Introduction
As a relatively common gastroenterological malignant
tumor, pancreatic adenocarcinoma (PAC) accounts for ∼8–10% of all
gastroenterological malignancies and its incidence increases 15%
every 10 years (1). Lymphatic
metastasis is an important prognostic and treatment factor in PAC;
post-mortem examination often reveals wide involvement of adjacent
organs in PAC patients. Local lymphatic metastases in mesenteric
lymph nodes, (lymph nodes next to the superior mesenteric artery),
gastroduodenal artery, hilar and portal vein lymph nodes comprise
the majority of lymphatic metastases. This may be due to the
anatomic position and structure of the pancreas. Most of the
pancreatic blood supply comes from the hepatic artery and
mesenteric artery superior (2).
Retroperitoneal and left supraclavicular lymph node metastases are
also common, similar to other gastroenterological malignancies.
Lymphatic spread is also found in up to 50% of so-called early
cancer of the pancreas with the presence of metastases to adjacent
or distant lymph nodes (3). Tumor
cells can spread multi-directionally via lymph nodes, and
pancreatic lymph reflux is part of the entire gastroenterological
lymph reflux, having direct or indirect association with adjacent
organs; this indicates the anatomic reason for the high metastatic
property of PAC. The metastasis and invasion of PAC may occur via
blood vessels, lymph node and direct spreading simultaneously.
CXCL12 is a member of the CXC chemokine subfamily,
working together with its chemokine receptor CXCR4 (4). CXCL12 can induce the adherence of
most circulating lymphocytes and pre-CD34 cells, and the secreted
protein on the cell surface. It can interact with integrin, causing
cells to migrate to a specific location (5). Endothelial cells express CXCR4, and
CXCL12 confers a strong chemostatic effect on them (6). CXCR4 expression is unregulated in
some breast cancer cells. CXCR4 antagonist can downregulate its
expression in breast cancer cells, thus repressing metastasis in
animal models (7,8). Kato et al (9) analyzed 79 cases of resected invasive
ductal carcinoma samples, all cancerous tissue expressing CXCR4.
Samples with high expression of CXCR4, particularly local high
expression of CXCR4, often had extensive lymph node metastasis,
indicating that CXCR4 may play pro-metastatic roles in the
lymphatic metastasis of breast cancer. Administration of the
monoclonal antibody to CXCR4 to SCID mice was found to effectively
regress lung metastasis from MDA-MB-231 xenografts, indicating the
key roles of chemokines and chemokine receptors in the
organ-specific metastasis of breast cancer. Liang et al
(10) found that CXCL12 caused a
2.2-fold increase in filamentous actin in breast cancer cells
within 20 sec in vitro, resulting in the formation of
pseudopods, induction of targeted migration and invasion of breast
cancer cells, which was dose-dependent. The anti-CXCR4 antibody can
block this effect. A better understanding of the molecular
mechanisms of the relationship between lymphangiogenesis and
lymphatic metastasis will provide the theoretical basis for the
blockage of lymphatic metastasis, which has important clinical
significance. To date, the clinical significance of the
CXCL12/CXCR4 axis in pancreatic cancer has not yet been clearly
elucidated. Thus, in the present study, we explored the molecular
roles of the CXCL12/CXCR4 axis in the organ-specific metastasis of
PAC.
Materials and methods
Patients and tissue samples
Tissue samples were obtained from 30 patients who
underwent macroscopically curative resection for pancreatic cancer
at Shandong Tumor Hospital (Jinan, China) between 2005 and 2007.
Samples of the pancreatic tumor, paracancerous tissues, normal
pancreas and lymph nodes surrounding the pancreas were immediately
frozen in liquid nitrogen or formalin-fixed after surgery and then
embedded in paraffin. Sections from each case were stained with
hematoxylin and eosin (H&E) for histological examination
according to the tumor node metastasis (TNM) classification system.
Tissue was collected based on the protocol approved by the Ethics
Committee of the Medical Faculty of Shandong Tumor Hospital (Jinan,
China).
All patients had complete clinical and pathological
data. The patients were comprised of 17 men and 13 women with a
median age of 57.2 years (range, 35-78). No patients received
preoperative chemotherapy or radiotherapy. Among the 30 patients,
17 well-differentiated and 13 poorly differentiated cases were
identified. The study included 12 Union for International Cancer
Control (UICC) stage I–II patients and 18 who were stage
III–IV.
Immunohistochemical staining
Tissue sections were analyzed using the standard
protocol for streptavidin-peroxidase immuno histochemical staining.
Sections (4-μm) were deparaffinized and rehydrated. After blocking
of endogenous peroxidase with methanol containing 0.3%
H2O2, the sections were autoclaved at 121°C
for 10 min in a citrate buffer (10 mmol/l sodium citrate; pH 6.0)
for antigen retrieval. After blocking with normal goat serum, the
sections were incubated overnight with the following primary
antibodies: CXCL12 (1:200), CXCR4 (1:200), VEGFR-3 (1:250) and CD34
(1:80) (DakoCytomation, Glostrup, Denmark). The sections were then
reacted sequentially with biotin-conjugated anti-mouse
immunoglobulin G antibodies (Vector Laboratories, Inc., Burlingame,
CA) and Vectastain Elite ABC reagent (Vector Laboratories, Inc.).
Diaminobenzidine was used as the chromogen, and the nuclei were
counterstained with hematoxylin. One breast cancer sample was used
as a positive control and phosphate-buffered solution (PBS) without
the primary antibody was employed as the negative control. Each
section was analyzed in terms of the staining intensity and the
proportion of positive tumor cells by two pathologists in a
double-blinded manner. The upper quartile was defined as the cutoff
point.
Microvessels were detected by morphological
observation and immunohistochemical labeling using the endothelial
marker CD34 for microvascular density (MVD) evaluation. All
independent CD34-positive vessels were counted regardless of the
presence of an identifiable lumen. The following methods were
employed to assess MVD. First, the area with the most intense
vascularization was determined under magnification, ×10. The
average MVD was then analyzed by randomly selecting 5 fields/tumor
at magnification, ×400. Blood vessels with lumens containing >8
red cells or with muscular layers were excluded. For each case, the
number of CD34-positive vessel structures in 5 high power fields
was recorded, and the average value was considered as the MVD. The
immunostaining results were assessed by two pathologists who were
blind to the clinicopathological findings. Meanwhile, VEGFR-3 was
used for micro-lymphatic vessel density (MLVD) evaluation. The
process used to calculate MLVD was the same as that for the MVD
assay discussed previously.
RT-PCR analysis
Reverse transcript-PCR was performed according to
the standard protocol. In brief, RNA extraction from frozen human
specimens was performed using the acid guanidinium thiocyanate
method. RNA was dissolved in diethylpyrocarbonate (DEPC)-treated
water. The prepared RNA (1 μg) was mixed with reverse transcription
reagents at a total volume of 20 μl and incubated for 30 min at
42°C to produce first-strand cDNA. A total of 1 μl cDNA was used
for PCR amplification. The primer sequences were as follows: CXCR4,
F, 5′-AGCTGTTGGTGAAAAGGTGGTCTATG-3′ and R,
5′-GCGCTTCTGGTGGCCCTTGGAGTGTG-3′; CXCL12, F,
5′-CCGCGCTCTGCCTCAGCGACGGGAAG-3′ and R,
5′-CTTGTTTAAAGCTTTCTCCAGGTACT-3′; β-actin, F,
5′-GTGGGGCGCCCCAGGCACCA-3′ and R, 5′-CTC
CTTAATGTCACGCACGATTT-3′.
Following the manufacturer’s instructions, reverse
transcription was performed at 42°C in the presence of 5 units AMV
reverse transcriptase and 1 μg RNA for 60 min. AMV RT inactivation
and RNA/cDNA/primer denaturation were performed at 95°C for 5 min
to activate modified Taq polymerase followed by 40 cycles at 95°C
for 10 min, 95°C for 10 sec and 56°C for 1 min and 1 cycle at 72°C
for 35 sec. The PCR product was separated by 2% agarose gel
electrophoresis. The gels were viewed using UV transillumination
and photographed by a Kodak 120 gel imaging system.
Statistical analysis
Data are shown as mean ± SE and analyzed by the SPSS
software program (version 13.0 for Windows; SPSS, Inc., Chicago,
IL). Comparison among different groups was performed by the
Chi-square test, the Fisher’s exact, one-way ANOVA and Spearman’s
rho tests. A value of P<0.05 was considered statistically
significant.
Results
CXCL12 and CXCR4 expression in pancreatic
cancer
The levels of CXCL12 and CXCR4 mRNA in the primary
tumor, paracancerous tissues, normal pancreas and lymph nodes
surrounding the pancreas were assessed using RT-PCR.
Immunohistochemical staining was performed to further determine the
levels and locations of CXCL12 and CXCR4 protein expression
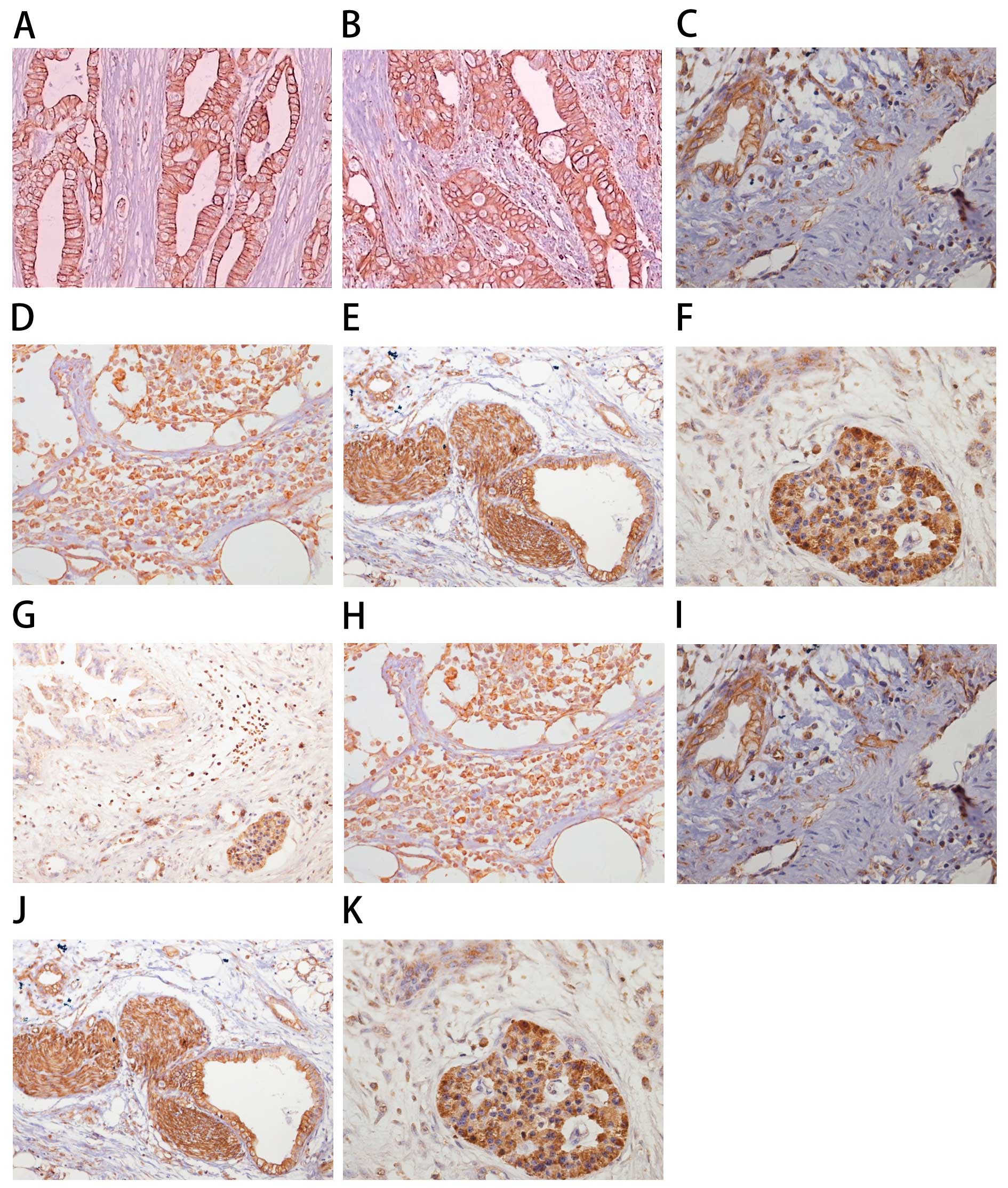
(Fig. 1). The rate of positive
expression of CXCL12 was lower in the pancreatic tumor tissues
compared with the positive rate in the other tissues (Table I). As shown in Table I, 80.0% of cancerous tissues
(Fig. 1B), 70.0% of paracancerous
tissues (Fig. 1C) and 73.3% of
lymph node tissues (Fig. 1D)
adjacent to the pancreas had positive expression of CXCR4. These
results were significantly different from that of the normal
pancreas tissue (Fig. 1A)
(P<0.001). Positive expression of CXCR4 was also detected in the
vascular endothelial cells of the pancreas (Fig. 1I), the peripancreatic lymph nodes
(Fig. 1H), the peripancreatic
neural tissue (Fig. 1J) and an
‘island’ of pancreatic cancer (Fig.
1K).
 | Table ICXCL12 and CXCR4 expression by
immunohistochemical staining in 4 groups. |
Table I
CXCL12 and CXCR4 expression by
immunohistochemical staining in 4 groups.
| CXCL12 expression
| CXCR4 expression
|
|---|
| Group | Negative | Positive n (%) | P-value | Negative | Positive n (%) | P-value |
|---|
| Cancerous
tissues | 26 | 4 (13.3) | | 6 | 24 (80.0) | |
| Paracancerous
tissues | 16 | 14 (46.7) | 0.011 | 9 | 21 (70.0) | 0.551 |
| Normal pancreas | 13 | 17 (56.7) | 0.001 | 22 | 8 (26.7) | <0.001 |
| Lymph nodes | 15 | 15 (50.0) | 0.006 | 8 | 22 (73.3) | 0.760 |
The level of CXCL12 mRNA was low in tumor tissues
and moderate in the normal pancreas, while expression of CXCL12 was
found in pancreatic cancer. A significant difference was found
between the pancreatic cancer and normal pancreas (P<0.01)
(Tables I and II). Moderate expression of CXCL12 was
observed in the paracancerous tissues adjacent to the pancreas at
the mRNA level, and this was significantly different from that of
the pancreatic cancer group (P<0.05) (Table II). CXCL12 mRNA was detected at
significantly high levels in paracancerous tissues, the normal
pancreas and lymph nodes (Table
II). The levels of CXCL12 protein exhibited the same trend as
those of the mRNA (Fig. 1A–F).
CXCR4 expression tended to be opposite that of CXCL12 at both the
mRNA and protein levels (Tables I
and II). Compared to the normal
pancreas, the expression of CXCR4 in cancerous tissues,
paracancerous tissues and lymph nodes was significantly higher
(P<0.001) (Table II).
 | Table IICXCL12 and CXCR4 mRNA expression by
RT-PCR. |
Table II
CXCL12 and CXCR4 mRNA expression by
RT-PCR.
| CXCL12 expression
| CXCR4 expression
|
|---|
| Group | CXCL12/β-actin | P-value | CXCR4/β-actin | P-value |
|---|
| Cancerous
tissues | 0.263±0.254 | | 0.789±0.300 | |
| Paracancerous
tissues | 0.429±0.136 | 0.003 | 0.701±0.291 | 0.254 |
| Normal
pancreas | 0.437±0.098 | <0.001 | 0.236±0.199 | <0.001 |
| Lymph nodes | 0.425±0.187 | 0.006 | 0.700±0.322 | 0.273 |
| Paracancerous
tissues | 0.429±0.136 | | 0.701±0.291 | |
| Cancerous
tissues | 0.263±0.254 | 0.003 | 0.789±0.300 | 0.254 |
| Normal
pancreas | 0.437±0.098 | 0.795 | 0.236±0.199 | <0.001 |
| Lymph nodes | 0.425±0.187 | 0.925 | 0.700±0.322 | 0.99 |
| Normal
pancreas | 0.437±0.098 | | 0.236±0.199 | |
| Cancerous
tissues | 0.263±0.254 | <0.001 | 0.789±0.300 | <0.001 |
| Caracancerous
tissues | 0.429±0.136 | 0.795 | 0.701±0.291 | <0.001 |
| Lymph nodes | 0.425±0.187 | 0.757 | 0.700±0.322 | <0.001 |
| Lymph nodes | 0.425±0.187 | | 0.700±0.322 | |
| Cancerous
tissues | 0.263±0.254 | 0.006 | 0.789±0.300 | 0.273 |
| Paracancerous
tissues | 0.429±0.136 | 0.925 | 0.701±0.291 | 0.99 |
| Normal
pancreas | 0.437±0.098 | 0.757 | 0.236±0.199 | <0.001 |
Correlation of CXCL12 or CXCR4 expression
with the clinicopathological factors of pancreatic cancer
To define the role of CXCL12 and CXCR4 in the
progression of pancreatic cancer, we analyzed the association of
expression profiles of CXCL12 and CXCR4 with tumor grade and stage.
This analysis revealed that higher CXCR4 expression and cytoplasmic
localization of CXCR4 were significantly associated with the lymph
node status of the tumor (Table
III). In addition, CXCR4 expression also correlated with stage
in pancreatic cancer patients (P=0.05) (Table III). No definitive correlation was
observed between CXCL12 expression and progression of pancreatic
cancer (Table III).
 | Table IIICorrelation between expression of
CXCL12 and CXCR4 and clinicopathological factors of pancreatic
cancer. |
Table III
Correlation between expression of
CXCL12 and CXCR4 and clinicopathological factors of pancreatic
cancer.
| CXCR4 expression
| CXCL12 expression
|
|---|
| Factors | n | Negative, n | Positive, n
(%) | P-value | Negative, n | Positive, n
(%) | P-value |
|---|
| Histology | | | | 0.311 | | | 0.810 |
|
Well-differentiated | 17 | 5 | 12 (70.6) | | 14 | 3 (17.6) | |
| Moderately/ | | | | | | | |
| Poorly
differentiated | 13 | 1 | 12 (92.3) | | 12 | 1 (7.8) | |
| TNM stage | | | | 0.050 | | | 0.324 |
| I–II | 12 | 5 | 7 (58.3) | | 9 | 3 (33.3) | |
| III–IV | 18 | 1 | 17 (94.4) | | 17 | 1 (5.6) | |
| Lymph node
metastasis | | | | 0.004 | | | 0.913 |
| Negative | 12 | 6 | 6 (50.0) | | 11 | 1 (8.3) | |
| Positive | 18 | 0 | 18 (100.0) | | 15 | 3 (16.7) | |
Correlations between MVD/MLVD and
clinicopathological factors of pancreatic cancer
Table IV shows the
varying degrees of angiogenesis and lymphangiogenesis, and the
relationships between these variables and clinical outcome were
determined. Compared to patients with lower intratumoral MVD,
patients with higher intratumoral MVD exhibited a significantly
lower degree of differentiation yet a higher class of TNM staging
(P<0.05). However, no relationship was found between lymph node
metastasis and blood vessel formation (P>0.05). Additionally, it
was found that MLVD status was correlated with tumor stage and
grade. The formation of lymphatic vessels significantly increased
in patients with higher TNM staging and lymph node metastasis, but
no significant change was observed in patients with a lower degree
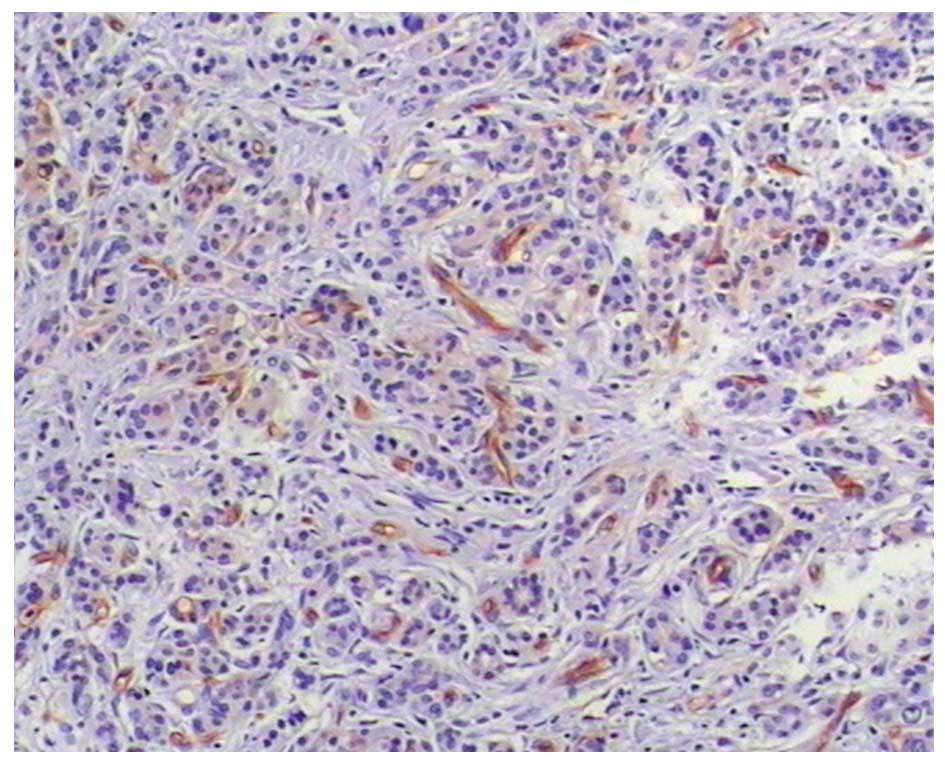
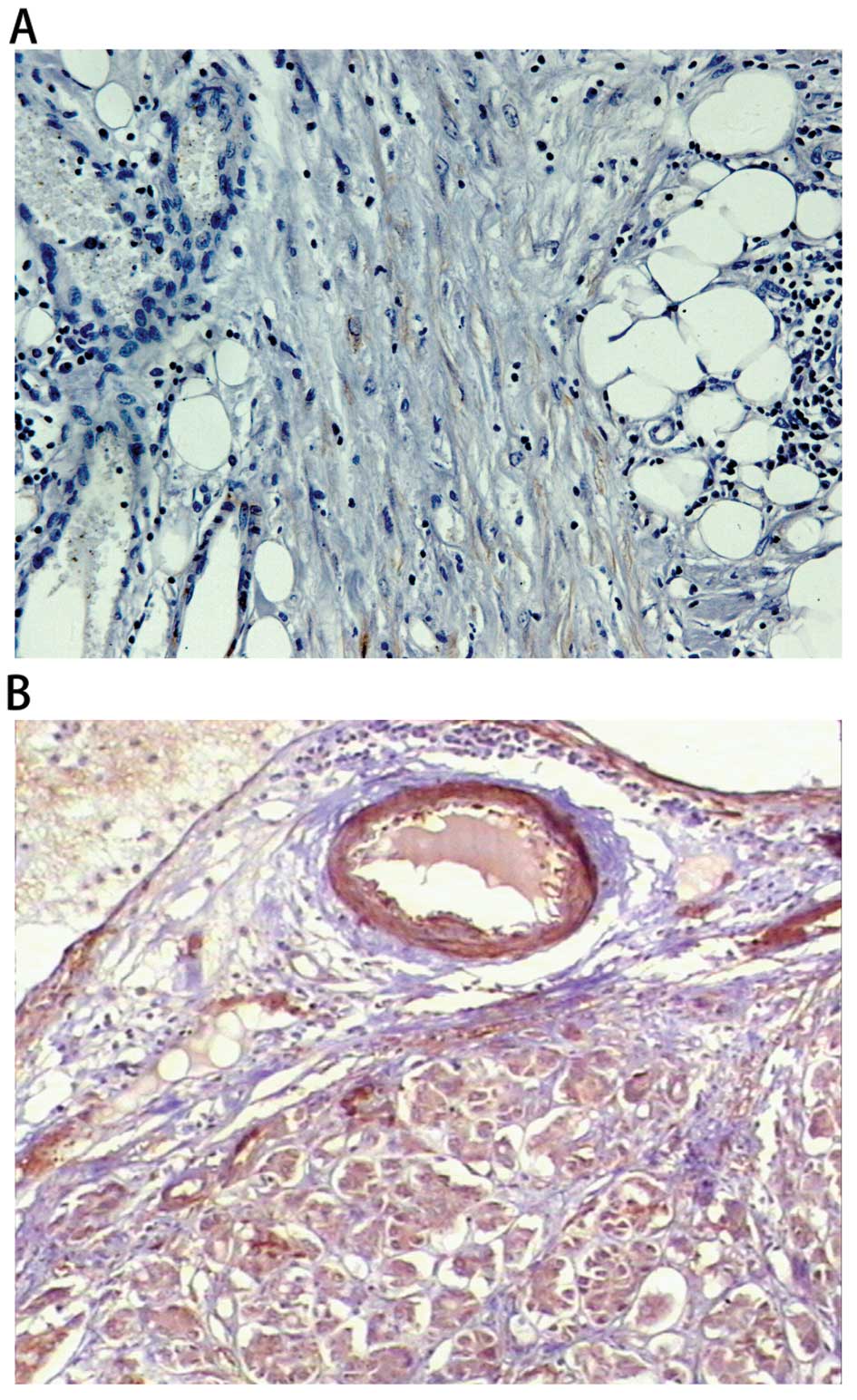
of differentiation (Figs. 2 and
3).
 | Table IVCorrelation between microvascular
density/micro-lymphatic vessel density and clinicopathological
factors of pancreatic cancer. |
Table IV
Correlation between microvascular
density/micro-lymphatic vessel density and clinicopathological
factors of pancreatic cancer.
| n | Microvascular
density | P-value | Micro-lymphatic
vessel density | P-value |
|---|
| Histology | | | <0.001 | | 0.194 |
|
Well-differentiated | 17 | 54±4.3 | | 5±5.9 | |
|
Moderately/Poorly-differentiated | 13 | 65±6.5 | | 8±6.4 | |
| TNM stage | | | <0.001 | | 0.017 |
| I–II | 12 | 52±7.4 | | 4±2.9 | |
| III–IV | 18 | 63±7.3 | | 8±4.9 | |
| Lymph node
metastasis | | | 0.456 | | 0.009 |
| Negative | 12 | 52±7.4 | | 4±2.9 | |
| Positive | 18 | 54±6.9 | | 9±5.7 | |
Analysis of the association of
CXCL12/CXCR4 with MVD/MLVD
As shown in Table
V, CXCL12 protein was negative in 26 samples, and the MVD in
these negative samples was higher than that in the positive samples
(P= 0.022). No significant correlation between the expression of
CXCL12 protein and MLVD of pancreatic cancer was found (P>0.05).
CXCR4 protein was positive in 24 samples, and the MLVD in these
positive samples was higher than that in the negative group
(P=0.003). These results indicate that lower expression of CXCL12
protein was strongly associated with angiogenesis of pancreatic
cancer, while higher expression of CXCR4 protein was significantly
associated with lymphangiogenesis of pancreatic cancer.
 | Table VCorrelation between CXCL12/CXCR4 and
microvascular density/micro-lymphatic vessel density. |
Table V
Correlation between CXCL12/CXCR4 and
microvascular density/micro-lymphatic vessel density.
| n | Microvascular
density | P-value | Micro-lymphatic
vessel density | P-value |
|---|
| CXCL12 | | | 0.022 | | 0.472 |
| Negative | 26 | 64±6.9 | | 7.9±5.3 | |
| Positive | 4 | 55±7.0 | | 5.9±3.1 | |
| CXCR4 | | | 0.818 | | 0.003 |
| Negative | 6 | 53±4.8 | | 6.1±5.8 | |
| Positive | 24 | 55±4.1 | | 12.1±4.0 | |
Discussion
Chemokine molecules constitute a superfamily of
inducible secreted proinflammatory proteins (11–14)
that are involved in a variety of immune responses. These molecules
primarily act as chemoattractants and activators of specific types
of leukocytes (11,15,16),
and they mediate these functions by binding to G-protein-coupled
receptors. It is becoming increasingly evident that chemokines play
an integral role in initiating specific immune responses (2). One such chemokine (CXCL12) is found
on high endothelial venules (HEV) and within T-cell zones of the
spleen and lymph nodes (3,17–19);
it is involved in the recruitment of naive T cells and dendritic
cells (DCs). In lymph nodes, CXCL12 plays an important role in the
initiation of immune responses by co-localizing naive T cells with
DC-presented antigens (20–23).
The receptor for this ligand, CXCR4, is expressed on all naive T
cells, some memory T cells, B cells and mature dendritic cells; it
plays a central role in lymphocyte trafficking and homing to lymph
nodes (24,25). Recent studies have shown the
involvement of the CXCL12/CXCR4 axis in the progression of several
types of cancer (4,26,27).
For example, Hassan et al (5) reported high levels of CXCL12/CXCR4
expression in breast cancer cells and linked receptor expression to
the metastatic destination of tumor cells. However, correlations
between the CXCL12/CXCR4 axis and clinical features of pancreatic
cancer have not been extensively studied. Therefore, in our study,
we evaluated the expression of CXCL12/CXCR4 and found a critical
relationship between CXCL12/CXCR4 and tumor stage and grade in
pancreatic cancer.
For patients with pancreatic carcinoma, CXCL12
expression was reduced in tumor tissues, but significant levels
were detected in paracancerous tissues, normal pancreas and lymph
nodes. CXCR4 expression showed the opposite trend. CXCR4 was
expressed in 80.0% of pancreatic carcinoma samples but only 26.7%
of normal samples. In addition, we found significant differences
between positive and negative CXCL12/CXCR4 samples in regards to
the following clinicopathological features: i) lymph node
metastasis and ii) tumor TNM staging.
To elucidate the underlying association between
CXCL12/CXCR4 expression and clinicopathological features, we
assessed MVD and MLVD in tumor tissues. We found that CXCL12
expression was significantly associated with the formation of blood
vessels, whereas it had no obvious relationship with MLVD. The
expression of CXCR4 was higher in patients with higher MLVD
compared to those with lower MLVD. CXCR4 had no relationship with
MVD.
Angiogenesis and lymphangiogenesis are required for
many pathological processes, including tumor growth, metastasis and
physiological organ/tissue maintenance. In general, the molecular
mechanisms that control carcinoma progression and metastasis are
related to mutations of various oncogenes, tumor-suppressor genes,
metastasis-suppressor genes, and growth factors and their
receptors, including Src, Ras, p16, KiSS-1, Nm23, FasL, vascular
endothelial growth factor (VEGF), basic fibroblast growth factor
(bFGF) and interleukin (IL)-6 (6,7,26,28).
These abnormalities affect the downstream signal transduction
pathways involved in the control of cell growth and other malignant
properties, such as tumor staging and degree of tumor
differentiation. Notably, one of the most recently recognized
events in this process involves the interaction between chemokines
and their receptors. Several studies have found that the chemokine
receptor was highly expressed in breast and ovarian carcinomas, and
the interaction between the receptor and its ligand resulted in
chemotaxis, or directed migration of tumor cells from their primary
site via the circulation to preferential sites of metastasis
(8,29–31).
These studies strongly support our hypothesis. The interaction
between CXCR4 and CXCL12 may play crucial roles in the metastasis
and progression of pancreatic cancer by its effects on the
formation of new blood vessels and lymphatic vessels.
In conclusion, our data suggest that the chemotactic
interaction between CXCR4 and its ligand CXCL12 may be a critical
event in the progression of pancreatic cancer. A potential
mechanism of action may be the induction of angiogenesis and
lymphangiogenesis by cancer cells. This hypothesis is supported by
findings that the expression pattern of CXCL12 and CXCR4 in
pancreatic cancer tissue significantly correlated to
clinicopathological features. Our data also suggest that the
CXCL12/CXCR4 axis is associated with the formation of lymphatic
vessels and blood vessels induced by pancreatic cancer cells.
Additional research is underway to determine the pathways
responsible for tumor cell chemokine and/or chemokine
receptor-associated angiogenesis and lymphangiogenesis. It is
likely that controlling such a poor prognostic feature would enable
more successful loco-regional tumor control and improve survival in
patients with pancreatic cancer.
Acknowledgements
This study was financially supported
by the National Natural Science Foundation of China (no. 30571712
and 30810403081) and the Department of Science and Technology of
Shandong Province of China (no. 2007GG20002022). We appreciate the
valuable comments from the other members of our laboratories.
References
|
1.
|
Oh JW, Olman M and Benveniste EN:
CXCL12-mediated induction of plasminogen activator inhibitor-1
expression in human CXCR4-positive astroglioma cells. Biol Pharm
Bull. 32:573–577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Sallusto F, Mackay CR and Lanzavecchia A:
The role of chemokine receptors in primary, effector, and memory
immune responses. Annu Rev Immunol. 18:593–620. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Birk D, Fortnagel G, Formentini A and
Beger HG: Small carcinoma of the pancreas. Factors of prognostic
relevance. J Hepatobiliary Pancreat Surg. 5:450–454. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Salmaggi A, Maderna E, Calatozzolo C, et
al: CXCL12, CXCR4 and CXCR7 expression in brain metastases. Cancer
Biol Ther. 8:1608–1614. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Hassan S, Ferrario C, Saragovi U, et al:
The influence of tumor-host interactions in the stromal
cell-derived factor-1/CXCR4 ligand/receptor axis in determining
metastatic risk in breast cancer. Am J Pathol. 175:66–73. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Welch DR and Goldberg SF: Molecular
mechanisms controlling human melanoma progression and metastasis.
Pathobiology. 65:311–330. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lu C and Kerbel RS: Interleukin-6
undergoes transition from paracrine growth inhibitor to autocrine
stimulator during human melanoma progression. J Cell Biol.
120:1281–1288. 1993.PubMed/NCBI
|
|
8.
|
Wu X, Lee VC, Chevalier E and Hwang ST:
Chemokine receptors as targets for cancer therapy. Curr Pharm Des.
15:742–757. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kato M, Kitayama J, Kazama S and Nagawa H:
Expression pattern of CXC chemokine receptor-4 is correlated with
lymph node metastasis in human invasive ductal carcinoma. Breast
Cancer Res. 5:R144–150. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Liang Z, Brooks J, Willard M, Liang K,
Yoon Y, Kang S and Shim H: CXCR4/CXCL12 axis promotes VEGF-mediated
tumor angiogenesis through Akt signaling pathway. Biochem Biophys
Res Commun. 359:716–722. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Morales J, Homey B, Vicari AP, et al:
CTACK, a skin-associated chemokine that preferentially attracts
skin-homing memory T cells. Proc Natl Acad Sci USA. 96:14470–14475.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Zlotnik A and Yoshie O: Chemokines: a new
classification system and their role in immunity. Immunity.
12:121–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Campbell JJ and Butcher EC: Chemokines in
tissue-specific and microenvironment-specific lymphocyte homing.
Curr Opin Immunol. 12:336–341. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Butcher EC, Williams M, Youngman K, Rott L
and Briskin M: Lymphocyte trafficking and regional immunity. Adv
Immunol. 72:209–253. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Klein RS, Rubin JB, Gibson HD, et al:
SDF-1 alpha induces chemotaxis and enhances Sonic hedgehog-induced
proliferation of cerebellar granule cells. Development.
128:1971–1981. 2001.PubMed/NCBI
|
|
16.
|
Peled A, Petit I, Kollet O, et al:
Dependence of human stem cell engraftment and repopulation of
NOD/SCID mice on CXCR4. Science. 283:845–848. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Eash KJ, Means JM, White DW and Link DC:
CXCR4 is a key regulator of neutrophil release from the bone marrow
under basal and stress granulopoiesis conditions. Blood.
113:4711–4719. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Salogni L, Musso T, Bosisio D, et al:
Activin A induces dendritic cell migration through the polarized
release of CXC chemokine ligands 12 and 14. Blood. 113:5848–5856.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Monterrubio M, Mellado M, Carrera AC and
Rodriguez-Frade JM: PI3Kgamma activation by CXCL12 regulates tumor
cell adhesion and invasion. Biochem Biophys Res Commun.
388:199–204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Sozzani S, Allavena P, D’Amico G, et al:
Differential regulation of chemokine receptors during dendritic
cell maturation: a model for their trafficking properties. J
Immunol. 161:1083–1086. 1998.PubMed/NCBI
|
|
21.
|
Bottazzi B, Walter S, Govoni D, Colotta F
and Mantovani A: Monocyte chemotactic cytokine gene transfer
modulates macrophage infiltration, growth, and susceptibility to
IL-2 therapy of a murine melanoma. J Immunol. 148:1280–1285.
1992.
|
|
22.
|
Seo KS, Park JY, Davis WC, et al:
Superantigen-mediated differentiation of bovine monocytes into
dendritic cells. J Leukoc Biol. 85:606–616. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Leng Q, Nie Y, Zou Y and Chen J: Elevated
CXCL12 expression in the bone marrow of NOD mice is associated with
altered T cell and stem cell trafficking and diabetes development.
BMC Immunol. 9:512008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Sasaki K, Natsugoe S, Ishigami S, et al:
Expression of CXCL12 and its receptor CXCR4 in esophageal squamous
cell carcinoma. Oncol Rep. 21:65–71. 2009.PubMed/NCBI
|
|
25.
|
Xu Q, Yuan X, Xu M, et al: Chemokine CXC
receptor 4-mediated glioma tumor tracking by bone marrow – derived
neural progenitor/stem cells. Mol Cancer Ther. 8:2746–2753.
2009.PubMed/NCBI
|
|
26.
|
Uchida D, Onoue T, Begum NM, et al:
Vesnarinone downregulates CXCR4 expression via upregulation of
Kruppel-like factor 2 in oral cancer cells. Mol Cancer. 8:622009.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Mirisola V, Zuccarino A, Bachmeier BE, et
al: CXCL12/SDF1 expression by breast cancers is an independent
prognostic marker of disease-free and overall survival. Eur J
Cancer. 45:2579–2587. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Sauter ER and Herlyn M: Molecular biology
of human melanoma development and progression. Mol Carcinog.
23:132–143. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Vandercappellen J, Van Damme J and Struyf
S: The role of CXC chemokines and their receptors in cancer. Cancer
Lett. 267:226–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Wendt MK, Johanesen PA, Kang-Decker N,
Binion DG, Shah V and Dwinell MB: Silencing of epithelial CXCL12
expression by DNA hypermethylation promotes colonic carcinoma
metastasis. Oncogene. 25:4986–4997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Kucia M, Jankowski K, Reca R, et al:
CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol
Histol. 35:233–245. 2004. View Article : Google Scholar : PubMed/NCBI
|