Introduction
Polysaccharide K (PSK; Kureha Chemical Industry Co.,
Ltd., Tokyo, Japan) is a protein-bound polysaccharide widely used
as a non-specific immunotherapeutic agent and is derived from the
cultured mycelia of Coriolus versicolor. This
protein-polysaccharide complex, which has a molecular weight of
approximately 940,000 Da, contains approximately 38% protein and a
saccharide portion consisting of a glucan with approximately 75%
glucose and smaller amounts of mannose, xylose and galactose
(1). To date, PSK has been
administered primarily to patients with gastric cancer, colon
cancer and other gastrointestinal malignancies. Torisu et al
reported that patients with curatively resected colon cancer had a
significantly improved survival rate when treated with PSK
(2). Yoshitani and Takashima
(3) and Ohwada et al
(4), who used PSK in combination
with anticancer agents to treat curatively resected patients, also
reported significantly improved survival in the patients who
received PSK compared with those who did not.
The following main mechanisms of action of PSK on
malignancies have been identified to date: i) direct apoptosis
induction, inhibition of cellular infiltration and enhancement of
MHC class-I expression; ii) enhancement of natural killer,
cytotoxic T and lymphokine-activated killer activation and
regulation of cytokine production; and iii) suppression of TGF-β
production and reduction of oxidative stress (5–8). PSK
also has a variety of immunostimulatory effects as a biochemical
response modifier. Liver, lung and other hematogenous metastases
are considered to be prognostic factors in colon cancer.
Hematogenous metastases of colon cancer are generally believed to
occur when cancer cells detach from the primary tumor, invade the
capillaries and spread systemically via the portal and greater
circulatory systems prior to adhering to vascular endothelial cells
in the target organ, escaping and infiltrating outside blood
vessels and proliferating (9,10).
Previous characterization of the mechanisms of metastasis has
identified key angiogenic growth factors in this process (11–13).
Therefore, we investigated the changes induced by PSK in angiogenic
growth factors, angiogenesis inhibitors and related genes in colon
cancer cells, and whether PSK suppresses angiogenesis.
Materials and methods
Cell culture and PSK stimulation
Human colorectal cancer cell lines, SW620, HT29 and
HCT116 (obtained from European collection of cell cultures, UK),
were cultured at 37°C in 5% CO2 in RPMI-1640 medium
containing 10% fetal bovine serum (14). Cells were seeded (5x105)
into 6-cm dishes in triplicate with PSK for 2 days.
Cell viability
Apoptosis was detected by flow cytometry using
Annexin V Detection kit (Nanjing KeyGen Biotech, Nanjing, China).
Briefly, cells were double stained with Annexin V-TIRIC for 15 min
at 37°C. After cells were washed thrice in PBS, we detected non-red
cells under a fluorescent microscope.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
The total RNA was extracted from the colorectal
cancer cells using guanidinium-thiocyanate (15,16).
Single strand cDNA was prepared from 3 μg of total RNA using
Moloney murine leukemia virus reverse transcriptase (Takara Bio,
Inc., Shiga, Japan). The primers for PCR amplification of the
HIF-1α gene-coding regions were as follows: 5′ primer; HIF-1α
-AX,GGACAAGTCACCACAGGA, 3′ primer; HIF-1α -BX,GGAGAAAATCAAGTCGTG.
GAPDH amplification was used as an internal PCR control with
5′-GGGGAGCCAAAAGGGTCATCATCT-3′ as the sense primer and
5′-GACGCCTGCTTCACCACCTTCTTG-3′ as the antisense primer. A total of
23 cycles of denaturation (94°C, 1 min), annealing (50°C, 1.5 min)
and extension (72°C, 2 min) were carried out in a thermal cycler
(PTC-100, Programmable Thermal Controller, NJ Research Inc., MA,
USA). The PCR products (10 μl) which demonstrated the relevant
bands in RT-PCR analysis were sequenced by electrophoresis in 1.2%
agarose gel. The sequencing was performed on PCR products that
showed the bands in RT-PCR analysis.
RT2 Profiler™ PCR array and real-time
PCR
Total RNA was extracted from colon cancer cells
using guanidiniumthiocyanate. Real-time PCR was performed according
to the manufacturer’s instructions included with the RT2 Profiler
PCR array system (angiogenic growth factors and angio-genesis
inhibitors; PCR array: catalog no. PAHS-072A; SA Bioscience,
Valencia, CA, USA). The data were analyzed using Excel-based PCR
array data analysis templates.
In vitro tube formation assay
Following preparation of the cells described above,
the medium was removed from all dishes and replaced with fresh
complete medium. After two days, each culture fluid was collected
and added to wells of an angiogenesis kit (Kurabo Company, Japan).
Fields from each sample were photographed and total tube length was
analyzed by the MacSCOPE program (Mitani Company, Tokyo, Japan).
The control tube areas were defined as 100% tube formation and the
percent increase in tube formation as compared with the control was
calculated for each sample (17).
Statistical considerations
Other characteristics of the two treatment methods
were compared using the Chi-square test. P<0.05 was considered
to indicate a statistically significant result.
Results
Cell viability
The colon cancer cells analyzed under a fluorescence
microscope using the Annexin-V assay demonstrated no increased cell
apoptosis and death in samples treated with PSK (100 or 300 μg/ml)
compared with untreated cells. Cells treated with 500 μg/ml
demonstrated an increase in cell apoptosis and death (Table I).
 | Table ICell viability following exposure to
PSK. |
Table I
Cell viability following exposure to
PSK.
| PSK (μg/ml) | Annexin V staining
(%) |
|---|
| 0 | 3.2 |
| 100 | 3.5 |
| 300 | 3.8 |
| 500 | 10.0 |
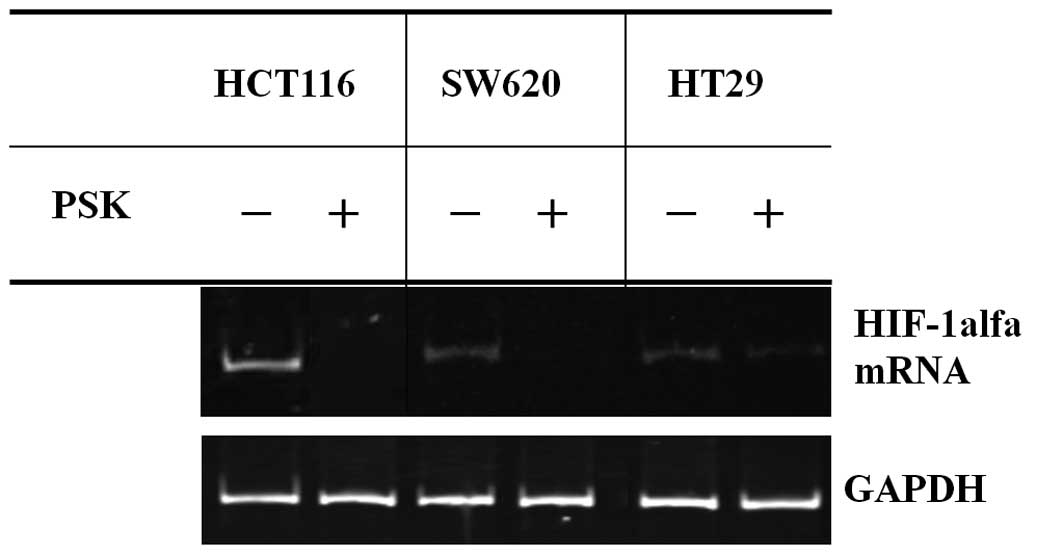
HIF-1α mRNA expression with PSK exposure
in colon cancer cell lines
RT-PCR was used to investigate HIF-1α mRNA
expression in colon cancer cell lines. The results are shown in
Fig. 1. Although the expression of
HIF-1α mRNA was detected in colon cancer cell lines, the addition
of PSK suppressed HIF-1α mRNA expression in colon cancer cell
lines.
Expression of angiogenic growth factors
in colon cancer cell lines treated with PSK
PCR array was used to investigate how the addition
of PSK to colon cancer cell lines affected levels of angiogenic
growth factors and related genes. A comparison of levels in these
cells to those in untreated colon cancer cell lines cultured is
listed in Table II. Typical genes
that were expressed at lower levels included gastrin-releasing
peptide (GRP), interleukin 8 (IL8) and platelet-derived growth
factor β polypeptide (PDGFB) in HCT116, EGF-like repeats and
discoidin I-like domains 3 (EDIL3) in SW620 and chemokine (C-X-C
motif) ligand 9 (CXCL9), fibroblast growth factor binding protein 1
(FGFBP1) and interleukin 8 (IL8) in the HT29 cell line. Numerous
other angiogenic growth factors and the expression of related genes
were reduced in all cell types.
 | Table IIRepresentative list of downregulated
genes in PSK-stimulated cells (angiogenic growth factors and
related genes). |
Table II
Representative list of downregulated
genes in PSK-stimulated cells (angiogenic growth factors and
related genes).
| Cell line | Gene Bank | Description | Ratio |
|---|
| HCT116 | Hs.153444 | GRP,
gastrin-releasing peptide | −5.2635 |
| Hs.624 | IL8, interleukin
8 | −4.0425 |
| Hs.1976 | PDGFB,
platelet-derived growth factor β polypeptide | −4.9113 |
| SW620 | Hs.482730 | EDIL3, EGF-like
repeats and discoidin I-like domains 3 | −11.0357 |
| HT29 | Hs.77367 | CXCL9, chemokine
(C-X-C motif) ligand 9 | −28.9895 |
| Hs.1690 | FGFBP1, fibroblast
growth factor binding protein 1 | −4.4097 |
| Hs.624 | IL8, interleukin
8 | −19.315 |
Expression of angiogenesis inhibitors in
colon cancer cell lines treated with PSK
PCR array was used to investigate how the addition
of PSK to colon cancer cell lines affected levels of angiogenesis
inhibitors and related genes. A comparison of levels in these cells
to those in untreated colon cancer cell lines cultured at 20% CO2
is listed in Table III. Typical
genes that were expressed at higher levels included TIMP
metallopeptidase inhibitor (TIMP1) in HCT116 and interleukin 12A
(IL12A) and troponin I type 3 (TNNI3) in the HT29 cell line. There
were no typical genes with an altered expression pattern in the
SW620 cell line.
 | Table IIIRepresentative list of upregulated
genes in PSK-stimulated cells (angiogenesis inhibitors and related
genes). |
Table III
Representative list of upregulated
genes in PSK-stimulated cells (angiogenesis inhibitors and related
genes).
| Cell line | Gene Bank | Description | Ratio |
|---|
| HCT116 | Hs.522632 | TIMP1, TIMP
metallopeptidase inhibitor 1 | 5.7541 |
| SW620 | - | - | - |
| HT29 | Hs.673 | IL12A, interleukin
12A (natural killer cell stimulatory factor 1, cytotoxic lymphocyte
maturation factor 1, p35) | 17.1 |
| Hs.644596 | TNNI3, troponin I
type 3 (cardiac) | 4.1713 |
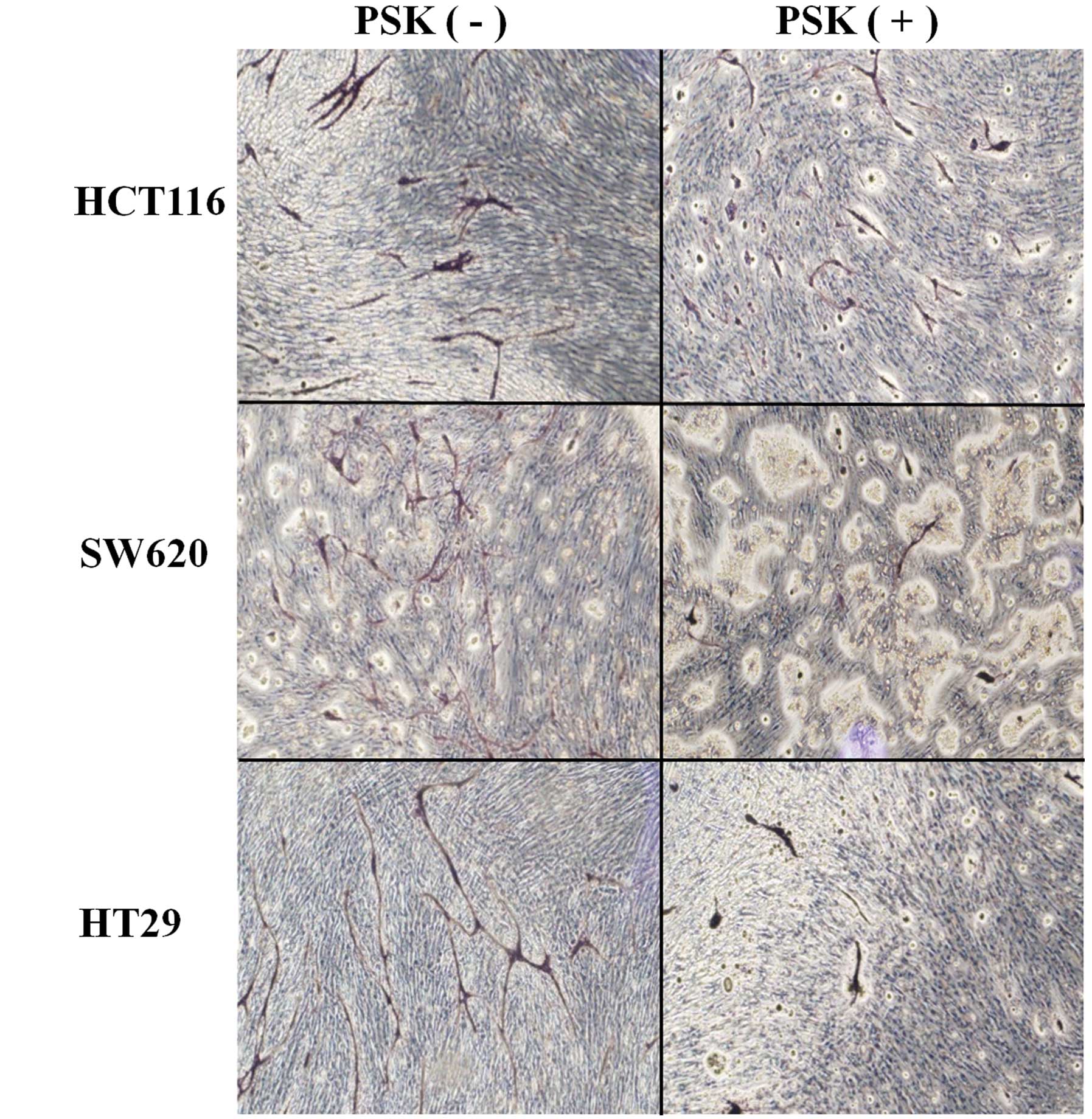
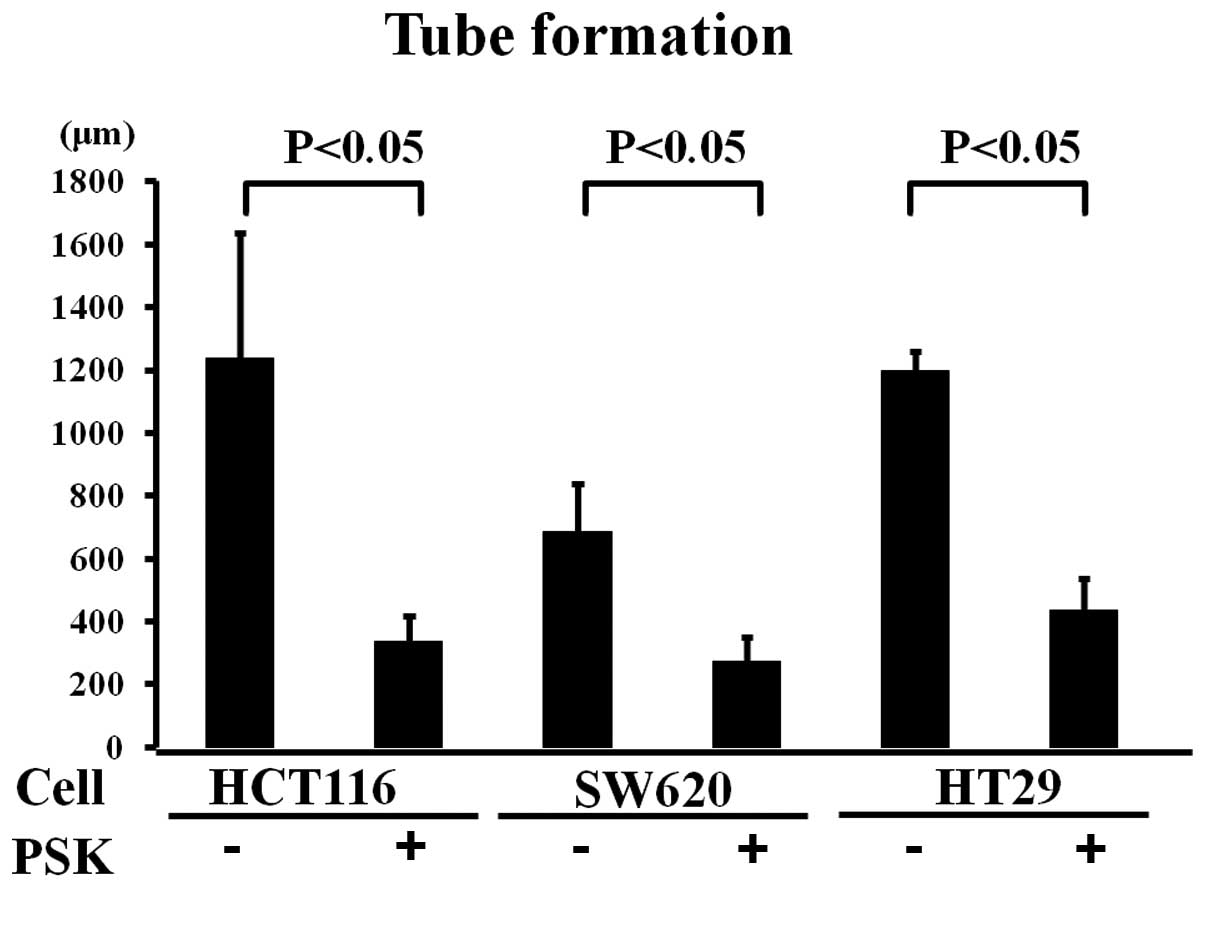
Tube formation in colon cancer cell lines
treated with or without PSK
The medium from PSK-treated colon cancer cell lines
was applied to the wells of a tube formation assay to investigate
the effects of PSK on the elongation of tube formation. Tube
elongation in the medium of untreated colon cancer cell lines was
taken to be 100%, elongation was 40% in SW620, 27% in HCT116 and
36.5% in HT29 cells cultured in the medium of PSK-treated colon
cancer cell lines (Figs. 2 and
3). Elongation was therefore
significantly less than that observed in the medium of non-treated
colon cancer cell lines.
Discussion
PSK, derived from the cultured mycelia of C.
versicolor, is widely used as a nonspecific immunotherapeutic
agent (1,5–8). The
efficacy of PSK has been demonstrated to increase survival in
patients with gastrointestinal malignancies, including gastric and
colon cancer. Hematogenous metastases are considered to be a
prognostic factor in colon cancer, and PSK is believed to act in
the process leading to these metastases, thereby increasing
survival (2–4). It has been reported that the
occurrence of hematogenous metastases in colon cancer is closely
correlated with increased angiogenesis, and angiogenic growth
factors and angiogenic growth inhibiting factors likely contribute
to the induction and propagation of angiogenesis and may eventually
promote hematogenous metastases (9–13).
We investigated how the addition of PSK to the
medium of cultured colon cancer cell lines affects the expression
of the HIF-1α gene, which is closely associated with the expression
of angiogenic growth factors, in addition to angiogenic growth
factors and angiogenesis (18–23).
The expression of HIF-1α mRNA was detected in colon
cancer cell lines, but the addition of PSK suppressed HIF-1α mRNA
expression. The HIF-1α gene is believed to activate the production
of numerous angiogenic growth factors, and has various effects on
cancer, regulating at least 70 genes, most of which promote cancer
(18–23). Also HIF-1α gene, oncogene and tumor
suppressor gene intricately linked with the expression of
angiogenic growth factors and angiogenesis inhibitors (24). A PCR array was then used to
investigate the affected angiogenic growth factors and angiogenesis
inhibitors. Although the suppression of genes differed between the
cell lines studied, the addition of PSK suppressed numerous
angiogenic growth factors and increased levels of angiogenesis
inhibitors.
When the untreated colon cancer cell lines were used
in a tube formation system, tube formation was promoted. By
contrast, when the PSK-treated colon cancer cell lines were used,
tube formation was reduced, which indicates that PSK acts to
suppress angiogenesis in the strains of colon cancer cells
studied.
The effects of PSK identified in the present study
include the suppression of HIF-1α gene expression, the suppression
of angiogenic growth factors and the enhancement of angiogenesis
inhibitors in colon cancer cells. These findings demonstrate the
potential of PSK to ultimately suppress angiogenesis.
References
|
1.
|
Tsukagoshi S, Hashimoto Y, Fujii G,
Kobayashi H, Nomoto K and Orita K: Krestin (PSK). Cancer Treat Rev.
11:131–155. 1984. View Article : Google Scholar
|
|
2.
|
Torisu M, Hayashi Y, Ishimitsu T, Fujimura
T, Iwasaki K, Katano M, Yamamoto H, Kimura Y, Takesue M, Kondo M
and Nomoto K: Significant prolongation of disease-free period
gained by oral polysaccharide K (PSK) administration after curative
surgical operation of colorectal cancer. Cancer Immunol Immunother.
31:261–268. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Yoshitani S and Takashima S: Efficacy of
postoperative UFT (Tegafur/Uracil) plus PSK therapies in elderly
patients with resected colorectal cancer. Cancer Biother
Radiopharm. 24:35–40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Ohwada S, Ikeya T, Yokomori T, Kusaba T,
Roppongi T, Takahashi T, Nakamura S, Kakinuma S, Iwazaki S,
Ishikawa H, et al: Adjuvant immunochemotherapy with oral
Tegafur/Uracil plus PSK in patients with stage II or III colorectal
cancer: a randomized controlled study. Br J Cancer. 90:1003–1010.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Araya S, Nio Y, Hayashi H, Masai Y,
Tsubono M, Ishigami S and Imamura M: Various plant-derived
polysaccharides augment the expression of HLA on Colo205 human
colonic cancer line. J Jpn Soc Cancer Ther. 29:1965–1973. 1994.(In
Japanese).
|
|
6.
|
Hirose K, Zachariae CO, Oppenheim JJ and
Matsushima K: Induction of gene expression and production of
immunomodulating cytokines by PSK in human peripheral blood
mononuclear cells. Lymphokine Res. 9:475–483. 1990.PubMed/NCBI
|
|
7.
|
Algarra I, Collado A, Garcia Lora A and
Garrido F: Differential effect of protein-bound polysaccharide
(PSK) on survival of experimental murine tumors. J Exp Clin Cancer
Res. 18:39–46. 1999.PubMed/NCBI
|
|
8.
|
Harada M, Matsunaga K, Oguchi Y, Iijima H,
Tamada K, Abe K, Takenoyama M, Ito O, Kimura G and Nomoto K: Oral
administration of PSK can improve the impaired anti-tumor
CD4+ T-cell response in gut-associated lymphoid tissue
(GALT) of specific-pathogen-free mice. Int J Cancer. 70:362–372.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Fidler IJ and Ellis LM: The implications
of angiogenesis for the biology and therapy of cancer metastasis.
Cell. 79:185–188. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Stoeltzing O, Liu W, Reinmuth N, Parikh A,
Ahmad SA, Jung YD, Fan F and Ellis LM: Angiogenesis and
antiangiogenic therapy of colon cancer liver metastasis. Ann Surg
Oncol. 10:722–733. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Ishigami SI, Arii S, Furutani M, Niwano M,
Harada T, Mizumoto M, Mori A, Onodera H and Imamura M: Predictive
value of vascular endothelial growth factor (VEGF) in metastasis
and prognosis of human colorectal cancer. Br J Cancer.
78:1379–1384. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Tokunaga T, Oshika Y, Abe Y, Ozeki Y,
Sadahiro S, Kijima H, Tsuchida T, Yamazaki H, Ueyama Y, Tamaoki N
and Nakamura M: Vascular endothelial growth factor (VEGF) mRNA
isoform expression pattern is correlated with liver metastasis and
poor prognosis in colon cancer. Br J Cancer. 78:998–1002. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Goi T, Yamaguchi A, Nakagawara G, Urano T,
Shiku H and Furukawa K: Reduced expression of deleted colorectal
carcinoma (DCC) protein in established colon cancers. Br J Cancer.
77:466–471. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Fujishima Y, Goi T, Kimura Y, Hirono Y,
Katayama K and Yamaguchi A: MUC2 protein expression status is
useful in assessing the effects of hyperthermic intraperitoneal
chemotherapy for peritoneal dissemination of colon cancer. Int J
Oncol. 40:960–964. 2012.
|
|
16.
|
Goi T, Fujioka M, Satoh Y, Tabata S,
Koneri K, Nagano N, Hirono Y, Katayama K, Hirose K and Yamaguchi A:
Angiogenesis and tumor proliferation/metastasis of human colorectal
cancer cell line SW620 transfected with endocrine
glands-derived-vascular endothelial growth factor, as a new
angiogenic factor. Cancer Res. 64:1906–1910. 2004. View Article : Google Scholar
|
|
17.
|
Nagano H, Goi T, Koneri K, Hirono Y,
Katayama K and Yamaguchi A: Endocrine gland-derived vascular
endothelial growth factor (EG-VEGF) expression in colorectal
cancer. J Surg Oncol. 96:605–610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Semenza GL: Oxygen homeostasis. Wiley
Interdiscip Rev Syst Biol Med. 2:336–361. 2010. View Article : Google Scholar
|
|
19.
|
Semenza GL: HIF-1 inhibitors for cancer
therapy: from gene expression to drug discovery. Curr Pharm Des.
15:3839–3943. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Liao D and Johnson RS: Hypoxia: a key
regulator of angiogenesis in cancer. Cancer Metastasis Rev.
26:281–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Chan DA and Giaccia AJ: Hypoxia, gene
expression, and metastasis. Cancer Metastasis Rev. 26:333–339.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Zhou J, Schmid T, Schnitzer S and Brüne B:
Tumor hypoxia and cancer progression. Cancer Lett. 237:10–21. 2006.
View Article : Google Scholar
|
|
23.
|
Harris AL: Hypoxia – a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002.
|
|
24.
|
Schmid T, Zhou J, Köhl R and Brüne B: p300
relieves p53-evoked transcriptional repression of hypoxia-inducible
factor-1 (HIF-1). Biochem J. 380:289–295. 2004. View Article : Google Scholar : PubMed/NCBI
|