Introduction
Acute lung injury (ALI) is one of the clinically
familiar critical emergencies. Studies have shown that ALI has a
high mortality rate and that 40–50% of patients succumb within
several days after onset of the disease (1) The pathogenesis of ALI is complicated
and remains to be elucidated. ALI is characterized by rapid
alveolar injury, inflammation, cytokine induction and neutrophil
accumulation (2). Although early
events in the pathogenesis of ALI have been defined, the mechanisms
underlying its resolution are unknown. At present, various studies
have started to explore the molecular mechanisms involved in the
pathogenesis and progression of ALI (2–3).
Studies on the pathological characteristics of ALI
have shown that it is an inflammatory reaction that induces lesions
in pulmonary capillary endothelial cells and the alveolar
epithelium (4). Pulmonary
capillary hyperpermeability leads to pneumonedema and the formation
of a transparent film accompanied by pulmonary interstitial
fibrosis (5). The inflammation of
lung tissue mediated by neutrophilic granulocytes is the
pathological foundation of pulmonary capillary hyperpermeability
and pneumonedema. Epidermal growth factor (EGF) is an effective
enhancer that stimulates various types of tissue cell to multiple
degradation. Previous studies on the biological effect of EGF in
the lung demonstrated that EGF promotes the DNA synthesis of
numerous types of cells in the lung, including fibroblast,
epithelial and granular alveolar cells (6). EGF also promotes water transportation
in the lung, which aids in the treatment of lung injury. EGF
augmented ALI by improving the transmembrane transport of alveolar
type II cells, resulting in the acceleration of alveolar liquid
clearance. Additionally, exogenous EGF adjusts and controls
pulmonary surfactant generation.
Currently there are no effective methods to cure
ALI. To investigate the effect of extrinsic EGF on lung fluid
transport in rabbits with ALI caused by endotoxin and evaluate its
therapeutic action, we constructed ALI rabbit models using
endotoxin and dripped EGF into trachea to investigate the curative
effect of EGF on ALI.
Materials and methods
Grouping of the laboratory animals
A total of 24 healthy rabbits (provided by Guangzhou
Medical College Animal Experimental Center, Guanzhou, China) of
either gender were selected. The weight of the animals varied from
1.90 to 2.50 kg. The rabbits were randomly divided into three
groups; control, simple ALI and EGF treatment (endotoxin-induced
ALI + EGF treatment) groups. Each group was divided into four
subgroups according to the time points of 1, 12, 24 and 48 h.
Reagents
Endotoxin (E. coli O111:B4) was produced by
Sigma (St. Louis, MO, USA) at a density of 0.1 mg/ml. The EGFs were
produced by Sigma. The reagents heparin, napental (3%) and
physiological saline used for the EGF solution were obtained from
Shanghai No. 1 Biochemical and Pharmaceutical Co., Ltd.
Construction of animal models with
endotoxin
The acute lung injury animal model was constructed
by injecting endotoxin lipopolysaccharide (LPS) intravenously into
the rabbit through the ear vein. Pathological indices confirmed the
accuracy of the animal model. Thus, following treatment with LPS,
the volume of the lung enlarged and dark red patch foci of unequal
sizes on the surface and light red or white frothy liquid overflow
from the section were observed. Leukocyte infiltration was observed
in lung tissue through light microscopy. Pulmonary interstitial and
alveolar edema, as well as pulmonary hemorrhage were evident.
Intervention of EGF
EGF was administered to the rabbits shortly after
the endotoxin had caused ALI. The method used was as follows:
rabbits were immobilized, EGF (100 μg/kg) was taken up with a 1-ml
injector and thinned to 1 ml with physiological saline, a syringe
needle was introduced into the windpipe through the glottis, the
liquid was sprayed quickly into the windpipe through the syringe
needle and the animals were returned to feeding.
Determination of the arterial partial
pressure of oxygen (PaO2)
The change in rabbit respiration, heart rate, color
of lips and other symptoms was observed. At each time point, 1–2 ml
of blood was phlebotomized from the rabbit ear central artery and a
blood gas analysis was performed immediately. Heparin was used to
moisten the wall of the injector.
Determination of the water content of
lung tissues
The rabbits were sacrificed following anaesthesia
with 3% napental. The left lung was dissociated and the wet weight
(W) was measured. At 24 h after anhydration under 0.06 ×70°C
conditions, the dry weight (D) was measured. The water content of
the lung tissues was then recorded as W/D.
Pathomorphological observation of lung
tissue
The left inferior lung lobe was removed, fixed and
embedded. It was then sliced, dyed with hematoxylin and eosin stain
(H&E) and observed under a light microscope.
Statistical analysis
The experimental data were processed by SPSS 13.0
and presented as the mean ± standard deviation. Analysis of
variance was used to the compare groups and the Student’s t-test
was used to compare the rabbit PaO2 and ratio of W/D in
the three groups at four periods of time. P<0.05 was considered
to indicate a statistically significant result.
Results
Characteristics of the rabbits
The rabbits in the control group were in a healthy
state, the breathing rhythm was even and the color of the skin and
mucous membranes were normal. In the simple ALI group, the
breathing rate increased and the skin and mucous membranes
developed cyanosis 10 min after the injection of endotoxin. The
breathing and heart rate became reduced and cyanosis decreased in
the EGF treatment group 30 min after therapy.
Arterial partial pressure of oxygen
(PaO2)
Compared with the control group, PaO2 in
the rabbits which had been injected with endotoxin 1 h before
decreased from 16.24±0.87 kPa to 6.99±1.45 kPa.
PaO2/FiO2 decreased from 580 to 249, while
the X-ray examination showed that there was diffuse leakage in both
lungs. The abovementioned symptoms were standard of ALI. Following
treatment with EGF, the breathing state continued to improve.
PaO2 increased to 13.78±0.62 kPa 24 h later (Table I).
 | Table IRabbit PaO2 (kPa) in three
groups at four periods of time. |
Table I
Rabbit PaO2 (kPa) in three
groups at four periods of time.
| Groups | T1 | T12 | T14 | T48 |
|---|
| Normal control | 16.24±0.87 | 16.94±0.82 | 16.43±0.78 | 16.65±0.80 |
| Simple ALI | 6.99±1.45a | 6.84±1.42a | 6.67±1.48a | 6.75±1.32a |
| EGF treatment | 10.78±0.46b | 11.16±0.54b | 13.78±0.62b | 13.84±0.56b |
W/D of lung tissues
The W/D of the simple ALI group was much higher than
that of the control group, indicating that there was a large
quantity of liquid overflow in the alveoli following
endotoxin-induced lung injury. At 24 h after the treatment with EGF
the pulmonary edema was reduced to a certain degree from 8.68±0.43
to 5.20±0.74 (Table II).
 | Table IIRatio of W/D in three groups of
rabbits at four periods of time. |
Table II
Ratio of W/D in three groups of
rabbits at four periods of time.
| Groups | T1 | T12 | T24 | T48 |
|---|
| Normal control | 4.41±0.46 | 4.39±0.50 | 4.31±0.52 | 4.28±0.55 |
| Simple ALI | 8.23±0.64a | 8.53±0.58a | 8.68±0.43a | 8.85±0.44a |
| EGF treatment | 5.86±0.78b | 5.23±0.65b | 5.20±0.74b | 5.06±0.72b |
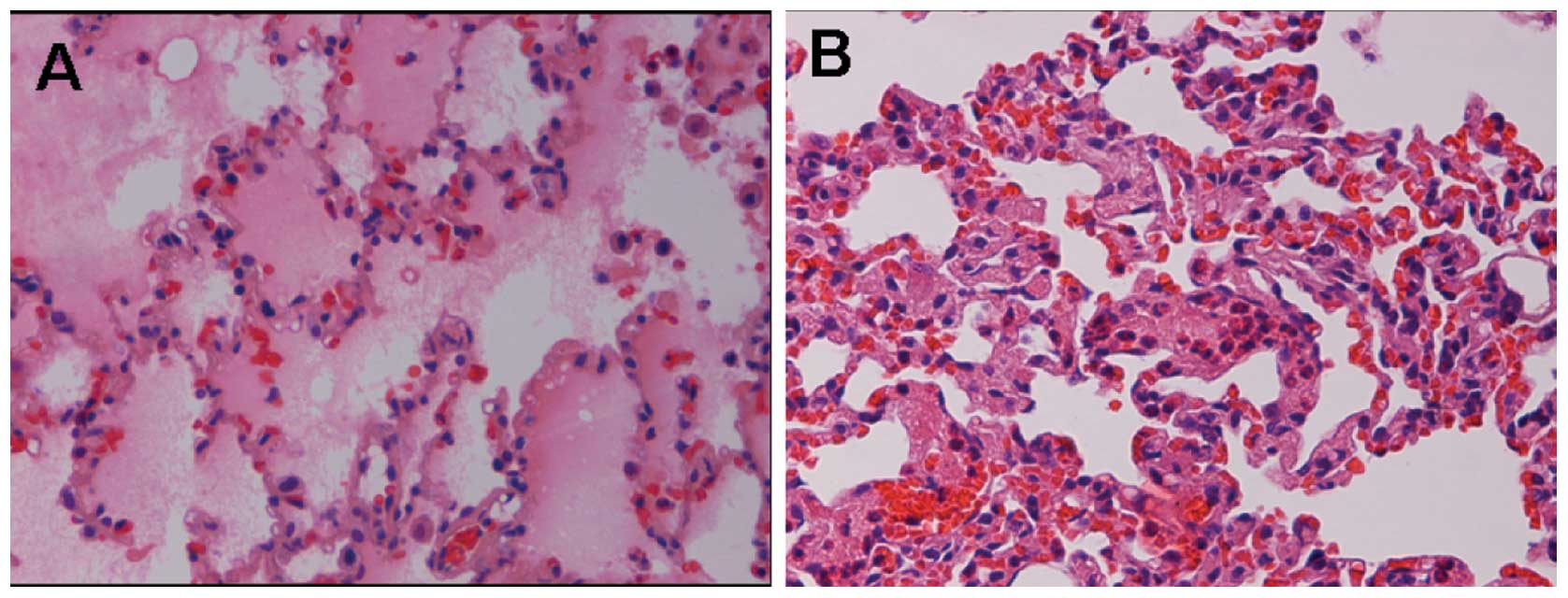
Lung tissue under light microscopy
The rabbit lungs in the normal control group
appeared to be a uniform pink, with a smooth envelope and good
flexibility, with no liquid overflow from the section. Results
obtained from light microscopy showed that the lung tissue was
structurally complete, the alveolar cavity was clear and the
alveolar septum had no special manifestation such as edema or
inflammation. The lungs in the simple ALI group had a dirty red
appearance, particularly the two lower sides of the lungs. There
was a large quantity of bloody frothy fluid overflowing from the
section. Destruction of the alveolar wall, edema and hemorrhage in
interstitial alveoli, and a decrease of alveoli and diffuse
neutrophilic granulocyte infiltration were observed under the light
microscope (Fig. 1). The lesions
in the EGF treatment group had a lighter appearance to a certain
degree. Results of the light microscopy showed that the majority of
the alveolar wall was restored, edema in interstitial alveoli was
alleviated and only a small quantity of neutrophilic granulocyte
infiltration was present (Fig. 2).
Discussion
Studies have shown that ALI has a high mortality
rate and 40–50% of patients succumb to multiple organ failure, not
hypoxia (1). The pathogenesis of
ALI is complicated and has yet to be clearly explained. The
majority of patients often succumb within several days following
onset of the disease. ALI remains a difficult clinical problem. At
present, studies have started to explore the molecular mechanisms
involved in the pathogenesis and progression of ALI (2–3).
Since ALI and gram-negative bacteria cause sepsis,
the endotoxin-induced injury ALI model is used in ALI pathogenesis
research. Endotoxins, including LPS, are constituents of
gram-negative bacterial cell walls. Through intravenous injection
or tracheal inhalation endotoxins can be used to construct ALI
animal models (4,7–9). The
construction of this model is key to the endotoxin or sepsis as it
induces alveolar capillary damage and pulmonary edema. Fiber
proteins, platelets and white blood cells are able to cause a
microthrombosis and increase in pulmonary arterial pressure. The
process of platelet aggregation releases vascular-active
substances, complement activation and aggregation of
polymorphonuclear neutrophil, and in theory, is similar to the
characteristics of the clinical cases (6).
PME cells, inflammatory factors and damaged
epithelial intercellular signaling are three main areas that play a
role in the pathogenesis of ALI (10). Studies on the pathological
characteristics of ALI have shown that it is the inflammatory
response that is responsible for the development of the lesion of
pulmonary capillary endothelial cells and alveolar epithelium,
while pulmonary capillary hyperpermeability leads to pneumonedema
and transparent film formation accompanied by pulmonary
interstitial fibrosis. The inflammation of lung tissue mediated by
neutrophilic granulocytes is the pathological basis of pulmonary
capillary hyperpermeability and pneumonedema.
EGF is a powerful cell division promoting factor and
stimulates the body responsible for the organization of cell
division and proliferation (11).
At present, the use of EGF to treat ALI has been studied in animal
models. Studies have confirmed that exogenous EGF treatment of ALI
of rat alveolar type II cells is able to significantly increase
cell proliferation. Thus, the exogenous epidermal growth factor is
capable of protecting alveolar epithelial function, so as to reduce
the lung injury (12). Studies on
the biological effect of EGF in the lung have demonstrated that EGF
promotes DNA synthesis of numerous types of cells in the lung, such
as fibroblast, epithelial and granular alveolar cells. EGF also
promotes water transportation in the lung, which aids in the
treatment of lung injury (13).
EGF increases alveolar liquid clearance rate (ALC) by improving the
ion transmembrane transport of AT-II, resulting in the acceleration
of liquid clearance in alveoli. Exogenetic EGF adjusts and
modulates pulmonary surfactant generation (14,15).
Results of the present study have shown that group W/D was
significantly higher than the normal control group following
endotoxin-induced lung injury. Additionally, there was extensive
fluid overflow from the alveoli. The W/D value decreased
significantly 24 h after EGF treatment, due to pulmonary edema,
with a certain degree of ease (16–18).
The present has shown how EGF stimulates vascular
endothelial cell migration and proliferation from a molecular
pathology point of view (19). The
results demonstrated that EGF promotes the restoration of the lung
vessels to repair lung, reduces pulmonary edema and improves the
reversal of ALI. Following treatment with EGF, the breathing and
heart rate had improved (20). An
increase in PaO2 was observed after 12 h, a marked
increase in PaO2 was noted 24 h later and further
improvement was evident 48 h later. Pathological examination and
the W/D measurement revealed that the lung injury in the EGF
treatment group was alleviated to a certain extent. The EGF
treatment group W/D was 5.86±0.78 and significantly different
compared with the simple ALI group W/D which was 8.23±0.94.
Continuous observation for 48 h confirmed the curative effect of
EGF on ALI. EGF may therefore provide a new treatment of ALI.
The literature reports that EGF promotes the
proliferation of type II alveolar cells and water transportation in
the lung, although its mechanism is complicated. The present study
only shows the therapeutic action of EGF via observation of the
pathology, improvement in symptoms and blood gas analysis. However,
the use of EGF requires more study before it can be safely used in
the clinic.
References
|
1
|
Abraham E, Matthay MA, Dinarello CA, et
al: Consensus conference definitions for sepsis, septic shock,
acute lung injury, and acute respiratory distress syndrome: time
for a reevaluation. Crit Care Med. 28:232–235. 2000. View Article : Google Scholar
|
|
2
|
Chen B, Xiao Y, Qian G, et al: Effect of
PMN in acute lung injury following cardiopulmonary bypass (CPB).
China J Emerg Med. 15:148–151. 2006.(In Chinese).
|
|
3
|
Cai X, Liu C, Du Y, et al: Comparison of
lung injury between neonatal and adult rats induced by
lopopolysaccharides. China J Emerg Med. 14:458–462. 2005.(In
Chinese).
|
|
4
|
Nam AJ, Brower RG, Fessler HE and Simon
BA: Biologic variability in mechanical ventilation rate and tidal
volume does not improve oxygenation or lung mechanics in canine
oleic acid lung injury. Am J Respir Crit Care Med. 161:1797–1804.
2000.PubMed/NCBI
|
|
5
|
Guo Z, Hong X, Mao B, et al: Effects of
nuclear factor-κB activation on acute lung injury. China J Emerg
Med. 12:148–151. 2003.
|
|
6
|
Xu RF, Xu LX, Xu SR, et al: Therapeutic
effect of hyperoxia solution on oleic acid induced acute lung
injury in rabbits. J Fourth Mil Med Univ. 24:1451–1453. 2003.
|
|
7
|
Zunic G, Pavlović R, Malicević Z, et al:
Pulmonary blast injury increases nitric oxide production, disturbs
arginine metabolism, and alters the plasma free amino acid pool in
rabbits during the early posttraumatic period. Nitric Oxide.
4:123–128. 2000. View Article : Google Scholar
|
|
8
|
Cakar N, der Kloot TV, Youngblood M, et
al: Oxygenation response to a recruitment maneuver during supine
and prone position in an oleic-induced lung injury model. Am J
Respir Crit Care Med. 161:1949–1956. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yancopoulos GD, Davis S, Gale NW, et al:
Vascular-specific growth factors and blood vessel formation.
Nature. 407:242–248. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li XP and Wang JX: The research progress
of acute lung injury animal model. Int J Respir. 25:506–508.
2005.(In Chinese).
|
|
11
|
Meng G, Zhao J, Wang HM, et al: Cell
injuries of the blood-air barrier in acute lung injury caused by
perfluoroisobutylene exposure. J Occup Health. 52:48–57. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawkitinarong K, Linz-McGillem L, Birukov
KG and Garcia JGN: Differential regulation of human lung epithelial
and endothelial barrier function by thrombin. Am J Respir Cell Mol
Biol. 31:517–527. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu S, Liu Q, Sheng Z, et al: Epidermal
growth factor alleviates pulmonary edema in rats with smoke
inhalation injury. China J Emerg Med. 13:24–26. 2004.(In
Chinese).
|
|
14
|
Harada C, Kawaguchi T, Ogata-Suetsugu S,
et al: EGFR tyrosine kinase inhibition worsens acute lung injury in
mice with repairing airway epithelium. Am J Respir Crit Care Med.
183:743–751. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bierman A, Yerrapureddy A, Reddy NM, et
al: Epidermal growth factor receptor (EGFR) regulates mechanical
ventilation-induced lung injury in mice. Transl Res. 152:265–272.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miyake Y, Kaise H, Isono K, et al:
Protective role of macrophages in noninflammatory lung injury
caused by selective ablation of alveolar epithelial type II Cells.
J Immunol. 178:5001–5009. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Geiser T: Mechanisms of alveolar
epithelial repair in acute lung injury - a translational approach.
Swiss Med Wkly. 133:586–590. 2003.PubMed/NCBI
|
|
18
|
Leikauf GD, McDowell SA, Bachurski CJ, et
al: Functional genomics of oxidant-induced lung injury. Adv Exp Med
Biol. 500:479–487. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eutamene H, Theodorou V, Schmidlin F, et
al: LPS-induced lung inflammation is linked to increased epithelial
permeability: role of MLCK. Eur Respir J. 25:789–796. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bazzoni G and Dejana E: Endothelial
cell-to-cell junctions: molecular organization and role in vascular
homeostasis. Physiol Rev. 84:8692004. View Article : Google Scholar : PubMed/NCBI
|