Introduction
Asthma is a chronic inflammatory disease of the
respiratory airways leading to episodes of wheezing, shortness of
breath, chest tightness and coughing (1). Approximately 300 million individuals
are affected by asthma globally, including 10 million children
(2). The pathogenesis of asthma is
extremely complex and not fully understood. The major risk factors
for the development and persistence of asthma are allergic disease,
reduced lung function and viral and bacterial infections (3–5). In
addition, variants in over 40 genes have been associated with
asthma (6). Parental asthma is a
strong predictor of childhood asthma, suggesting a strong genetic
basis of pediatric asthma (7).
The regulated upon activation, normal T cells
expressed and secreted (RANTES) protein is one of the most
extensively studied chemokines in allergic and infectious diseases
(8). RANTES is likely to be
significant in airway inflammation, since blocking antibodies to
RANTES reportedly inhibit airway inflammation in a murine model of
allergic airway disease (8). Two
polymorphisms in the RANTES promoter region (−28 C/G and −403 G/A)
have been demonstrated to affect the transcription of the RANTES
gene and exacerbate asthma severity (9,10).
However, the published results have been controversial. To aid the
clarification of the inconsistent findings, with the publication of
several more recent studies, this meta-analysis of RANTES gene
polymorphisms (−403G/A and −28C/G) and the risk of pediatric asthma
was conducted.
Materials and methods
Selection of studies
The data were independently gathered in duplicate by
two investigators on the basis of a standard protocol (Y.M.L. and
L.F.C.). Discrepancies between the investigators were settled by
discussion until consensus was reached. Literature databases were
searched, including PubMed, Google Scholar and the China National
Knowledge Infrastructure (CNKI) databases. The following key words
and subject terms were searched: ‘RANTES’, ‘−403G/A’, ‘−28C/G’,
‘pediatric asthma’ and ‘gene polymorphism’. The reference lists of
retrieved reviews and articles were hand-searched. The search was
without restriction on language and was conducted on human
subjects. The literature search was updated on 30 May 2012.
Selection criteria
The titles and abstracts of all citations identified
by the literature search were reviewed. Selection criteria were
then applied to all potentially relevant studies. The selection
criteria for inclusion in the meta-analysis were: i) case-control
studies conducted to evaluate the association between RANTES gene
polymorphisms (−403G/A and −28C/G) and pediatric asthma risk; ii)
sufficient genotype data were presented to calculate the odds
ratios (ORs) and 95% confidence intervals (CIs); iii) the study
should clearly describe pediatric asthma diagnoses and the sources
of cases and controls. Studies were excluded when: i) they were
non-case-control studies that evaluated the association between
RANTES gene polymorphisms and pediatric asthma risk; ii) they were
case reports, letters, reviews or editorial articles; iii) they
were studies based on incomplete raw data and no usable data were
reported; iv) they contained duplicate data.
Data extraction
The following characteristics were collected from
each study: first author, year of publication, region of the first
or corresponding author, ethnicity, number of cases and controls,
number of genotypes and evidence of Hardy-Weinberg equilibrium
(HWE; Table I). Ethnicities were
classified as Asian or Caucasian. If original genotype frequency
data were unavailable in the relevant articles, an email was sent
to the corresponding author for additional data. For conflicting
evaluations, an agreement was reached following a discussion.
 | Table ICharacteristics of the included
studies for meta-analysis. |
Table I
Characteristics of the included
studies for meta-analysis.
| A, −403G/A
polymorphism |
|
| First author
(Refs.) | Year | Area | Ethnicity | Cases/controls | Case genotypes
| Control genotypes
| HWE test |
| GG | GA | AA | GG | GA | AA |
|
| Szalai (11) | 2001 | Hungary | Caucasian | 160/303 | 122 | 32 | 6 | 211 | 84 | 8 | 0.92 |
| Yao (12) | 2003 | China | Asian | 182/107 | 98 | 65 | 19 | 60 | 41 | 6 | 0.77 |
| Liu (13) | 2005 | China | Asian | 32/32 | 17 | 13 | 2 | 16 | 14 | 2 | 0.64 |
| Leung (14) | 2005 | China | Asian | 129/66 | 60 | 53 | 16 | 37 | 21 | 8 | 0.09 |
| Tölgyesi (15) | 2006 | Hungary | Caucasian | 144/174 | 107 | 34 | 3 | 131 | 40 | 3 | 0.98 |
| Sohn (16) | 2008 | Korea | Asian | 326/253 | 109 | 146 | 71 | 97 | 107 | 49 | 0.05 |
|
| B, −28C/G
polymorphism |
|
| First author
(Refs.) | Year | Area | Ethnicity | Cases/controls | Case genotypes
| Control genotypes
| HWE test |
| CC | CG | GG | CC | CG | GG |
|
| Szalai (11) | 2001 | Hungary | Caucasian | 160/303 | 144 | 16 | 0 | 284 | 19 | 0 | 0.57 |
| Yao (12) | 2003 | China | Asian | 182/107 | 134 | 39 | 9 | 83 | 23 | 1 | 0.67 |
| Wang (17) | 2004 | China | Asian | 100/90 | 65 | 31 | 4 | 72 | 17 | 1 | 1.00 |
| Liu (13) | 2005 | China | Asian | 32/32 | 25 | 6 | 1 | 29 | 3 | 0 | 0.78 |
| Sohn (16) | 2008 | Korea | Asian | 326/253 | 218 | 93 | 15 | 174 | 66 | 13 | 0.05 |
Statistical analysis
A meta-analysis was performed using the STATA
package version 12.0 (Stata Corporation, College Station, TX, USA).
The OR corresponding to the 95% CI was used to assess the intensity
of the association between RANTES gene polymorphisms (−403G/A and
−28C/G) and pediatric asthma under a homozygote comparison (AA vs.
aa), a heterozygote comparison (AA vs. Aa), a dominant model and a
recessive mode between groups. In the current study, the dominant
model was defined as Aa+aa vs. AA, where ‘A’ and ‘a’ are major and
minor alleles, respectively, and the recessive model as aa vs.
AA+Aa. The distribution of genotypes in the included studies was
tested for HWE using the χ2 test. The effect of
heterogeneity by the Q-test and I2 test was also
quantified. I2 ranges between 0 and 100% and I2 values
of 25, 50 and 75% were defined as low, moderate and high estimates,
respectively. When a significant Q-test (P<0.10) or
I2>50% indicated heterogeneity across studies, the
random-effects model was used for meta-analysis, otherwise the
fixed-effects model was calculated. Sensitivity analysis was
performed to assess the stability of the results by excluding the
study by Sohn et al (16)
with genotype distributions not in HWE. Begg’s test was used to
provide evidence of publication bias, shown as a funnel plot.
P<0.05 was considered to indicate a statistically significant
result.
Results
Characteristics of studies
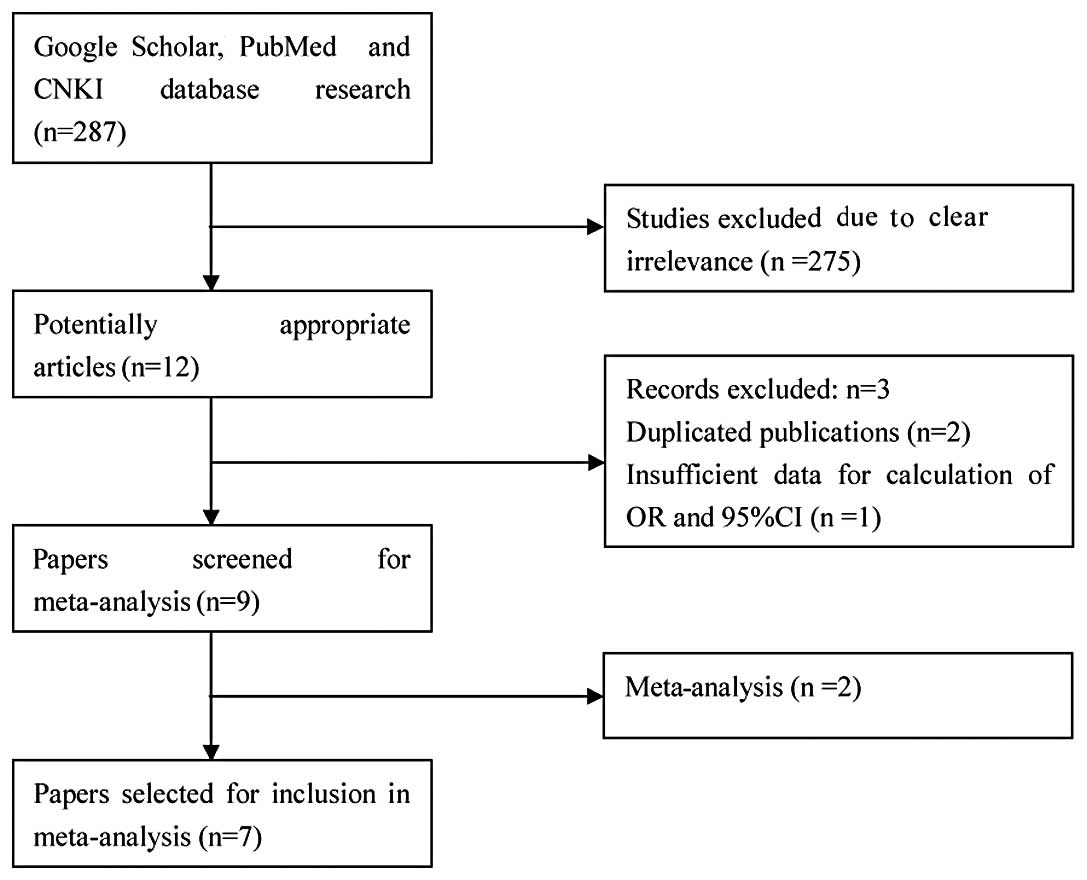
There were 287 studies relevant to the search words.
Through screening the title and reading the abstract and the entire
article, 7 eligible articles were selected for this meta-analysis
(Fig. 1). All the studies were
case-control studies that evaluated the association between RANTES
gene polymorphisms and pediatric asthma risk (11–17).
For −28C/G, the sample population in the Europe group was
inadequate and ethnicity-specific meta-analysis was not conducted
on Caucasians. The HWE test was performed on the genotype
distribution of the controls in all included studies; all of them
were in HWE except that in the study by Sohn et al (16). The main characteristics of the
included studies are listed in Table
I.
Results of meta-analysis
A summary of the meta-analysis findings of the
association between RANTES gene polymorphisms (−403G/A and −28C/G)
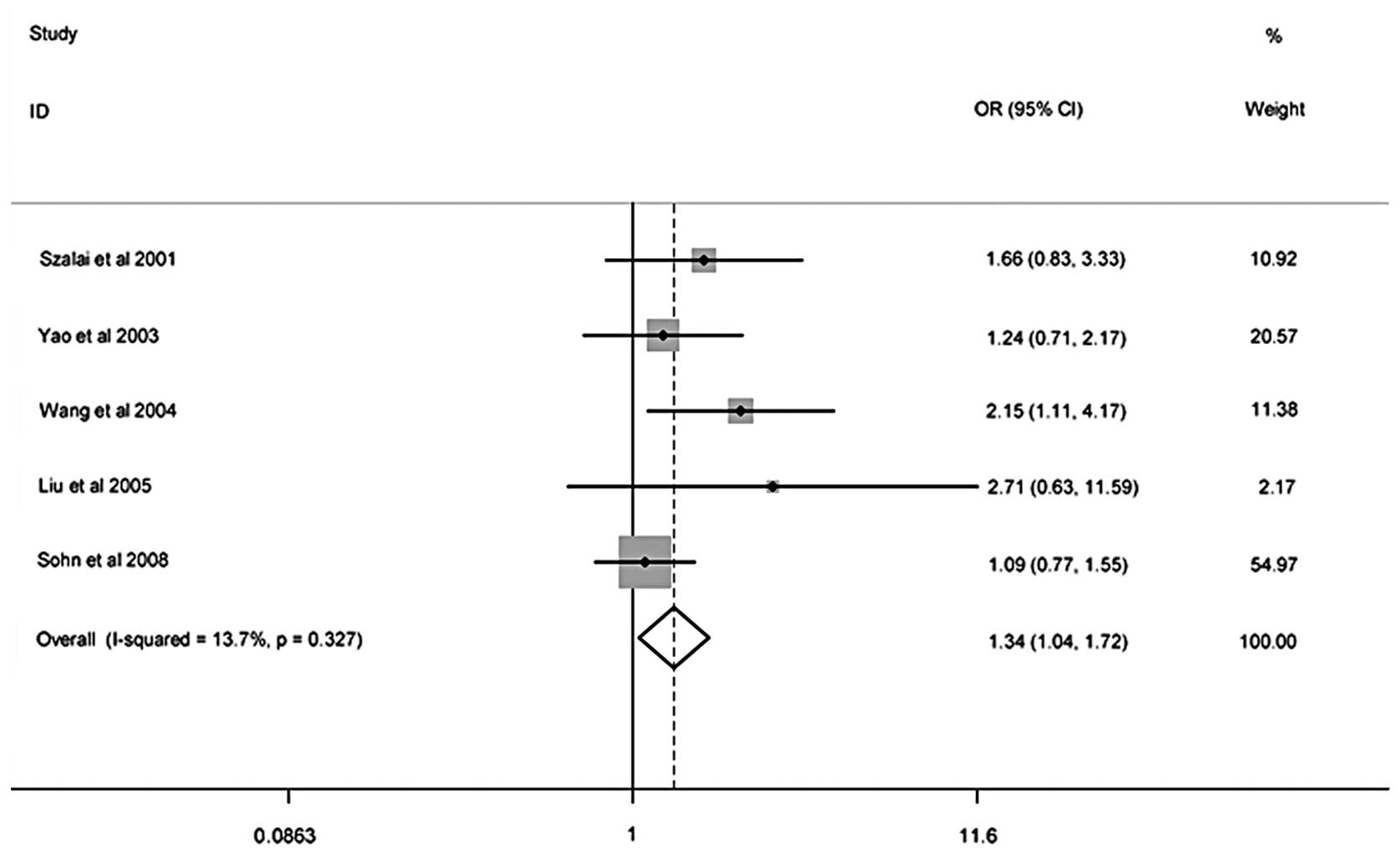
and pediatric asthma risk is shown in Table II. Meta-analysis results showed
that there was no association between the −403G/A polymorphism and
the risk of pediatric asthma, the meta-analysis indicated that the
−28C/G polymorphism was associated with an increased risk of
pediatric asthma in the general population (recessive model: OR,
1.34; 95% CI, 1.04–1.72). On the basis of the potential
overestimation of the true effect of the polymorphism on the
pediatric asthma risk, these studies were stratified according to
ethnicity. In the stratified analysis, no significant associations
were identified in Asian and European populations. Sensitivity
analysis was performed to assess the stability of the results by
excluding one study by Sohn et al (16) with genotype distributions not in
HWE and the exclusion of any single study did not alter the
significance of the final decision, suggesting that the outcomes
were robust. The funnel plot and Begg’s test were used to assess
the publication bias. There was no evidence of publication bias
visually from the funnel plot (Fig.
2, Table II). The difference
was not significant in Begg’s test (all P>0.05), suggesting that
the publication bias was low in the present meta-analysis.
 | Table IISummary ORs and 95% CIs of RANTES gene
polymorphisms and pediatric asthma risk. |
Table II
Summary ORs and 95% CIs of RANTES gene
polymorphisms and pediatric asthma risk.
| Subgroup | Genetic model | Sample size
| Type of model | Heterogeneity
| Association
| Publication bias
|
|---|
| Case | Control | I2
(%) | P-value | OR | 95% CI | z | P-value |
|---|
| −403G/A | | | | | | | | | | |
| Overall | AA vs. GG | 973 | 935 | Fixed | 0.0 | 0.98 | 1.34 | 0.95–1.89 | 0.00 | 1.00 |
| AA vs. GA | | | Fixed | 0.0 | 0.75 | 1.18 | 0.84–1.66 | 0.00 | 1.00 |
| Dominant | | | Fixed | 0.0 | 0.94 | 0.81 | 0.59–1.11 | 0.00 | 1.00 |
| Recessive | | | Fixed | 3.6 | 0.39 | 1.06 | 0.87–1.29 | 0.00 | 1.00 |
| Caucasian | AA vs. GG | 304 | 477 | Fixed | 0.0 | 0.95 | 1.27 | 0.52–3.13 | 0.00 | 1.00 |
| AA vs. GA | | | Fixed | 0.0 | 0.62 | 1.66 | 0.65–4.24 | 0.00 | 1.00 |
| Dominant | | | Fixed | 0.0 | 0.86 | 0.73 | 0.30–1.80 | 0.00 | 1.00 |
| Recessive | | | Fixed | 22.2 | 0.26 | 0.84 | 0.60–1.17 | 0.00 | 1.00 |
| Asian | AA vs. GG | 669 | 458 | Fixed | 0.0 | 0.87 | 1.35 | 0.93–1.96 | 0.34 | 1.00 |
| AA vs. GA | | | Fixed | 0.0 | 0.61 | 1.12 | 0.78–1.62 | 0.34 | 1.00 |
| Dominant | | | Fixed | 0.0 | 0.75 | 0.82 | 0.58–1.15 | 0.34 | 1.00 |
| Recessive | | | Fixed | 0.0 | 0.81 | 1.21 | 0.95–1.54 | 0.34 | 1.00 |
| −28C/G | | | | | | | | | | |
| Overall | GG vs. CC | 800 | 785 | Fixed | 30.0 | 0.23 | 1.53 | 0.81–2.90 | 0.24 | 1.00 |
| GG vs. CG | | | Random | 82.5 | 0.00 | 4.93 | 0.48–50.53 | 0.24 | 1.00 |
| Dominant | | | Fixed | 26.4 | 0.25 | 0.69 | 0.37–1.29 | 0.24 | 1.00 |
| Recessive | | | Fixed | 13.7 | 0.33 | 1.34 | 1.04–1.72 | 0.24 | 1.00 |
| Asian | GG vs. CC | 640 | 482 | Fixed | 30.0 | 0.23 | 1.53 | 0.81–2.90 | 1.02 | 0.31 |
| GG vs. CG | | | Random | 82.5 | 0.00 | 4.93 | 0.48–5053 | 1.02 | 0.31 |
| Dominant | | | Fixed | 26.4 | 0.25 | 0.69 | 0.37–1.29 | 1.02 | 0.31 |
| Recessive | | | Fixed | 28.6 | 0.24 | 1.30 | 1.00–1.70 | 1.02 | 0.31 |
Discussion
Asthma is the most common chronic childhood disease
(18). The pathogenesis of asthma
is extremely complex and not fully understood, although the
correlation of a variety of genetic loci and multiple environmental
factors have been suggested as significant determinants of this
disease (19,20). A number of research studies have
evaluated the association of RANTES gene polymorphisms (−403G/A and
−28C/G) and pediatric asthma risk, but the results are
controversial, making it difficult to speculate the true
correlation between gene polymorphisms and pediatric asthma. The
studies included were conducted in various populations, sample
sizes were relatively small and various criteria were used for the
phenotype definition (21).
To the best of our knowledge, this is the first
meta-analysis to consider RANTES gene polymorphisms and pediatric
asthma. This meta-analysis quantitatively assessed the association
between RANTES gene polymorphisms (−403G/A and −28C/G) and
susceptibility to pediatric asthma. Additonally, the results of our
meta-analysis did not show any significant association between
−403G/A polymorphism and pediatric asthma risk. As for −28C/G, the
meta-analysis indicated a significant association between −28C/G
polymorphism and pediatric asthma susceptibility among the total
population (recessive model: OR, 1.34; 95% CI, 1.04–1.72). However,
when the subgroup analyses were performed by ethnicity, no
significant associations were identified in Asian and European
populations. This result suggests that the −28C/G polymorphism may
not be associated with pediatric asthma risk and the observed
increase in the risk of pediatric asthma may be due to a bias of
racial differences. Sensitivity analysis was performed to assess
the stability of the results by excluding the study by Sohn et
al (16) with genotype
distributions that were not in HWE, suggesting that the results of
the meta-analysis were reliable. No evidence revealed publication
bias in this meta-analysis for the association between RANTES gene
polymorphisms and susceptibility to pediatric asthma.
There were certain limitations to this
meta-analysis. First, the linkage disequilibrium of −403G/A and
−28C/G of the RANTES gene may synergistically increase the risk of
asthma (22). There was
insufficient individual information on genotypes of the RANTES
−403G/A and −28C/G polymorphisms and combined analysis of linkage
disequilibrium was therefore not performed. Consequently, more
studies with larger sample sizes and providing more detailed
information are required. Second, the effect of gene-gene and
gene-environment interactions was not addressed in this
meta-analysis. Third, the significance between study-heterogeneity
was observed. Although the random-effect model was used to pool
ORs, it may have affected the precision of results.
In conclusion, these results suggest that RANTES
gene polymorphisms (−403G/A and −28C/G) may not be involved with
the susceptibility of pediatric asthma. Owing to the previously
mentioned limitations, the findings should be verified by further
research in the near future.
References
|
1
|
Zhang J, Paré PD and Sandford AJ: Recent
advances in asthma genetics. Respir Res. 9:42008. View Article : Google Scholar
|
|
2
|
Bloom B, Cohen RA and Freeman G: Summary
Health Statistics for U.S. Children: National Health Interview
Survey, 2009. Vital Health Stat. 10:1–82. 2010.PubMed/NCBI
|
|
3
|
Schwerk N, Brinkmann F, Soudah B, et al:
Wheeze in preschool age is associated with pulmonary bacterial
infection and resolves after antibiotic therapy. PLoS One.
6:e279132011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Covar RA, Strunk R, Zeiger RS, et al
Childhood Asthma Management Program Research Group: Predictors of
remitting, periodic, and persistent childhood asthma. J Allergy
Clin Immunol. 125:359–366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bisgaard H, Hermansen MN, Bønnelykke K, et
al: Association of bacteria and viruses with wheezy episodes in
young children: prospective birth cohort study. BMJ. 341:c49782010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Purcell S, Neale B, Todd-Brown K, Thomas
L, Ferreira MA, Bender D, et al: PLINK: a tool set for whole-genome
association and population-based linkage analyses. Am J Hum Genet.
81:559–575. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Litonjua AA, Carey VJ, Burge HA, Weiss ST
and Gold DR: Parental history and the risk for childhood asthma.
Does mother confer more risk than father? Am J Respir Crit Care
Med. 158:176–181. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lukacs NW, Strieter RM, Warmington K,
Lincoln P, Censue SW and Kunkel SL: Differential recruitment of
leukocyte populations and alteration of airway hyperreactivity by
C-C family chemokines in allergic airway inflammation. J Immunol.
158:4398–4404. 1997.PubMed/NCBI
|
|
9
|
Nickel RG, Casolaro V, Wahn U, Beyer K,
Barnes KC, Plunkett BS, et al: Atopic dermatitis is associated with
a functional mutation in the promoter of the C-C chemokine RANTES.
J Immunol. 164:1612–1616. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu H, Chao D, Nakayama EE, Taguchi H,
Goto M, Xin X, et al: Polymorphism in RANTES chemokine promoter
affects HIV-1 disease progression. Proc Natl Acad Sci USA.
96:4581–4585. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Szalai C, Kozma GT, Nagy A, et al:
Polymorphism in the gene regulatory region of MCP-1 is associated
with asthma susceptibility and severity. J Allergy Clin Immunol.
108:375–381. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao TC, Kuo ML, See LC, et al: The RANTES
promoter polymorphism: a genetic risk factor for near-fatal asthma
in Chinese children. J Allergy Clin Immunol. 111:1285–1292. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu M, Li HL, Huang YK, et al: The SNPs of
chemokine RANTES promoter in children with asthma. Zhong Guo You
Sheng Yu Yi Chuan Za Zhi. 13:20–24. 2005.(In Chinese).
|
|
14
|
Leung TF, Tang NL, Lam CW, et al: RANTES
G-401A polymorphism is associated with allergen sensitization and
FEV1 in Chinese children. Respir Med. 99:216–219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tölgyesi G, Keszei M, Ungvari I, et al:
Involvement of TNFalpha-308A promoter polymorphism in the
development of asthma in children infected with Chlamydophila
pneumoniae. Pediatr Res. 60:543–548. 2006.PubMed/NCBI
|
|
16
|
Sohn MH, Kim SH, Kim KW, et al: RANTES
gene promoter polymorphisms are associated with bronchial
hyperresponsiveness in Korean children with asthma. Lung.
186:37–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang LJ, Li YR, Chen JH, et al:
Polymorphism of regulated upon activation, normal T cell expressed
and secreted promoter region-28 position in Chinese allergic
asthmatic children. Zhonghua Jie He He Hu Xi Za Zhi. 27:394–397.
2004.(In Chinese).
|
|
18
|
Richards W: Hospitalization for children
with status asthmaticus: A review. Pediatrics. 84:111–118.
1989.
|
|
19
|
Maddox L and Schwartz DA: The
pathophysiology of asthma. Annu Rev Med. 53:477–498. 2002.
View Article : Google Scholar
|
|
20
|
Sengler C, Lau S, Wahn U and Nickel R:
Interactions between genes and environmental factors in asthma and
atopy: new developments. Respir Res. 3:72002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Angles MR, Ocaña DB, Medellín BC and
Tovilla-Zárate C: No association between the HTR1A gene and
suicidal behavior: a meta-analysis. Rev Bras Psiquiatr. 34:38–42.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hizawa N, Yamaguchi E, Konno S, et al: A
functional polymorphism in the RANTES gene promoter is associated
with the development of late-onset asthma. Am J Respir Crit Care
Med. 166:686–690. 2002. View Article : Google Scholar : PubMed/NCBI
|