Introduction
Rheumatoid arthritis affects approximately 1% of the
general population and is characterized by the inflammatory
propagation of synovial cells caused by articular injuries,
resulting in an almost complete functional defect (1). Non-steroidal anti-inflammatory drugs
(NSAIDs) are commonly used for the treatment of rheumatoid
arthritis despite their gastric and renal toxicities (2). Although NSAIDs are efficient at
reducing symptoms, including pain and edema, they have no effect on
the basic disease process and do not protect against tissue or
joint injury (3). Furthermore,
NSAID treatment has been shown to enhance joint destruction in
osteoarthritis (4) and inhibit the
synthesis of glycosaminoglycans by articular chondrocytes (5).
Glucosamine (GlcN), a naturally occurring amino
monosaccharide, is present in the connective and cartilage tissues
and contributes to the maintenance of the strength, flexibility and
elasticity of these tissues. GlcN has been widely used to treat
osteoarthritis in humans (6). A
number of short- and long-term clinical trials have demonstrated
the significant symptom-modifying effects of GlcN in osteoarthritis
(7–9). According to previous biochemical and
pharmacological findings, the administration of GlcN normalizes
cartilage metabolism by inhibiting degradation (10) and stimulating the synthesis of
proteoglycans (11,12), and restores articular function.
Additionally, GlcN has been reported to have an anti-inflammatory
effect through inhibition of the production of inflammatory
mediators, including nitric oxide (NO) and prostaglandin
E2 (PGE2) (13).
Methylsulfonylmethane (MSM), a sulfur compound, is
effective at treating osteoarthritis and, in combination with GlcN,
demonstrates greater efficacy in the reduction of pain and swelling
and the improvement of the functional ability of joints compared to
the individual agents in humans (14). MSM also has an anti-inflammatory
effect on type II collagen-induced arthritis in rats (15). Furthermore, MSM inhibits the
lipopolysaccharide (LPS)-induced production of NO and
PGE2 in the mouse macrophage-like cell RAW264.7
(16). Chondroitin sulfate,
another sulfur compound, reduces pain, prevents narrowing of the
knee joint space in humans (17)
and inhibits the nuclear translocation of nuclear factor κB (NF-κB)
in interleukin (IL)-1β-stimulated chondrocytes (18). Since sulfur compounds, such as MSM
and chondroitin sulfate, have anti-inflammatory effects,
methionine, a sulfur-containing amino acid, is also expected to
exhibit an anti-inflammatory effect. However, no experimental data
on the anti-inflammatory effects of methionine are currently
available. Thus, in the present study, we evaluated the effect of
methionine combined with GlcN on inflammation in the adjuvant
arthritis model in rats.
Materials and methods
Animals
Female Lewis rats were purchased from Charles River
Laboratories Japan, Inc. (Kanagawa, Japan). The animals were housed
under specific pathogen-free conditions (controlled temperature of
24±3°C and humidity of 55±15%) and fed standard laboratory food and
water ad libitum. To induce arthritis, rats were injected
with Freund’s complete adjuvant (FCA), as described below. Animals
received proper care and maintenance in accordance with
institutional guidelines (Juntendo University, Graduate School of
Medicine, Tokyo, Japan). The experiments adhered to the guidelines
of the International Association for the Study of Pain (19).
Induction of adjuvant arthritis
Adjuvant arthritis was induced in 8-week-old rats by
a single intradermal injection of 0.1 ml FCA containing 0.5 mg
heat-killed M. tuberculosis H37Ra emulsified in liquid
paraffin (Wako Pure Chemical Industries, Osaka, Japan) into the
footpad of the right hind paw (20,21).
Methionine (20 mg/ml) and GlcN (40 mg/ml; Protein
Chemical Co., Ltd., Tokyo, Japan) were dissolved in 0.5% sodium
carboxymethyl cellulose (CMC). Methionine and/or GlcN were orally
administered by gavage to adjuvant-injected rats twice a day for 21
days at a dose of 200 mg/kg/day methionine and 400 mg/kg/day GlcN.
As a vehicle control, 0.5% CMC solution was administered orally to
rats with adjuvant arthritis instead of methionine or GlcN. Naïve
control rats received orally administered 0.5% CMC, but were not
injected with adjuvant. Six animals were used in each experimental
group (naïve control, vehicle control, methionine, GlcN or
methionine combined with GlcN).
Evaluation of arthritis
The swelling of hind paws was monitored using a
plethysmometer (TK-105, Muromachi Kikai Co., Ltd., Tokyo, Japan)
prior to (day 0) and following (days 5, 8, 12, 15, 19 and 21) the
FCA injection. The progression of adjuvant arthritis was clinically
evaluated for the characteristic signs and symptoms, using an
arthritis score that grades each paw from 0 to 4 points based on
erythema and swelling of the joint (0 points, no erythema or
swelling; 1 point, erythema or swelling of one toe; 2 points,
erythema or swelling of two or more of the toes; 3 points, erythema
and swelling of the entire paw; 4 points, complete erythema and
swelling of the entire paw and an inability to bend the ankle)
(21). Three paws, excluding the
FCA-injected right hind paw (FCA-uninjected left hind paw and right
and left fore paws) were scored and the highest possible score was
12.
Histopathological evaluation of knee
joints
Animals were sacrificed on day 22. The FCA-injected
right and -uninjected left legs were resected above the ankle
joints, and fixed in neutral 20% formalin. Following
decalcification in formic acid, the knee joints were sectioned
longitudinally and tissue sections (10 μm) were mounted on glass
slides and stained with hematoxylin and eosin. Articular lesions
were observed under a light microscope. Synovitis (synovial
hyperplasia) was evaluated by measuring the area of the synovial
membrane attached to the articular meniscus.
Quantification of NO, PGE2 and
hyaluronic acid (HA) in rat plasma
Blood samples were collected from the abdominal
aorta under ether anesthesia on day 22. The heparin-anticogulated
blood was centrifuged at 1500 x g for 10 min at 4°C to separate the
plasma.
The total NO (nitrite and nitrate) level in the
plasma was measured using a nitrate/nitrite colorimetric assay kit,
according to the manufacturer’s instructions (Cayman Chemical
Company, Ann Arbor, MI, USA). The sensitivity of the assay was
<2.5 μM. The level of PGE2 in the plasma was measured
using an enzyme-linked immunosorbent assay method, according to the
manufacturer’s instructions (Cayman Chemical Company). The assay
revealed no cross reactivity with other prostanoids, and the
sensitivity was <15 pg/ml. HA levels in the plasma were measured
using a hyaluronan assay kit, according to the manufacturer’s
instructions (Seikagaku Biobusiness Corporation, Tokyo, Japan). The
detection limit of the assay was 12.5 ng/ml.
Statistical analysis
Statistical analyses of paw volume and arthritis
score were performed using the Dunnett and Mann-Whitney U tests,
respectively. Synovial membrane area and the levels of NO,
PGE2 and HA were statistically analyzed using the
Student’s t-test. Data were presented as the mean ± SE. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of methionine and GlcN on the
inflammatory reaction in FCA-induced rat adjuvant arthritis
The swelling of hind paws was examined by measuring
changes in paw volume. As shown in Fig. 1A, the swelling of the
adjuvant-injected right hind paws increased rapidly after the
injection, reached a maximum on day 5, and maintained an almost
constant level until day 21 in the vehicle control rats.
Administration of methionine and/or GlcN did not substantially
change the swelling of adjuvant-injected right hind paws during the
observation period, although GlcN administration slightly, but
significantly, suppressed the swelling on days 12 and 21.
 | Figure 1Effects of individual and combined
administrations of methionine and GlcN on the swelling of hind paws
in rat adjuvant arthritis. Adjuvant arthritis was induced by a
single intradermal injection of FCA into the footpad of the right
hind paw. Methionine (200 mg/kg/day; ▪), GlcN (400 mg/kg/day; ▴) or
methionine combined with GlcN (♦) was administered orally for 21
days. Swelling of (A) FCA-injected right and (B) uninjected left
hind paws was monitored using a plethysmometer before (day 0) and
after (days 5, 8, 12, 15, 19 and 21) the FCA injection. As a
vehicle control (•), 0.5% CMC solution was administered orally to
rats with adjuvant arthritis instead of methionine or GlcN. Naïve
control rats received orally administered 0.5% CMC, but were not
injected with adjuvant (○). Data are the mean ± SE of six animals
per experimental group. Values were compared between vehicle
control and methionine, GlcN, or methionine combined with GlcN
administration in adjuvant arthritis. *P<0.05,
**P<0.01. GlcN, glucosamine; FCA, Freund’s complete
adjuvant; CMC, carboxymethyl cellulose. |
By contrast, the swelling of adjuvant-uninjected
left hind paws gradually increased between days 12 and 21 (Fig. 1B). However, GlcN administration
moderately suppressed the swelling (P<0.05) compared with the
vehicle control. The administration of methionine combined with
GlcN further suppressed the swelling on days 19 and 21 (P<0.01
compared with vehicle control). These observations suggest that the
combined administration of methionine and GlcN suppresses the
inflammatory reaction in FCA-induced adjuvant arthritis more
efficiently compared with the single administration of methionine
or GlcN.
Effects of methionine and GlcN on an
arthritis score in adjuvant arthritis
The arthritis score was based on the severity and
extent of erythema and swelling of the periarticular tissues, and
the enlargement, distortion or ankylosis of the joints (21,22).
In rats with adjuvant arthritis, the arthritis score increased
progressively, and reached 11 points on day 21 (Fig. 2). Administration of methionine or
GlcN slightly suppressed the increase in arthritis scores between
days 12 and 21. Notably, the administration of methionine combined
with GlcN further suppressed the increase in arthritis score
between days 15 and 21, although the suppression was not
statistically significant. Thus, the combined administration of
methionine and GlcN more potently relieved the clinical signs and
symptoms of adjuvant arthritis than individual administrations of
methionine or GlcN.
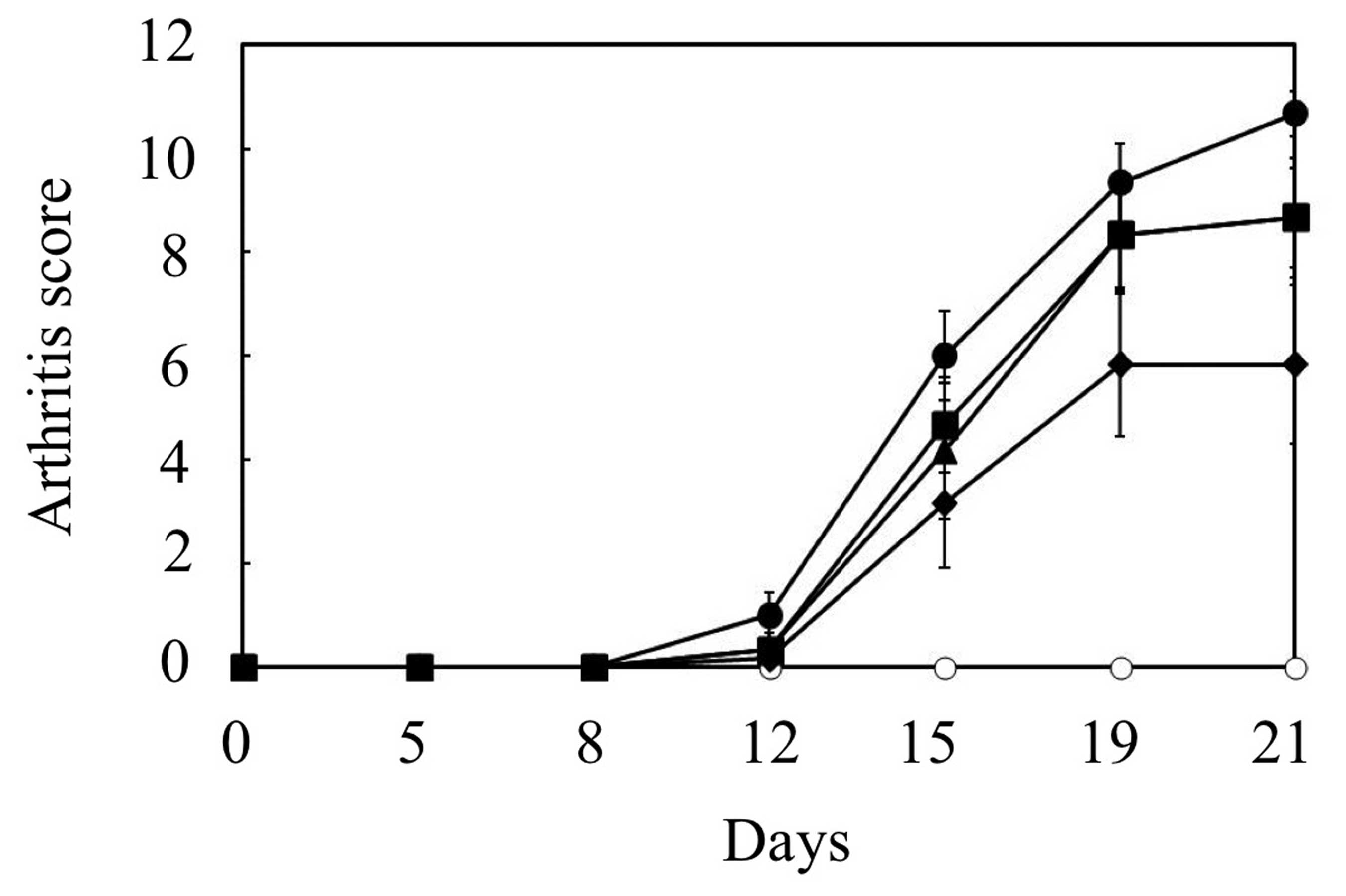 | Figure 2Effects of individual and combined
administrations of methionine and GlcN on arthritis scores in rat
adjuvant arthritis. Adjuvant arthritis was induced by a single
intradermal injection into the footpad of the right hind paw.
Methionine (200 mg/kg/day; ▪), GlcN (400 mg/kg/day; ▴) or
methionine combined with GlcN (♦) was administered orally for 21
days. Arthritis was clinically evaluated using an arthritis score
by grading each paw (excluding the FCA-injected right paw) from 0
to 4 points based on erythema and swelling of the joint before (day
0) and after (days 5, 8, 12, 15, 19 and 21) the FCA injection. As a
vehicle control (•), 0.5% CMC solution was administered orally to
rats with adjuvant arthritis instead of methionine or GlcN. Naïve
control rats received orally administered 0.5% CMC, but were not
injected with adjuvant (○). Data are the mean ± SE of six animals
per experimental group. GlcN, glucosamine; FCA, Freund’s complete
adjuvant; CMC, carboxymethyl cellulose. |
Effects of methionine and GlcN on
histopathological changes in the joints in adjuvant arthritis
Histopathological examination indicated that the
cartilage surface and articular meniscus were destroyed by synovial
hyperplasia in the knee joints of the FCA-injected right hind paws
(Fig. 3B). Although synovial
hyperplasia was observed, the destruction of the cartilage surface
and articular meniscus was apparently suppressed in
methionine-administered rats (Fig.
3C). Of note, the administration of GlcN or methionine combined
with GlcN potently suppressed synovial hyperplasia and the
destruction of the cartilage surface and articular meniscus in rats
with adjuvant arthritis (Fig. 3D and
E). The area of the synovial membrane was measured to evaluate
the inflammatory reaction. The synovial membrane area was found to
be increased by ∼2-fold in rats with adjuvant arthritis compared
with naïve control rats (Fig. 3F).
The administration of GlcN or methionine combined with GlcN notably
suppressed the increase in synovial membrane area, whereas
methionine administration only slightly abrogated the increase in
synovial membrane area (Fig. 3F).
These observations indicate that the administration of GlcN, or
methionine combined with GlcN markedly suppressed the
histopathological changes induced by inflammatory reactions in the
knee joints of rats with adjuvant arthritis.
 | Figure. 3.Effects of individual and combined
administrations of methionine and GlcN on histopathological changes
in FCA-injected right hind paw joints. Adjuvant arthritis was
induced by a single intradermal injection of FCA into the footpad
of the right hind paw. (C) Methionine (200 mg/kg/day), (D) GlcN
(400 mg/kg/day or (E) methionine combined with GlcN was
administered orally for 21 days. (B) As a vehicle control, 0.5% CMC
solution was administered orally to rats with adjuvant arthritis
instead of methionine or GlcN. (A) Naïve control rats received
orally administered 0.5% CMC, but were not injected with adjuvant.
On day 22, the right hind legs were resected, fixed and
decalcified. The knee joints were longitudinally sectioned, and
tissue sections (10 μm) were mounted on glass slides and stained
with hematoxylin and eosin. Images are representative of 4 rats per
group. (B) Synovial hyperplasia and destruction of the cartilage
surface and articular meniscus are indicated by arrows and
arrowheads, respectively. (F) Synovial hyperplasia was evaluated by
measuring the area of the synovial membrane attached to the
articular menisci using a K400 image analysis system. Data are the
mean ± SE of four animals per experimental group. GlcN,
glucosamine; FCA, Freund’s complete adjuvant; CMC, carboxymethyl
cellulose. |
Effects of methionine and GlcN on the
plasma levels of NO, PGE2 and HA in adjuvant
arthritis
NO is a gaseous-free radical which has been shown to
be present at increased levels in the sera and synovial fluids of
patients with rheumatoid arthritis (23). As shown in Fig. 4A, plasma NO levels in the rats with
FCA-induced adjuvant arthritis were increased compared with the
naïve control rats. Combined methionine and GlcN administration
markedly reduced the levels of NO in the plasma of rats with
adjuvant arthritis (P<0.05 compared with vehicle control
rats).
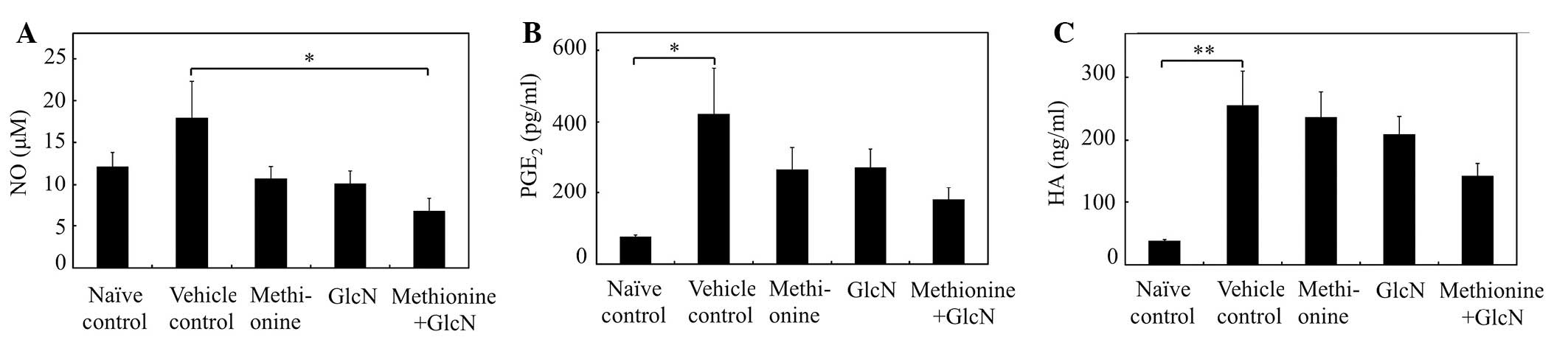 | Figure. 4.Effects of individual and combined
administrations of methionine and GlcN on the plasma levels of NO,
PGE2 and HA in rat adjuvant arthritis. Adjuvant
arthritis was induced by a single intradermal injection of FCA into
the footpad of the right hind paw. Methionine (200 mg/kg/day), GlcN
(400 mg/kg/day) or methionine combined with GlcN was administered
orally for 21 days. As a vehicle control, 0.5% CMC solution was
administered orally to rats with adjuvant arthritis instead of
methionine or GlcN. Naïve control rats received orally administered
0.5% CMC, but were not injected with adjuvant. On day 22, blood
samples were collected for the preparation of plasma. (A) NO, (B)
PGE2 and (C) HA levels in the plasma were measured using
nitrate/nitrite colorimetric assay kit, an enzyme-linked
immunosorbent PGE2 assay kit and hyaluronan assay kit,
respectively. Data are the mean ± SE of six animals per
experimental group. Values were compared between vehicle control
and methionine, GlcN, or combined methionine and GlcN
administration in rats with adjuvant arthritis.
*P<0.05, **P<0.01. GlcN, glucosamine;
NO, nitric oxide; PGE2, prostaglandin E2; HA,
hyaluronic acid; FCA, Freund’s complete adjuvant; CMC,
carboxymethyl cellulose. |
Plasma PGE2 levels were elevated
significantly in rats with FCA-induced adjuvant arthritis compared
with naïve control rats (P<0.05; Fig. 4B). Combined methionine and GlcN
administration markedly suppressed the increase in plasma
PGE2 levels in rats with adjuvant arthritis, although
the change was not significant (P<0.08). The HA level in the
plasma was evaluated as a marker of synovial inflammation. HA
levels were elevated significantly in rats with FCA-induced
adjuvant arthritis compared with naïve control rats (P<0.01;
Fig. 4C). Combined methionine and
GlcN administration markedly suppressed the increase in plasma HA
levels in rats with adjuvant arthritis. Thus, combined methionine
and GlcN administration more potently suppressed the production of
inflammatory mediators (NO and PGE2) and synovial
inflammation (HA) in adjuvant arthritis compared with the
individual administration of methionine or GlcN.
Discussion
In the present study, we utilized adjuvant
arthritis, a model of rheumatoid arthritis, to evaluate the effects
of anti-inflammatory substances (20). Methionine, GlcN and a combination
of the two were orally administered to rats with adjuvant
arthritis, and the effects on the arthritis were microscopically
and biochemically evaluated. Our results showed that the combined
methionine and GlcN administration more potently suppressed not
only the increase in swelling of the joints (Fig. 1) and arthritis score (Fig. 2), but also the histopathological
changes in the joints in adjuvant arthritis (identified as synovial
hyperplasia and destruction of cartilage surface and articular
meniscus; Fig. 3). Furthermore,
the combined methionine and GlcN administration markedly inhibited
increases in the plasma levels of NO, PGE2 and HA in
rats with adjuvant arthritis (Fig.
4). These observations suggest that the administration of a
combination of methionine and GlcN is more effective than the
individual administration of methionine or GlcN in suppressing the
progression of adjuvant arthritis by inhibiting synovial
inflammation and the production of inflammatory mediators.
The suppressive effects of GlcN on rat adjuvant
arthritis have already been reported (24,25).
Moreover, the administration of GlcN to patients with rheumatoid
arthritis has been shown to significantly reduce the pain and
swelling of arthritic joints compared with a placebo (26). Additionally, NO produced by
synovial cells plays a role in the pathogenesis of rheumatoid
arthritis (27), and
PGE2 is also one of the important inflammatory mediators
in rheumatoid arthritis (28).
GlcN reportedly suppresses the IL-1β-mediated activation of
synoviocytes, including IL-8, NO and PGE2, production
(13). Although
S-adenosylmethionine, a metabolite of methionine, has been
demonstrated to be as effective as NSAIDs in the symptomatic
management of osteoarthritis patients (29), the anti-inflammatory effect of
methionine, one of the main sources of sulfur in the body, has not
been reported. In the present study, we demonstrated that
methionine slightly suppressed the progression of adjuvant
arthritis by inhibiting the inflammatory reaction and the
production of inflammatory mediators. Thus, the combination of
methionine and GlcN is likely to exert a synergistic effect on
adjuvant arthritis, possibly by suppressing synovial inflammation
and the production of inflammatory mediators.
Additionally, we preliminarily evaluated the effect
of cystine (200 mg/kg), a sulfur amino acid, on adjuvant arthritis.
Cystine demonstrated suppressive effects on adjuvant arthritis to
the same extent as methionine; i.e., cystine suppressed the
swelling of the FCA-injected right and -uninjected left paws, the
arthritis score, the histopathological changes in joints, NO and
PGE2 production and synovial inflammation (HA level;
data not shown). However, the combination of cystine and GlcN
revealed no inhibitory effect on adjuvant arthritis, although the
combination of methionine and GlcN potently suppressed adjuvant
arthritis, as demonstrated in the present study. These observations
suggest that methionine and cystine act on the adjuvant arthritis,
possibly through different mechanisms, despite the fact that
methionine and cystine are classified as sulfur amino acids.
In summary, the administration of methionine to rats
with adjuvant arthritis slightly suppressed the arthritis. However,
the combined administration of methionine and GlcN markedly
suppressed the arthritis, possibly by a synergistic effect, thereby
inhibiting synovial inflammation and the production of inflammatory
mediators in adjuvant arthritis.
References
|
1
|
Firestein GS: Etiology and pathogenesis of
rheumatoid arthtitis. Kelley’s Textbook of Rheumatology. Firestein
GS, Budd RC, Harris ED Jr, Mclnnes IB, Ruddy S, Harris JED and
Sergent JS: 2. Saunders Elsevier; Philadelphia: pp. 1035–1086.
2009
|
|
2
|
Schuna AA and Megeff C: New drugs for the
treatment of rheumatoid arthritis. Am J Health Syst Pharm.
57:225–234. 2000.PubMed/NCBI
|
|
3
|
Ballou LR and Wang BWE: Nonsteroidal
anti-inflammatory drugs. Kelley’s Textbook of Rheumatology.
Firestein GS, Budd RC, Harris ED Jr, Mclnnes IB, Ruddy S, Harris
JED and Sergent JS: 1. Saunders Elsevier; Philadelphia: pp.
833–861. 2009
|
|
4
|
Newman NM and Ling RS: Acetabular bone
destruction related to non-steroidal anti-inflammatory drugs.
Lancet. 2:11–14. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anastassiades T, Chopra R, Law C and Wong
E: In vitro suppression of transforming growth factor-β induced
stimulation of glycosaminoglycan synthesis by acetylsalicylic acid
and its reversal by misoprostol. J Rheumatol. 25:1962–1967.
1998.
|
|
6
|
Crolle G and D’Este E: Glucosamine
sulphate for the management of arthrosis: a controlled clinical
investigation. Curr Med Res Opin. 7:104–109. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pavelká K, Gatterová J, Olejarová M,
Machacek S, Giacovelli G and Rovati LC: Glucosamine sulphate use
and delay of progression of knee osteoarthritis: a 3-year,
randomized, placebo-controlled, double-blind study. Arch Intern
Med. 162:2113–2123. 2002.PubMed/NCBI
|
|
8
|
McAlindon TE, LaValley MP, Gulin JP and
Felson DT: Glucosamine and chondroitin for treatment of
osteoarthritis: a systematic quality assessment and meta-analysis.
JAMA. 283:1469–1475. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reginster JY, Deroisy R, Rovati LC, Lee
RL, Lejeun E, Bruyere O, Giacovelli G, Henrotin Y, Dacre JE and
Gossett C: Long-term effects of glucosamine sulfate on
osteoarthritis progression: a randomized, placebo-controlled
clinical trial. Lancet. 357:251–256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fenton JI, Chlebec-Brown KA, Peters TL,
Caron JP and Orth MW: Glucosamine HCl reduces equine articular
cartilage degradation in explant culture. Osteoarthritis Cartilage.
8:258–265. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oegema TR Jr, Deloria LB, Sandy JD and
Hart DA: Effect of oral glucosamine on cartilage and meniscus in
normal and chymopapain-injected knees of young rabbits. Arthritis
Rheum. 46:2495–2503. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gouze JN, Bordji K, Gulberti S, Terlain B,
Netter P, Magdalou J, Fournel-Giqleux S and Ouzzine M:
Interleukin-1β down-regulates the expression of
glucuronosyl-transferase I, a key enzyme priming glycosaminoglycan
biosynthesis: influence of glucosamine on Interleukin-1β-mediated
effects in rat chondrocytes. Arthritis Rheum. 44:351–360. 2001.
|
|
13
|
Hua J, Sakamoto K, Kikukawa T, Abe C,
Kurosawa H and Nagaoka I: Evaluation of the suppressive actions of
glucosamine on the interleukin-1β-mediated activation of
synoviocytes. Inflamm Res. 56:432–438. 2007.PubMed/NCBI
|
|
14
|
Usha PR and Naidu MU: Randomised,
double-blind, parallel, placebo-controlled study of oral
glucosamine, methylsulfonylmethane and their combination in
osteoarthritis. Clin Drug Invest. 24:353–363. 2004. View Article : Google Scholar
|
|
15
|
Hasegawa T, Ueno S, Kumamoto S and
Yoshikai Y: Suppressive effect of methylsulfonylmethane (MSM) on
type II collagen-induced arthritis in DBA/1J mice. Jpn Pharmacol
Ther. 32:421–427. 2004.(In Japanese).
|
|
16
|
Kim YH, Kim DH, Lim H, Baek DY, Shin HK
and Kim JK: The anti-inflammatory effects of methylsulfonylmethane
on lipopolysaccharide-induced inflammatory responses in murine
macrophages. Biol Pharm Bull. 32:651–656. 2009. View Article : Google Scholar
|
|
17
|
Iouv M, Dumais G and du Souich P:
Anti-inflammatory activity of chondroitin sulfate. Osteoarthritis
Cartilage. 16(Suppl 3): S14–S18. 2008. View Article : Google Scholar
|
|
18
|
Volpi N: Anti-inflammatory activity of
chondroitin sulphate: new functions from an old natural
macromolecule. Inflammopharmacology. 19:299–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zimmermann M: Ethical guidelines for
investigations of experimental pain in conscious animals. Pain.
16:109–110. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gryglewski RJ: Some experimental models
for the study of inflammation and anti-inflammatory drugs. Agents
Actions Suppl. 17–23. 1977.PubMed/NCBI
|
|
21
|
Hirano S, Wakazono K, Agata N, et al:
Effects of cytogenin, a novel anti-arthritic agent, on type II
collagen-induced arthritis in DBA/1J mice and adjuvant arthritis in
Lewis rats. Int J Tissue React. 16:155–162. 1994.PubMed/NCBI
|
|
22
|
Wood FD, Pearson CM and Tanaka A: Capacity
of mycobacterial wax D and its subfractions to induce adjuvant
arthritis in rats. Int Arch Allergy Appl Immunol. 35:456–467. 1969.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jang D and Murrell GAC: Nitric oxide in
arthritis. Free Radic Biol Med. 24:1511–1519. 1998. View Article : Google Scholar
|
|
24
|
Setnikar I, Pacini MA and Revel L:
Antiarthritic effects of glucosamine sulfate studied in animal
models. Arzneimittelforschung. 41:542–545. 1991.PubMed/NCBI
|
|
25
|
Hua J, Suguro S, Hirano S, Sakamoto K and
Nagaoka I: Preventive actions of a high dose of glucosamine on
adjuvant arthritis in rats. Inflamm Res. 54:127–132. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakamura H, Masuko K, Yudoh K, Kato T,
Kamada T and Kawahara T: Effects of glucosamine administration on
patients with rheumatoid arthritis. Rheumatol Int. 27:213–218.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McInnes IB, Leung BP, Field M, Wei XQ,
Huang FP, Sturrock RD, Kinninmonth A, Weidner J, Mumford R and Liew
FY: Production of nitric oxide in the synovial membrane of
rheumatoid and osteoarthritis patients. J Exp Med. 184:1519–1524.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Crofford LJ, Wilder RL, Ristimäki AP, Sano
H, Remmers EF, Epps HR and Hla T: Cyclooxygenase-1 and -2
expression in rheumatoid synovial tissues. Effects of interleukin-1
beta, phorbolester, and corticosteroids. J Clin Invest.
93:1095–1101. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Najm WI, Reinsch S, Hoehler F, Tobis JS
and Harvey PW: S-adenosyl methionine (SAMe) versus celecoxib for
the treatment of osteoarthritis symptoms [ISRCTN36233495]: a
double-blind cross-over trial. BMC Musculoskelet Disord.
5:62004.PubMed/NCBI
|


















