Introduction
Various types of chronic liver fibrosis exhibit a
common pathological process of liver disease. There is no specific
treatment for liver fibrosis, and its end stage is liver cirrhosis.
Administration of traditional treatments to attenuate the
degradation process is challenging, since the mechanisms involved
in liver fibrosis activate hepatic stellate cells (HSCs). Activated
HSCs express α-actin (α-smooth muscle actin, α-SMA). The synthesis
of large numbers of cells in the extracellular matrix (ECM) and
collagen plays a key role in liver fibrosis (1). Inhibition of HSC activation or
promotion of the induction of apoptosis reduces the secretion of
ECM, and collagen synthesis is the key to the prevention and
treatment of liver fibrosis (2).
Bone marrow mesenchymal stem cells (BMSCs) are classified as being
beyond non-hematopoietic stem cells in the bone marrow
hematopoietic stem cells. Studies have shown that BMSCs possess
differentiation potential (3–5), and
that these cells may act as valuable cell sources for stem cell
transplantation. Studies have also shown that MSCs effectively
repair various types of liver injury, inhibit ECM deposition and
reduce the degree of liver fibrosis (6,7).
In eukaryotic cells, the material bases of cell
cycle regulation are the cell cycle proteins (cyclins),
cyclin-dependent kinases (CDKs), cyclin-dependent kinase inhibitors
(CKIs), and other intermediate factors. As a CKI member of the
group, P27 plays a key regulating role in cell proliferation
(8,9) and cell cycle G1/S phase transition
(10). RhoA is a member of the
RhoGTP kinase family, which regulates cytoskeletal dynamics, gene
transcription, cell cycle progression, and cell transformation.
RhoA and P27 are closely linked. RhoA activation may reduce P27
expression levels (11). MSCs
co-cultured with HSCs may significantly inhibit the proliferation
of the latter (12,13), although the specific mechanism is
unclear. The aims of the present study were to observe the effect
of MSCs on RhoA signaling factors, cell cycle protein kinase cyclin
D1, and cell cycle inhibitor P27 expression, and to investigate the
mechanism of MSCs in inhibiting HSC proliferation.
Materials and methods
Cells and animals
Six healthy Sprague-Dawley (SD) rats, 6–8 weeks old,
were obtained from the Experimental Animal Center, Guangxi Medical
University, Nanning, Guangxi, China. The HSC-T6 and fibroblast cell
lines were purchased from the Cancer Cell Bank of the Affiliated
Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China.
MSC isolation, culture, and functional
identification
According to a previously used method (12), 12 SD rat femur bone marrow cells
were isolated under sterile conditions, cultured at 37°C, and
incubated under 5% CO2. The MSCs were purified by
passage, and cell morphology was observed under microscopy. Passage
4 cells were digested by 2.5 g/l trypsin, and the cell
concentration was adjusted to 2x105/cm2. The
cells were inoculated in 50-ml disposable culture flasks. Then, 20
μg/l (final concentration) HGF was added to the cells to induce
MSCs. The solution was then cultured at 37°C and incubated under 5%
CO2. The medium was changed once every 3 days and
continuously cultured for 14 days. Cell morphology was observed
under inverted phase contrast microscopy.
HSC-T6 culture, passage, and activation
identification
Rat HSC-T6 was cultured in an L-DMEM medium
containing 100 ml/l fetal calf serum at 37°C in a 5% CO2
incubator. The cells grew at 8 h, and 80–90% of the cells adhered
to the bottom of the bottle after 2–3 days for passage. The active
3rd–4th generation cells were used in the experiments. The α-SMA
expression was examined using immunohistochemistry. The
morphological changes of the living cells were observed under
inverted phase contrast microscopy.
Cell co-culture
According to a previously described method (14,15),
the MSCs or fibroblasts were inoculated in a semi-permeable
membrane (transwell insert) in the upper part (2x105
cells/well) of a cell culture 6-well plastic box. The HSC-T6 cells
were inoculated in the lower part (2x105 cells/well) to
establish the upper and lower double-cell co-culture system. The
experiments were divided as follows: i) control group, HSCs
cultured alone (only the upper layer contained the medium); ii)
negative control group, HSCs cultured with fibro-blasts; iii) MSC
experimental group, MSCs cultured with HSCs. The three groups were
observed at 0, 6, 12, 24, 48, and 72 h. The dynamic morphology of
the living cells was observed through an inverted phase contrast
microscope.
HSC proliferation rate
After each period of co-culture, the adherent cells
were digested with 2.5 g/l trypsin. The cell concentration was
adjusted to 2x105 cells/ml and mixed thoroughly. Then,
100 μl of these cells was added into each well of 96-well plates,
followed by the addition of 10 μl CCK-8 solution. The solution was
incubated for 1 h and then examined at 450 nm. The mean value was
obtained.
Cell cycle detection
The MSCs and HSCs were co-cultured at
2x105 cells/well. Cells were obtained at different
intervals, and adherent cells were digested by trypsin, washed with
PBS and fixed with 70% pre-cooled ethanol at 4°C overnight. An
equal amount of PBS was added twice for washing. Up to 100 μl RNase
A was added at 37°C for 30 min, followed by the addition of
propidium iodide at 4°C in the dark for 30 min. Cell cycle was
analyzed by flow cytometry using the MCYCLE software (Beckman, New
York, NY, USA).
RNA extraction and RT-PCR
The HSCs were collected and counted at each period.
TRIzol was added to extract the total RNA according to the
instructions in the kit. The target gene was amplified according to
the following conditions: 95°C pre-denaturation for 5 min, 95°C
denaturation for 45 sec, 55°C annealing for 45 sec, 72°C for 1 min
for 35 cycles, and 72°C for 5 min. GAPDH was used as an internal
reference. The primers used were as follows: RhoA upstream,
5′-TGGTGA TGGAGCTTGTGGTAAG-3′; downstream, 5′-AACATCAGT
GTCTGGGTAGGAG-3′; P27 upstream, 5′-TGCAACCGA CGATTCTTCTACTCAA-3′;
downstream, 5′-CAAGCAGTG ATGTATCTGATAAACAAGGA-3′; cyclin D1
upstream, 5′-TGTTCGTGGCCTCTAAGATG-3′; downstream, 5′-ACT
CCAGAAGGGCTTCAATC-3′; and GAPDH upstream,
5′-GCCAGTAGACTCCACGACAT-3′; downstream, 5′-GCA AGTTCAACGGCACAG-3′.
A total of 6 μl PCR products was examined using 1.7% agarose gel
electrophoresis and scanned under a gel image analysis system to
observe the gray ratio of the target gene/GAPDH, representing the
target gene mRNA levels.
Western blot analysis
The HSC total proteins from each period were
extracted with cell lysate. Protein concentration was determined
using the Coomassie brilliant blue colori-metric method. Proteins
(80 μg) were run on 15% SDS-PAGE gel electrophoresis, transferred
onto the PVDF membrane, and then blocked. Anti-mouse anti-RhoA, P27
mAb, and cyclin D1 antibody (1:500 dilution) were added
sequentially and then incubated at 4°C overnight. A secondary
antibody labeled by horseradish peroxidase-conjugate was added for
hybridization. The solution was then incubated with ECL
luminescence agent for 1–5 min, exposed, developed, and fixed.
Digital image analysis software (Bio-Rad, New York, NY, USA) was
used to analyze the results. The target protein/GAPDH ratio
indicated the relative target protein expression level.
Statistical analysis
Data are expressed as the means ± SD and analyzed
using the statistical software SPSS13.0. P<0.05 was considered
to indicate statistical significance.
Results
Identification of the HSC-T6
activity
The immunohisto-chemical staining results showed the
positive HSC α-SMA expression following 48 h of culture. The
cytoplasm was stained brown with a thin streak. The HSC was
star-shaped, with a large cell body and stretched membrane. The
positive rate of α-SMA was >95%.
HSC morphological changes
The HSCs showed no significant change in morphology
following co-culture with MSCs from 0 to 24 h. The cells were
mostly oval with weakened membrane stretching, smaller refractive
index particles were exhibited, cell adhesion decreased, and cell
number decreased at 48 h. The HSCs were round or oval without
membrane stretching, the refractive index particle became dense,
adhesion was poor, and the cell number decreased significantly at
72 h. Following co-culture, the HSCs showed no significant
morphological change between the blank and the negative control
groups. The HSCs appeared as stars, with large cell bodies and
membrane stretching and with low refractive-index particles.
Detection of HSC proliferation rate
The cells in the control group were used as
reference values. The MSCs caused mild inhibition at 24 h, with an
inhibition rate of 5.15±2.1%. Afterward, cell proliferation
inhibition was significantly enhanced, with 16.23±2.35 and
32.91±1.8% at 48 and 72 h, respectively. The proliferation
inhibition appeared to be time-dependent. A significant difference
was observed between the MSC experimental (2.85±0.12%, 2.77±0.25%)
and negative control (2.89±0.11%) groups at 24 h (P<0.01). No
difference was observed between the negative and the blank control
groups throughout the co-culture process.
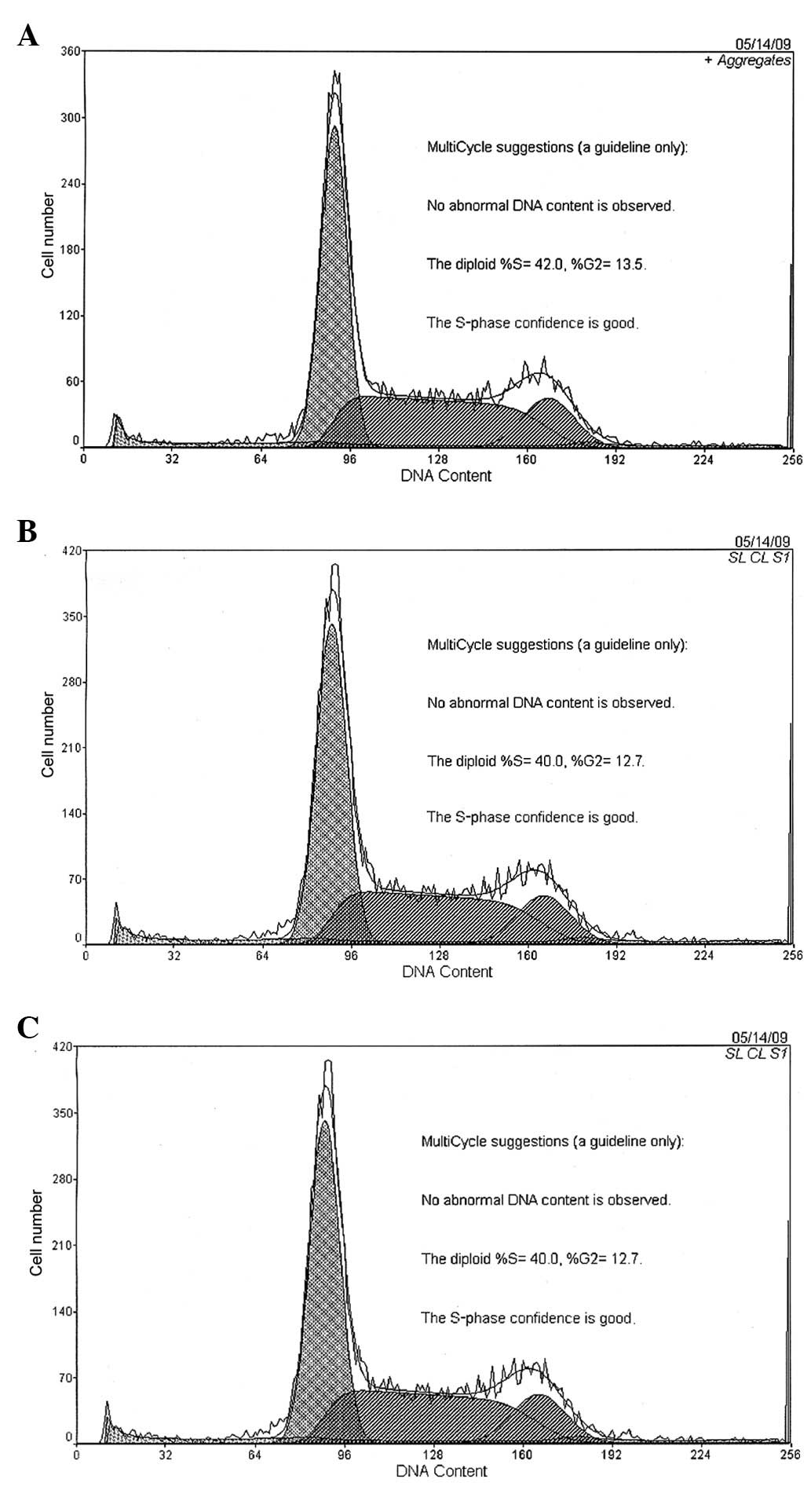
HSC cell cycle
Following 12 h of co-culture with MSCs, the cell
number of the HSCs blocked in the G0/G1 phase increased
significantly (P<0.01) in the experimental group, and the
S-phase cells were significantly reduced (P<0.01) compared with
the control and negative control groups. The G0/G1-phase cells were
49.45±0.95, 54.28±0.99, and 58.64±1.10%, whereas the S-phase cells
were 38.86±1.17, 35.42±0.94 and 33.5±0.78% at 24, 48, and 72 h,
respectively. The results revealed no difference between the
negative and blank control groups throughout the co-culture process
(Fig. 1).
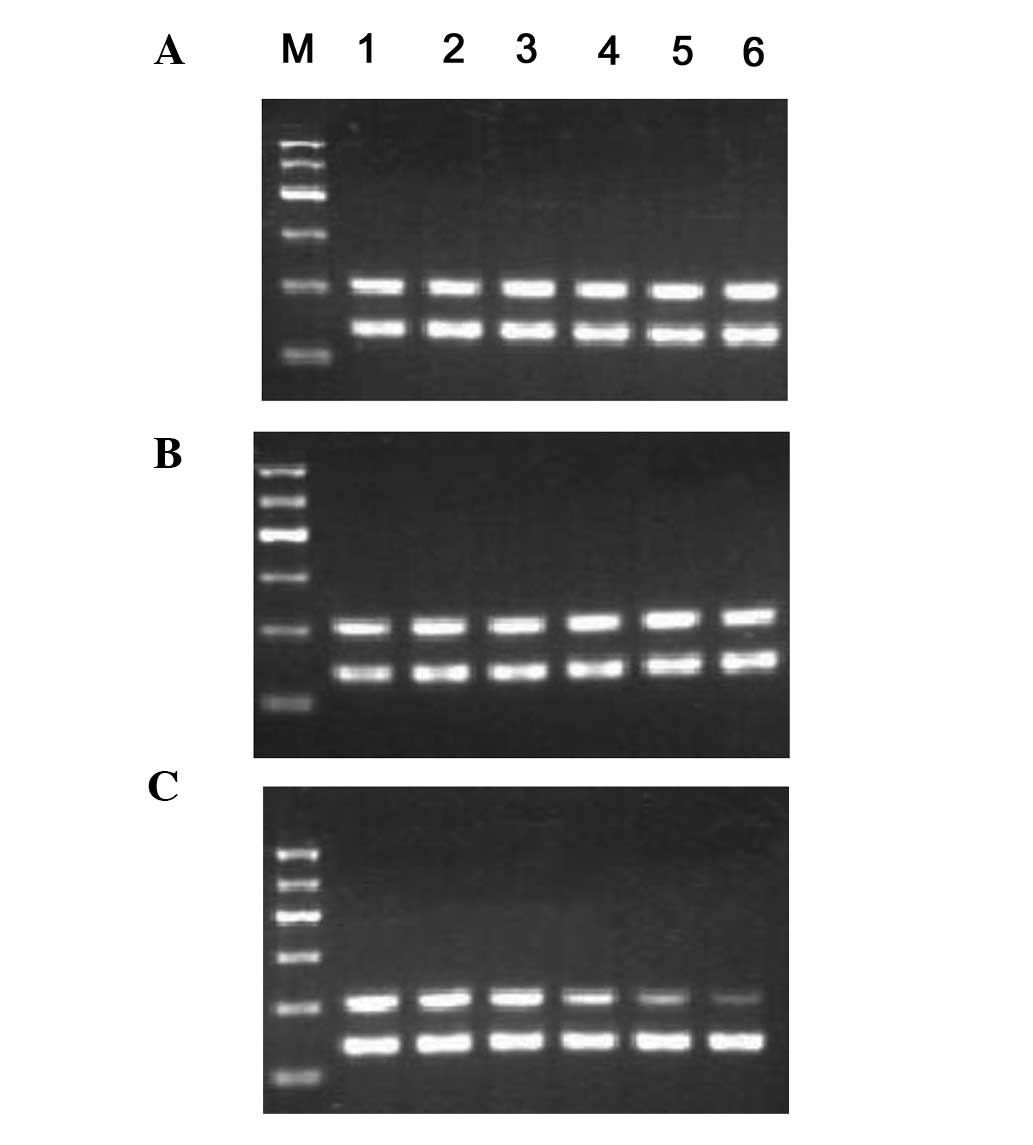
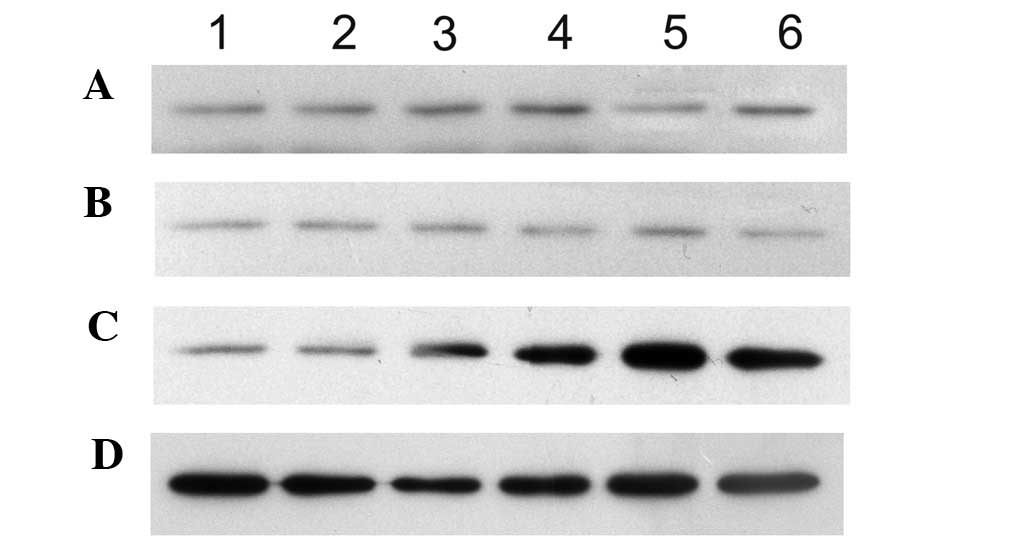
RhoA, cyclin D1, and P27 mRNA
expression
Following 12 h of co-culture, the MSC RhoA
mRNA expression in the experimental group (0.89±0.02%) was
significantly lower than that of the control group (1.06±0.02%)
(P<0.01). The expression then decreased rapidly and achieved its
minimum level at 72 h (0.37±0.05%). During the co-culturing period,
the RhoA mRNA expression in the negative control (1.07±0.03,
1.03±0.05, 1.06±0.03, 1.04±0.07, 1.01±0.06 and 0.96±0.10%) and
control groups (1.08±0.02, 1.04±0.03, 1.06±0.02, 0.96±0.08,
1.00±0.06 and 0.92±0.07%) exhibited no difference (Fig. 2). Following 24 h of co-culture, the
cyclin D1 mRNA expression began to decrease in the MSC group
(0.71±0.03, 0.57±0.03, 0.40±0.01 and 0.28±0.02%), was markedly
lower than that of the control (0.72±0.01, 0.71±0.01, 0.71±0.02,
0.70±0.02, 0.70±0.01 and 0.72±0.02%) and the negative control
(0.69±0.03, 0.71±0.02, 0.70±0.01, 0.72±0.01, 0.71±0.01 and
0.70±0.02) groups at 72 h, and significant difference (P<0.01)
was observed. The P27 mRNA expression in each group showed
no difference (Fig. 3) throughout
co-culture period. No significant correlation (r=−0.105) between
RhoA and P27 mRNA expression was observed.
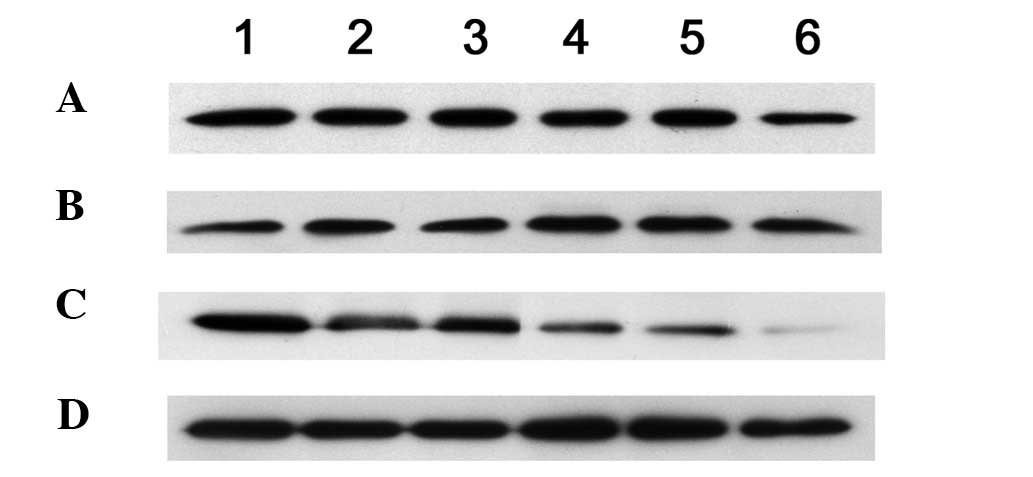
RhoA, cyclin D1, and P27 protein
expression
After 12 h of co-culture, RhoA protein expression
(0.86±0.07%) was significantly lower in the MSC experimental group
compared with that in the control group (1.11±0.12%) (P<0.01).
The expression then decreased slowly and reached its lowest level
at 72 h (Table I and Fig. 4).
 | Table IHSC RhoA protein/GAPDH gray ratio
following co-culture (n=3, mean ± SD). |
Table I
HSC RhoA protein/GAPDH gray ratio
following co-culture (n=3, mean ± SD).
| Group | Time (h)
|
|---|
| 0 | 6 | 12 | 24 | 48 | 72 |
|---|
| Blank control | 1.17±0.04 | 1.14±0.09 | 1.11±0.12 | 1.09±0.08 | 1.09±0.05 | 1.07±0.05 |
| Negative control | 1.07±0.16 | 1.03±0.25 | 1.06±0.16 | 1.06±0.17 | 1.05±0.28 | 0.99±0.27 |
| MSCs | 1.18±0.10 | 1.03±0.15 | 0.86±0.07a | 0.60±0.11a | 0.46±0.03a | 0.18±0.03a |
After the MSCs had been co-cultured for 24 h, the
cyclin D1 protein (0.65±0.09%) began to decrease, and its
expression (0.11±0.06%) was significantly lower than that in the
control and experimental control (P<0.01) groups at 72 h. After
12 h of co-culture, the P27 protein expression in the MSC
experimental group (0.39±0.03%) increased compared with that in the
control group (0.20±0.04%) (P<0.05). After 24 h of co-culture,
the P27 protein (0.73±0.07%) expression significantly increased in
the experimental group MSCs compared with that in the control group
(0.20±0.04%) (P<0.01) and maintained high expression (Table II and Fig. 5). No difference was observed in the
RhoA and P27 protein expression between the negative and blank
control groups at the various co-culture time points. A significant
negative correlation (r=−0.943, P<0.01) was observed in the RhoA
and P27 protein expression.
 | Table IIHSC p27 protein/GAPDH gray ratio
following co-culture (n=3, mean ± SD). |
Table II
HSC p27 protein/GAPDH gray ratio
following co-culture (n=3, mean ± SD).
| Group | Time (h)
|
|---|
| 0 | 6 | 12 | 24 | 48 | 72 |
|---|
| Blank control | 0.19±0.02 | 0.19±0.03 | 0.20±0.04 | 0.20±0.04 | 0.21±0.04 | 0.22±0.04 |
| Negative control | 0.22±0.03 | 0.22±0.05 | 0.21±0.04 | 0.20±0.02 | 0.21±0.02 | 0.22±0.03 |
| MSCs | 0.13±0.03 | 0.14±0.03 | 0.39±0.03a | 0.73±0.07b | 1.07±0.02b | 0.96±0.06b |
Discussion
The molecular bases of cell proliferation, achieved
through the operation of the cell cycle, include cell cycle
proteins (cyclin A–H), cyclin-dependent protein kinases (CDK1-7),
and cyclin-dependent protein kinase inhibitors (including P21, P27
and P18). These control elements are closely linked and form a
center of the cell cycle CDK regulatory network. The G0/G1-S-phase
check points are regulated by G1-phase cyclin D1 (14,15).
The ability of cells to pass from the G1 to the S
phase through the restriction point depends largely on cyclin D1
accumulation during the G1 phase. Cyclin D1 combines with the CDK
to form complexes, conduct phosphorylation mediated by the CDK
kinase and promote expression of certain genes. These gene
expression products promote the passage of the cells through the
G1-S regulation point and induce the cells to undergo the process
of cell self-division (16). By
contrast, if cyclin D1 expression is blocked, the cells cannot pass
from the G1 to the S phase.
P27 is a member of the CKI family, which mainly
inhibits CDK by combining with cyclin. The P27 inhibition of CDK
involves two aspects: P27 inhibits cyclin CDK activity or inhibits
the activation of CDK, which ultimately inhibits the cell cycle
G1→S transition (17,18). In the present study, the MSCs and
HSCs were cultured for 24 h. The results showed that the percentage
of cells in the S phase after 24 h decreased significantly compared
with that in the control group. Proliferation was significantly
inhibited, cyclin D1 mRNA and cyclin D1 protein expression
significantly decreased, and P27 protein increased significantly.
There was a statistically significant difference between the MSC
experimental and the control groups. This condition indicates that
the inhibition of the proliferation of HSCs by MSCs may be through
downregulation of cyclin D1 expression and upregulation of P27
protein expression. The cell cycle was arrested at the G0/G1 phase,
thereby inhibiting rat HSC proliferation.
RhoA is a member of the RhoGTP kinase family, which
regulates cytoskeletal dynamics, gene transcription, cell cycle
progression, and cell transformation functions (19,20).
Seasholtz et al (21) found
that the RhoA activation of the PI3K pathway can be reduced by P27
protein expression and leads to changes of its own DNA synthesis,
thereby regulating cell proliferation and migration. The Rho
pathway inhibitor, lovastatin, or the exoenzyme C3 are capable of
enhancing the efficiency of the translation of P27 mRNA. RhoA also
regulates the Skp2-P27 pathway and promotes cell cycle G1/S-phase
transition (22). P27 regulates
cell migration through combination with RhoA to inhibit RhoA
activity (23). The present study
also found that, in cells cultured for 12 h, the RhoA protein
expression of the HSCs was significantly reduced, whereas the P27
protein expression was significantly increased. There was a
significant negative correlation between the P27 protein and the
RhoA protein expression. MSCs suppressed HSC RhoA expression, and
the decreased RhoA activity led to decreased P27 protein
degradation. A large amount of P27 protein accumulated in the
intracellular matrix, resulting in a large number of HSCs being
arrested in the cell cycle in the G0/G1 phase. The cell cycle was
arrested during the early period of DNA synthesis, eventually
leading to HSC cell division and proliferation reduction, reduced
activity, and promotion of apoptosis. The changes were
significantly time-dependent.
However, the P27 mRNA expression did not change
significantly in any of the co-culture groups throughout the
process. P27 is not regulated at the transcriptional level
(24), and P27 upregulation may be
associated with the blocking of P27 degradation in the cytoplasm.
The main regulation of P27 protein expression occurs in the
post-translational level.
In the co-culture model, the HSC cell morphology,
activity, growth inhibition rate, and protein, as well as mRNA,
expression levels of RhoA and P27 did not change significantly at
the 0, 6 and 12-h periods. This was probably related to the
paracrine nature of the two cells or to their secretion of certain
cytokines and growth factors, such as IL-10, TNF-α, GM-CSF
(25), HGF (26) and NGF (27), among others. These active factors
may interact and lead to changes in the microenvironment.
In conclusion, BMSCs may regulate HSCs and cyclin D1
via the RhoA-P27 pathway, which causes the cell cycle G1/S phase
transition, inhibits HSC proliferation and promotes apoptosis.
Acknowledgements
This study was supported by the
Natural Science Foundation of Guangxi (0897008) and Guangxi ‘New
Century Talents Project’ special funds (2006206). We would like to
thank XiaoCong Kuang and Weiping Chen from Guangxi Medical
University for their help in the cell culture.
References
|
1.
|
Friedman SL: Hepatic stellate cells:
protean, multifunctional, and enigmatic cells of the liver. Physiol
Rev. 88:125–172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Friedman SL: Mechanisms of hepatic
fibrogenesis. Gastroenterology. 134:1655–1669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Anderson PAW, Muller-Borer BJ, Esch GL,
Coleman WB, Grisham JW and Malouf NN: Calcium signals induce liver
stem cells to acquire a cardiac phenotype. Cell Cycle. 6:1565–1569.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Iop L, Chiavegato A, Callegari A, et al:
Different cardiovascular potential of adult-and fetal-type
mesenchymal stem cells in a rat model of heart cryoinjury. Cell
Transplant. 17:679–694. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lysy PA, Campard D, Smets F, et al:
Persistence of a chimerical phenotype after hepatocyte
differentiation of human bone marrow mesenchymal stem cells. Cell
Prolif. 41:36–58. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Polyak K, Lee MH, Erdjument-Bromage H, et
al: Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a
potential mediator of extracellular antimitogenic signals. Cell.
78:59–66. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ishikawa T, Terai S, Urata Y, et al:
Fibroblast growth factor2 facilitates the differentiation of
transplanted bone marrow cells into hepatocytes. Cell Tissue Res.
323:221–231. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Toyoshima H and Hunter T: p27, a novel
inhibitor of G1 cyclin-Cdk protein kinase activity, is related to
p21. Cell. 78:67–74. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Slingerland J and Pagano M: Regulation of
the cdk inhibitor p27 and its deregulation in cancer. J Cell
Physiol. 183:10–17. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Weber JD, Hu W, Jefcoat SC Jr, Raben DM
and Baldassare JJ: Ras-stimulated extracellular signal-related
kinase 1 and RhoA activities coordinate platelet-derived growth
factor-induced G1 progression through the independent regulation of
cyclin D1 and p27. J Biol Chem. 272:32966–32971. 1997. View Article : Google Scholar
|
|
11.
|
Russo FP, Alison MR, Bigger BW, et al: The
bone marrow functionally contributes to liver fibrosis.
Gastroenterology. 130:1807–1821. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Parekkadan B, van Poll D, Megeed Z, et al:
Immunomodulation of activated hepatic stellate cells by mesenchymal
stem cells. Biochem Biophys Res Commun. 363:247–352. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Shi L, Li G, Wang J, et al: Bone marrow
stromal cells control the growth of hepatic stellate cells in
vitro. Dig Dis Sci. 53:2969–2974. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Cattam P, Hohaus S, Bellacosa A, et al:
Association between Cyclin Dl (CCND1) gene amplification and human
papillomavirus infection in human laryngeal squamous cell
carcinoma. Clin Cancer Res. 4:2585–2589. 1998.PubMed/NCBI
|
|
15.
|
Calbo J, Parreno M, Sotillo E, et al: Gl
cyclin/cyclin-dependent kinase-coordinated phosphorylation of
endogenous pocket proteins differentially regulates their
interactions with E2F4 and E2FI and gene expression. Biol Chem.
277:50–63. 2002. View Article : Google Scholar
|
|
16.
|
Lents NH, Keenan SM, Bellon C and
Baldassare JJ: Stimulation of the Raf/MEK/ERK cascade is necessary
and sufficient for activation and Thr-l60 phosphorylation of a
nuclear-targeted CDK2. Biol Chem. 277:47–69. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Gardner LB, Li Q, Park MS, Flanagan WM,
Semenza GL and Dang CV: Hypoxia inhibits G1/S transition through
regulation of p27 expression. J Biol Chem. 276:7919–7926. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Kuo MY, Hsu HY, Kok SH, et al: Prognostic
role of p27 (Kip1) expression in oral squamous cell carcinoma in
Taiwan. Oral Oncol. 38:172–178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Ridley AJ: Rho proteins and cancer. Breast
Cancer Res Treat. 84:13–19. 2004. View Article : Google Scholar
|
|
20.
|
Hall A: The cytoskeleton and cancer.
Cancer Metastasis Rev. 28:5–14. 2009. View Article : Google Scholar
|
|
21.
|
Seasholtz TM, Zhang T, Morissette MR,
Howes AL, Yang AH and Brown JH: Increased expression and activity
of RhoA are associated with increased DNA synthesis and reduced
p27(Kip1) expression in the vasculature of hypertensive rats. Circ
Res. 89:488–495. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Mammoto A, Huang S, Moore K, Oh P and
Ingber DE: Role of RhoA, mDia, and ROCK in cell shape-dependent
control of the Skp2-p27kip1 pathway and the G1/S transition.
J Biol Chem. 279:26323–26330. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Besson A, Dowdy SF and Roberts JM: CDK
inhibitors: cell cycle regulators and beyond. Dev Cell. 14:159–169.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Singh SP, Lipman J, Goldman H, et al: Loss
or altered subcellular localization of p27 in Barrett's associated
adenocarcinoma. Cancer Res. 58:1730–1735. 1998.
|
|
25.
|
Yannaki E, Athanasiou E, Xagorari A, et
al: G-CSF-primed hematopoietic stem cells or G-CSF per se
accelerate recovery and improve survival after liver injury,
predominantly by promoting endogenous repair programs. Exp Hematol.
33:108–119. 2005. View Article : Google Scholar
|
|
26.
|
Oyagi S, Hirose M, Kojima M, et al:
Therapeutic effect of transplanting HGF-treated bone marrow
mesenchymal cells into CCl4-injured rats. J Hepatol. 44:742–748.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Li Y, Chen J, Chen XG, Wang L, et al:
Human marrow stromal cell therapy for stroke in rat: neurotrophins
and functional recovery. Neurology. 59:514–523. 2002. View Article : Google Scholar : PubMed/NCBI
|