
transformation. Plasmid extraction was performed with the Minipreps
DNA purification system and the products were identified by
HindIII and MluI.
Identification of the transfected cells
by fluorescence staining
C6, COS-7 and MEF cells were used for transfection.
In brief, the cells were washed in 0.01 mol/l PBS (pH 7.4) thrice
(5 min for each) and then fixed in 4% paraformaldehyde at 4°C for
20 min. Then, the solution was removed and the cells were dried for
0.5 h in air. After washing in 0.01 mol/l PBS (pH 7.4) thrice (5
min for each), cells were blocked in 5% normal goat serum
containing 0.1% Triton X-100 for 1 h at room temperature and then
with an antibody against nestin (1:1,000) at 4°C overnight. After
being kept at room temperature for 0.5 h, cells were washed in 0.01
mol/l PBS (pH 7.4) thrice (5 min for each) and then treated with
fluorescence-conjugated secondary antibody (1:200) at 4°C
overnight. After being kept at room temperature for 0.5 h, cells
were washed in 0.01 mol/l PBS (pH 7.4) thrice (5 min for each) and
observed under a fluorescence microscope.
Expression of the target gene in cells
transfected with recombinant plasmid
Six different recombinant plasmids were prepared
with the EndoFree Plasmid Maxi kit and then used to transfect MEF,
C6 and COS-7 cells, independently, by using
Lipofectamine™ 2000 reagent. Transfection was performed
in triplicate (6-well plate) 4 times. Forty-eight hours after
transfection, representative images were captured under a
fluorescence microscope. Then, cells were washed in PBS twice (3
min for each) and then digested in trypsin followed by cell
collection through centrifugation at 1,200 rpm for 5 min. The cells
in each well were diluted with 400 μl of PBS and subjected
to flow cytometry for the analysis of EGFP-positive cells.
Statistical analysis
Statistical analysis was carried out with SPSS. Data
were expressed as mean ± SD. One-way analysis of variance (ANOVA)
was employed to analyze the intra-group difference and least
significant difference test to analyze the inter-group difference.
A value of P<0.05 was considered statistically significant.
Results
PCR amplification
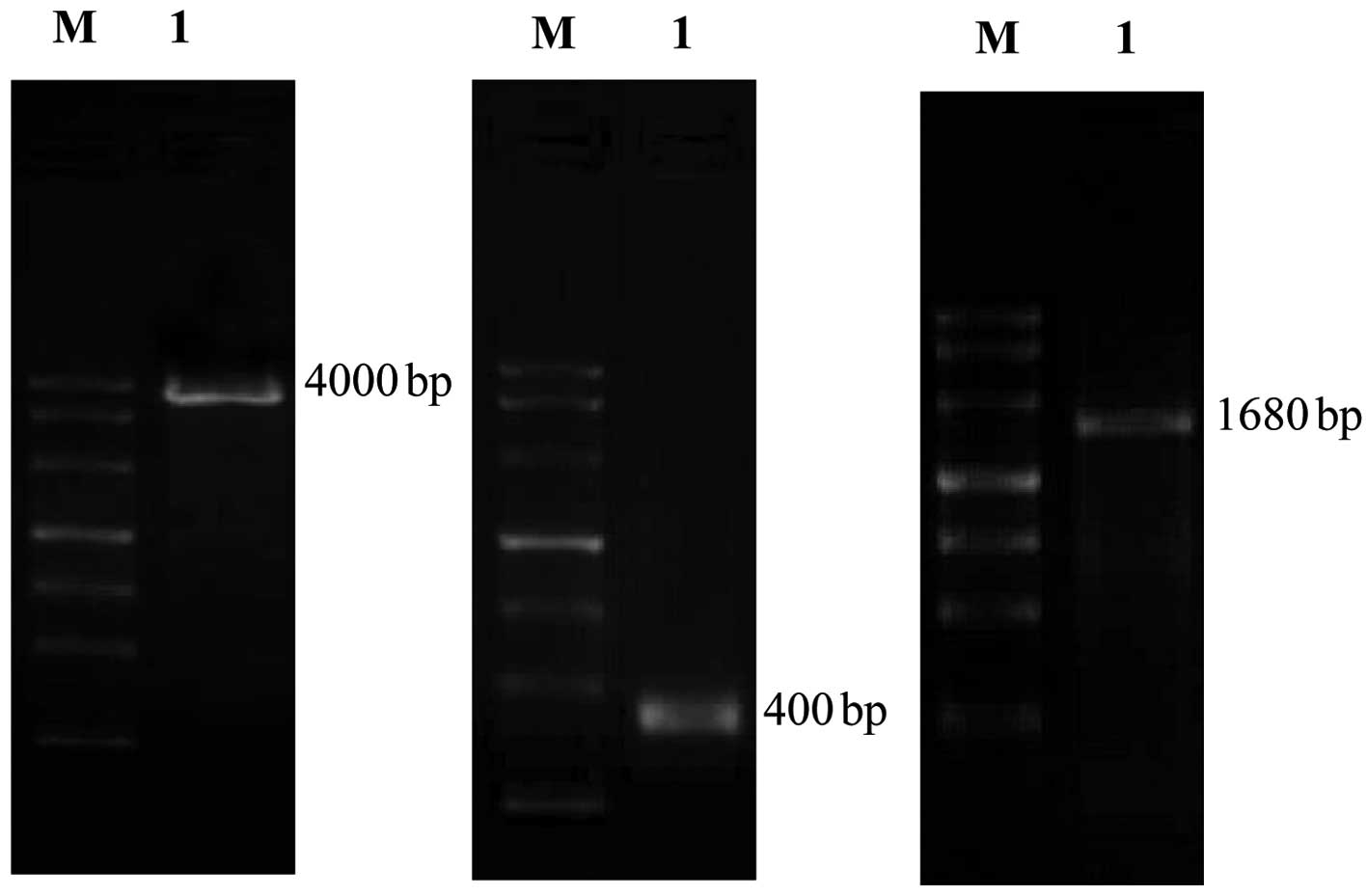
Following amplification of the core and full
sequence of the nestin promoter by PCR, the products were
identified at 4,000 and 400 bp and then retrieved for sequencing
(Fig. 2). Results showed that the
size and base composition of these products were identical to those
published in GenBank. The products of PCR assay were identified at
1,680 bp and then retrieved for sequencing (Fig. 2). Results showed that the size and
base composition of these products were identical to those
published in GenBank.
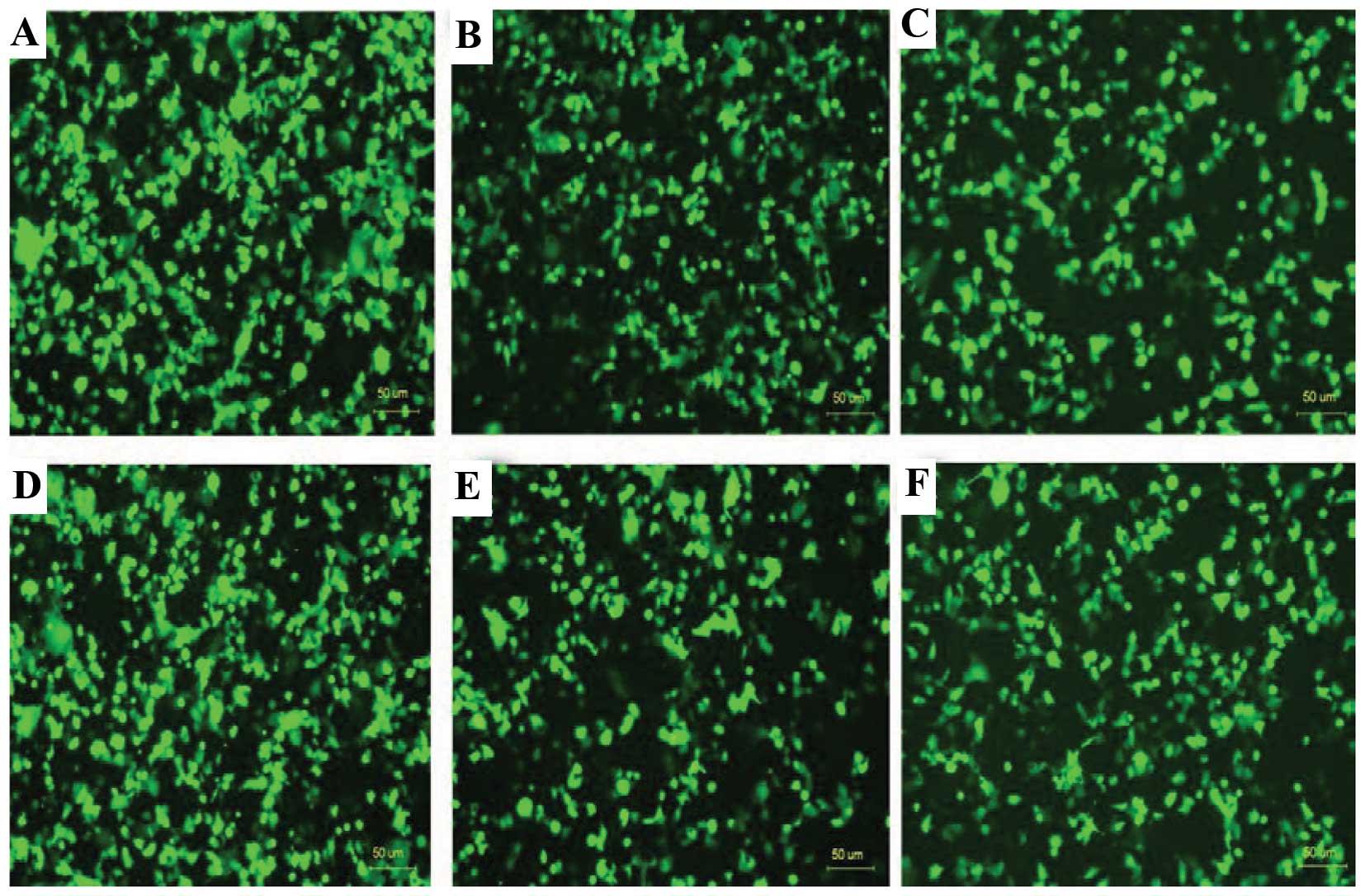
Expression of EGFP in the COS-7 cells
transfected with the different recombinant plasmids
Six different recombinant plasmids were used to
transfect COS-7 cells independently. The promoters in the six
recombinant plasmids regulated the EGFP expression in COS-7 cells
(Fig. 3). Flow cytometry showed
that the expression rate of EGFP in COS-7 cells transfected with
P1, P2, P3, P4, P5 and P6 plasmids to be 86.3±1.5, 82.1±2.4,
78.0±3.2, 85.3±4.1, 79.6±4.5 and 78.3±4.6%, respectively, showing
no significant difference.
Expression of EGFP in the C6 cells
transfected with the different recombinant plasmids
Six different recombinant plasmids were used to
transfect C6 cells independently. The promoters in the six
recombinant plasmids regulated the EGFP expression in COS-7 cells.
Flow cytometry showed that the expression rate of EGFP in COS-7
cells transfected with P1, P2, P3, P4, P5 and P6 plasmids to be
37.5±1.1, 23.1±1.6, 17.1±1.5, 26.0±1.4, 18.3±1.5 and 17.8±1.3%,
respectively. The EGFP expression in the P3 group was significantly
lower than that in the P1 and P4 groups, but there was no marked
difference among the P2, P3, P5 and P6 groups.
Expression of EGFP in the MEF cells
transfected with different recombinant plasmids
Six different recombinant plasmids were used to
transfect MEF cells independently. The promoters in six recombinant
plasmids regulated EGFP expression in the MEF cells. Flow cytometry
showed the expression rate of EGFP in the MEF cells transfected
with P1, P2, P3, P4, P5 and P6 plasmids to be 4.4±0.3, 3.9±0.4,
0.1±0.5, 3.9±0.4, 3.7±0.6 and 0.2±0.4%, respectively. The EGFP
expression in the P3 and P6 groups was significantly lower than
that in the remaining groups.
Discussion
Studies on the regulation of gene expression may
provide clues for the investigation of gene modification. The
regulation of gene expression can occur at different levels
including replication, amplification, gene activation,
transcription, post-transcription, translation and
post-translation. The initiation of transcription is a basic
control point in the regulation of gene expression. Thus, the
promoter of the target gene is critical for the regulation of gene
expression and particularly the regulation of transcription
(6).
Eukaryotic genes include a core element and an
upstream promoter element (7). In
the core sequence, the TATA box mediates the formation of
transcription-initiation complexes involving RNA polymerase II (Pol
II) and the initiation of basic transcription. It also mediates the
regulation of downstream elements and determines the site where
transcription starts. Based on the analysis using TFSEARCH
software, the promoter of the cloned nestin gene has the TATA-like
box. The functions of promoters of eukaryotic genes depend on not
only the TATA box, but also on one or more upstream regulation
elements (UPE). The core sequence of the nestin promoter has the
binding sites of basic regulation elements including GATA-1,
GATA-3, AP1, AP3 and SP1, which assures that the core sequence of
the nestin promoter has the characteristics of the promoter of
eukaryotic genes (8).
Results showed that the promoter of the nestin gene
had the regulation potential in nestin-positive and nestin-negative
cells. To investigate the possibility of cell-specific regulation
elements in the 5′ upstream of the mouse nestin gene, we prepared
the full sequence (4,000 bp) and core sequence of the nestin
promoter (400 bp) as promoters to regulate GEFP expression in
different cells. Results showed that the two promoters had
non-specific regulations and the activities of the promoters were
comparable with that of the CMV promoter. Therefore, we speculated
that there were no cell-specific regulation elements in the
promoter of the nestin gene. Our results also demonstrated that the
full sequence and core sequence of the mouse nestin promoter had
the activity of transcription initiation in different mammalian
cell lines and could regulate the expression of reporter genes or
exogenous genes in different cell lines.
The intron-2 of the mouse nestin gene was 1,686 bp
in length and has 51.4 and 81.1% homology to the intron-2 of human
and rat nestin genes, respectively (http://seqtool.sdsc.edu). Josephson et al
(9) confirmed that the POU site in
the intron-2 of the rat nestin gene was essential for the normal
expression of the nestin gene in the whole central nervous system
(CNS), and that the HRE/RXR/TTF1 sites were only found in a few
regions of the CNS including the forebrain and dorsal
mesencephalon. Tanaka et al (10) found that Sox could coordinate with
transcription factors in the POU family to regulate nestin
expression during neurogenesis. This coordination occurred between
class III, POU factors in Brn2 and SoxB1 subfamily (Sox1, Sox2 and
Sox3) and Sox11, as well as between Sox2, class III POU factors
(Brn1, Brn2, Brn4 and Oct6) and class V POU factor Oct4. The site
1,387–1,396 bp of intron-2 of the mouse nestin gene is a binding
site of transcription factors in the Sox family, the site of
1,402–1,410 bp is a binding site of POU factors and the site of
1,435–1,443 bp is a binding site of HRE/RXR/TTF1. Results showed
that the intron-2 of the mouse nestin which was fused with the
promoter of the nestin gene specifically regulated EGFP expression
in nestin-positive cells, while intron-2 which was fused with the
promoter CMV had no specific regulation. These findings suggest
that, in NSCs, the promoter of the nestin gene can coordinate with
intron-2 of the nestin gene to specifically regulate protein
expression in NSCs.
Our findings confirm that the promoter of the nestin
gene can coordinate with intron-2 of the nestin gene to regulate
the expression of exogenous genes in nestin-positive cells. In the
CNS, nestin is a NSC-specific protein. The promoter can coordinate
with intron-2 to regulate the expression of exogenous genes in
NSCs. Our findings may provide evidence for studies on the gene
regulation as well as the targeting and positioning of drugs in
NSCs.
Acknowledgements
The study was supported by Natural
Science Foundation of Jiangsu Province (BK2010180); Social
Development Foundation for Xuzhou City (XM09B116); Superintendent
Foundation of Xuzhou Medical College (2010KJ10).
References
|
1.
|
Reynolds BA and Weiss S: Generation of
neurons and astrocytes from isolated cells of the adult mammalian
central nervous system. Science. 255:1707–1710. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Eriksson PS, Perfilieva E, Björk-Eriksson
T, Alborn AM, Nordborg C, Peterson DA and Gage FH: Neurogenesis in
the adult human hippocampus. Nat Med. 4:1313–1317. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Nakano T: Establishment maintenance and
differentiation induction of embryonic stem cells. Nihon Rinsho.
61:385–389. 2003.PubMed/NCBI
|
|
4.
|
Cheng L, Jin Z, Liu L, Yan Y, Li T, Zhu X
and Jing N: Characterization and promoter analysis of the mouse
nestin gene. FEBS Lett. 565:195–202. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lothian C and Lendahl U: An evolutionarily
conserved region in the second intron of the human nestin gene
directs gene expression to CNS progenitor cells and to early neural
crest cells. Eur J Neurosci. 9:452–462. 1997. View Article : Google Scholar
|
|
6.
|
Trinklein ND, Aledred SJ, Saldanha AJ and
Myers RM: Identification and functional analysis of human
transcriptional promoters. Genome Res. 13:308–312. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Sun HX, Lu DX and Liu FP: Theory and
Application of Transgenic Technology Henan Medical. University
Press; Zhengzhou: pp. 2342000
|
|
8.
|
Diamond MI, Miner JN, Yoshinaga SK and
Yamamoto KR: Transcription factor interactions: selectors of
positive or negative regulation from a single DNA element. Science.
249:1266–1272. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Josephson R, Müller T, Pickel J, Okabe S,
Reynolds K, Turner PA, Zimmer A and McKay RD: POU transcription
factors control expression of CNS stem cell-specific genes.
Development. 125:3087–3100. 1998.PubMed/NCBI
|
|
10.
|
Tanaka S, Kamachi Y, Tanouchi A, Hamada H,
Jing N and Kondoh H: Interplay of SOX and POU factors in regulation
of the nestin gene in neural primordial cells. Mol Cell Biol.
24:8834–8846. 2004. View Article : Google Scholar : PubMed/NCBI
|