Introduction
Cancer is a disease in which cells lose their normal
control mechanisms and exhibit unorganized growth, thus it may
develop in several tissues or organs, growing and invading
contiguous tissues and extending to the whole body (1). Cancer is associated with a high rate
of mortality due to its capacity to disseminate rapidly and the
lack of effective treatments (2–5). A
number of cancer types originate from cancer stem cells (2,6–10).
These cancer stem cells are important in tumor proliferation and
resistance to chemotherapy and radiotherapy (11–13).
Each type of tumor has a unique combination of markers that define
the subpopulation of stem cells with the highest tumorigenic
potential (14). For example, the
stem cell marker CD133 is expressed in fetal liver but not in
normal adult liver, and is re-expressed in cancer livers. This
upregulation of CD133 is a factor associated with poor prognosis,
suggesting that CD133 plays an oncogenic role in hepatocellular
carcinoma (12,15–18).
CD133 (or Prominin 1) is a membrane glycoprotein of
120 kDa in size in humans and 115 kDa in mice (19). Cancer stem cells that are positive
for CD133 exhibit the activation of a number of mechanisms
responsible for tumor growth and recurrence (8–10)
and inhibition of apoptosis (16,18,20,21).
Observation of CD133+ cancer stem cells aids the
classification, diagnosis and treatment of cancer, and a high
expression of CD133 protein has been associated with lymph and
visceral metastasis (22),
malignancy and poor prognosis (23). The aim of this study was to
determine the effect of suppression of the CD133 protein in cancer
cell lines and its role in chemosensitization, with a view to
contributing to our understanding of CD133 in cancer stem cells as
a possible therapeutic target.
Materials and methods
Cell culture
The B16F10 murine melanoma and MCF7 breast cancer
cell lines were obtained from the American Type Culture Collection
(ATCC, Manassas, VA, USA) and the INER51 lung cancer cell line was
obtained from the National Institute of Respiratory Diseases (INER)
in Mexico City, Mexico.
Cell lines were cultured and maintained in
Dulbecco’s modified Eagle’s medium (DMEMF-12, Life Technologies,
Invitrogen, Burlington, ON, Canada). The medium was supplemented
with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) and
cells were incubated at 37°C in a 5% CO2 atmosphere.
Immunocytochemistry
B16F10, MCF7 and INER51 cells were grown on glass
slides in 6-well plates (1×105 cells/well) with 3 ml
DMEMF-12 supplemented with 10% FBS for 24 h at 37°C and 5%
CO2, and fixed with a 1:1 acetone-methanol solution for
10 min at −20°C. The cells were rehydrated in phosphate-buffered
saline (PBS) and processed for antigen retrieval by a standard
microwave heating technique prior to incubation with anti-CD133
antibody (Ab-CD133; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) at a dilution of 1:100. The reaction was developed using the
Dako Liquid DAB Substrate-Chromogen system (Dako, Carpinteria, CA,
USA) and the cells were counterstained with hematoxylin and
eosin.
Construction of vector expressing
antisense specific for CD133 (As-CD133)
Two primers specific for CD133 were designed using
the published sequence for Mus musculus CD133 (GenBank
accession, NM_008935; NCBI Nucleotide): CD133-1, forward:
5′-GGATCCGCTTGAGAGATC AGGCCAAC-3′ with the restriction site
for BamHI (in bold) and reverse: 5′-GAATTCAACAATCCC
AGCATTGAAGG-3′ with the restriction site for EcoRI
(in bold). A 200-bp product was amplified from cDNA of B16F10 cells
by 30 cycles of PCR (95°C for 60 sec, 60°C for 60 sec and 72°C for
6 sec) using TaqDNA polymerase (Invitrogen, Carlsbad, CA, USA) in a
PTC-200 Peltier Thermal Cycler (MJ Research, Inc., Watertown, MA,
USA). The resulting product was cloned into the vector pEGFP-N3
(Gene Therapy Systems, Inc., San Diego, CA, USA).
Transfection with As-CD133
B16F10, MCF7 and INER51 cell lines were transfected
with As-CD133 and pEGFP-N3 plasmid as a control (Clontech
Laboratories, Inc., Palo Alto, CA, USA) using the cationic branched
polymer polyethylenimine 25 kDa (PEI) (Sigma-Aldrich, St. Louis,
MO, USA). A stock solution of PEI was prepared at a concentration
of 6.45 μg/ml in H2O. The charge ratio, expressed as PEI
nitrogen:DNA phosphate, was 5 (N:P=5). The cells were seeded at
3×103 cells/well in 100 μl DMEMF-12 supplemented with
10% FBS in a 96-well plate 24 h before transfection. For each well,
0.1–0.6 μg of As-CD133 was diluted into 10 μl 150 mmol/l NaCl and
0.01–0.06 μl of the PEI solution was added to another 10 μl of 150
mmol/l NaCl. The PEI-NaCl solution was added to the DNA-NaCl
solution, agitated and incubated for 30 min at room temperature.
Then, 20 μl of the mixture was added to each well and incubated at
37°C in a 5% CO2 atmosphere. Cell viability was
evaluated by MTT assay after 48 h.
Analysis of CD133 expression by RT-PCR
(reverse transcription-polymerase chain reaction)
B16F10, MCF7 and INER51 cell lines were plated in a
6-well plate at 1×105 cells/well in 3 ml DMEMF-12
supplemented with 10% FBS and incubated for 48 h at 37°C. Cells
were harvested and total RNA was extracted using 1 ml TRIzol
reagent (Invitrogen) according to the manufacturer’s instructions.
For RT-PCR, 5 μg of total RNA was reverse transcribed using RT and
oligo(dT) (Invitrogen).
The downregulation of CD133 mRNA in B16F10 cells
transfected with As-CD133 was confirmed by PCR using a second pair
of primers: CD133-2, forward: 5′-TCCAAG GAGATTGCCCTCTA-3′ and
reverse: 5′-CATGGTGCATT CTGCTTCTG-3′, designed using the published
sequence for Mus musculus CD133 described above.
Amplification was performed for 35 cycles (95°C for 60 sec, 58.2°C
for 60 sec and 72°C for 60 sec), generating a 200-bp fragment. As a
control a 350-bp product of G3PDH was amplified using the primers:
forward: 5′-ACCACAGTCCATGCCATCAC-3′ and reverse:
5′-TCCACCACCCTGTTGCTGTA-3′. PCR products were analyzed by
electrophoresis on a 0.8% agarose gel and visualized under UV light
in a transilluminator (Chemi Doc, Image Lab Software, Bio-Rad,
Hercules, CA, USA).
Cell viability analysis by
3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay
The transfected cells were seeded in 96-well plates
at a density of 3×103 cells/well and allowed to attach
for ∼24 h at 37°C. For the MTT assay, 0.025 g MTT (Sigma-Aldrich)
was added to 5 ml PBS at a concentration of 5 mg/ml MTT. The cells
were incubated with 20 μl MTT solution at 37°C for 1 h. The medium
was then removed, 100 μl dimethylsulfoxide was added to each well
and the samples were incubated for 10 min. The optical density (OD)
at 570 nm was determined using a microplate reader (Microplate
Autoreader EL311, BioTek Instruments, Inc., Winooski, VA, USA). The
data are shown as the percentage viability with the standard
error.
Determination of DNA integrity by
acridine orange staining
B16F10 cells (3×103 cells/well in a
96-well plate) were transfected with 0.4 and 0.6 μg of As-CD133 and
incubated at 37°C in a 5% CO2 atmosphere. After 48 h,
the cells were stained with 20 μl of a solution of ethidium bromide
(1 mg/ml) and acridine orange (1 mg/ml) in PBS. The cells were
incubated for 5 min in the dark at room temperature and then washed
with PBS. The samples were photographed using fluorescence
microscopy (TE-Eclipse 300, Nikon).
RT-PCR of apoptotic genes
cDNA of B16F10 cells was amplified using the MPCR
kit for mouse apoptotic gene set-1 (Maxim Biotech, Inc., San
Francisco, CA, USA) according to the manufacturer’s instructions,
using a PTC-200 Peltier Thermal Cycler. The PCR products were
analyzed by electrophoresis on a 0.8% agarose gel and visualized
under UV light in a ChemiDoc transilluminator.
Synergistic effect of As-CD133 and
cisplatin combination treatment on cancer cell viability
B16F10, MCF-7 and INER51 cells were seeded in a
96-well plate at 3×103 cells/well in 100 μl DMEMF-12
supplemented with 10% FBS 24 h prior to transfection. Subsequent to
the previous procedure, the cells were transfected with 0.4 μg
As-CD133 and the addition of cisplatin at the time of transfection
(2–14 ng/μl resuspended in DMEMF-12 supplemented with 10% FBS).
Cells were incubated for 48 h at 37°C in a 5% CO2
atmosphere and analyzed by MTT assay.
Results
Expression of CD133 in cancer cell
lines
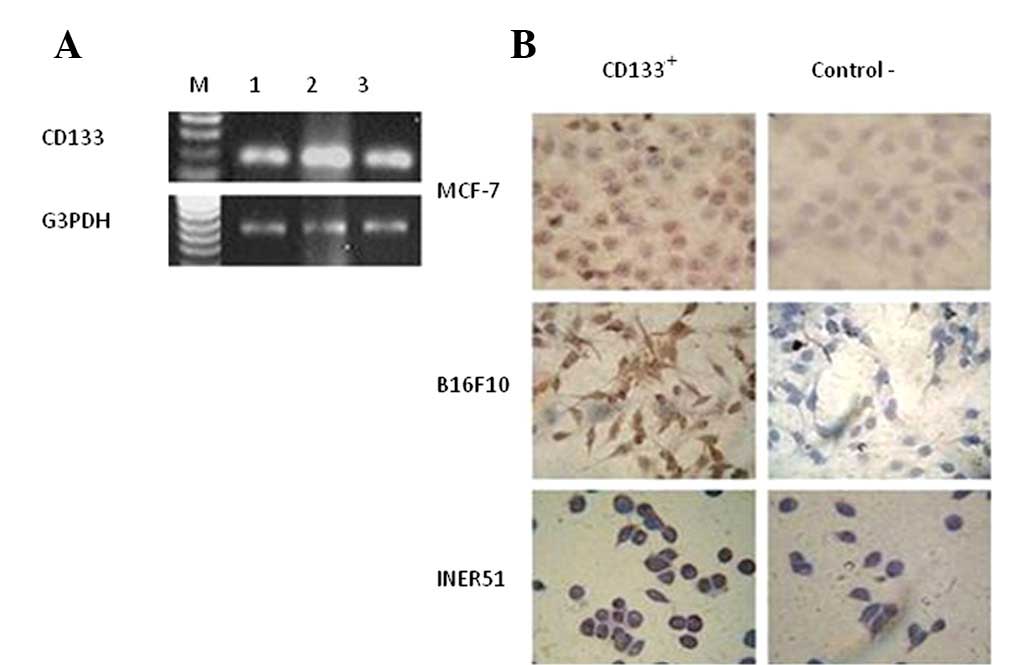
The RT-PCR analysis revealed that the three cancer
cell lines analyzed, B16F10, MCF7 and INER51, all expressed high
levels of CD133 mRNA (Fig. 1A).
These results correlate with the immunocytochemistry, which showed
that 70% of the cells were CD133+ (Fig. 1B).
Effect of CD133 downregulation by
As-CD133 on cancer cell viability
To determine the effect of CD133 protein
downregulation in cancer cells, the three cell lines were
transfected with As-CD133 or control pEGFP-N3. To determine the
transfection efficiency, green fluorescent protein expression was
visualized by UV microscopy, demonstrating that 70–80% of B16F10
cells were transfected, compared with only 20–30% of MCF7 and
INER51 cells (Fig. 2A).
Forty-eight hours after transfection with As-CD133, the three cell
lines exhibited a decrease in cell viability and morphological
changes. The MTT assay of cells treated with 0.6 μg As-CD133
indicated 48, 53 and 78% viability for the B16F10, MCF-7 and INER51
cancer cell lines, respectively (Fig.
2B), indicating a statistically significant difference between
the control and treated B16F10 and MCF7 cell lines (P<0.05).
These effects were dose-dependent (P=0.4). However, the decrease in
the viability of INER51 cells was not statistically significant
(P>0.05; Fig. 2B).
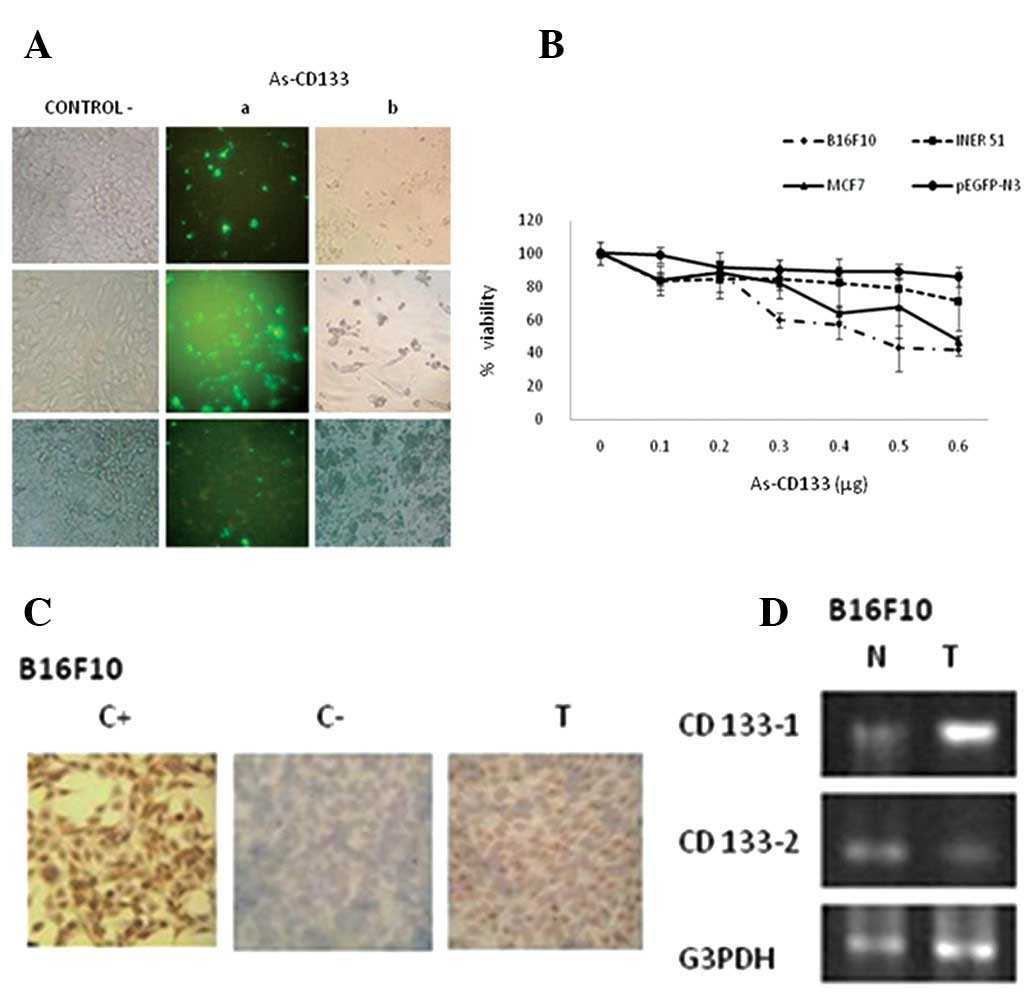 | Figure 2Effect of CD133 downregulation by
As-CD133 on cancer cell viability. (A) Reduced viability of B16F10,
MCF7 and INER51 cells subsequent to transfection with As-CD133.
Images were captured with a confocal fluorescent microscope under
visible light (left and right) and ultraviolet light (center).
Viable cells express the GFP reporter as a control for
transfection. (B) Cell viability analysis of cancer cells treated
with As-CD133 is shown. B16F10, MCF7 and INER51 cells were
transfected with various concentrations (0.1–0.6 μg) of As-CD133 or
with pEGFP-N3 as a control. Cell viability was evaluated by the MTT
assay. Values are means of the average cell viability from three
independent experiments ± SD (P<0.05). (C) Immunocytochemical
analysis of downregulation of the CD133 protein in B16F10 cells
after transfection with As-CD133. C+, stained with Ab-CD133; C-,
lacking Ab-CD133; T, transfected with As-CD133 and stained with
Ab-CD133. (D) RT-PCR analysis of the downregulation of CD133 mRNA
in B16F10 cells subsequent to transfection with As-CD133.
Amplification was performed with CD133-1 and CD133-2 primers, or
with G3PDH primers as a control. T, transfected with the As-CD133;
N, not transfected, negative control. RT-PCR, reverse
transcription-polymerase chain reaction. |
To investigate the correlation between the decrease
in cell viability and CD133 expression, immunocytochemical and
RT-PCR analyses of CD133 expression were conducted.
Immunocytochemical staining showed a decrease in the CD133 protein
in B16F10 cells transfected with As-CD133 (Fig. 2C). RT-PCR with primers CD133-1
corroborate the antisense expression in transfected cells, while
the primers CD133-2 indicated a decrease in CD133 mRNA expression
when the cells were transfected with As-CD133 (Fig. 2D).
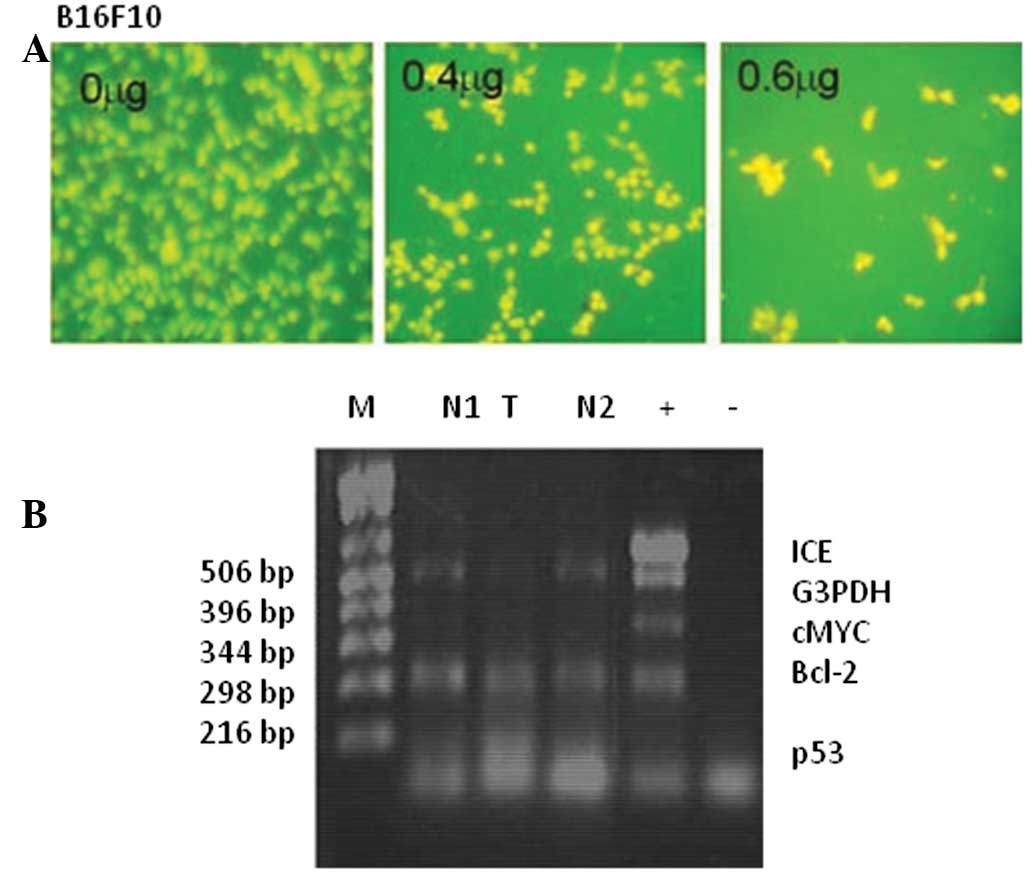
Analysis of DNA integrity in B16F10
cancer cells transfected with As-CD133
The analysis of DNA integrity with acridine orange
showed staining of a high percentage (70–80%) of transfected B16F10
cells compared with the control, indicating that the transfected
cells contained degraded DNA. This staining was dose-dependent with
respect to the antisense vector (Fig.
3A), suggesting that the cell death mechanism induced by
As-CD133 is apoptosis.
Analysis of apoptotic gene expression in
B16F10 cells transfected with As-CD133
Analysis of the expression of apoptotic genes by
multiplex RT-PCR revealed overexpression of the p53 gene in cells
transfected with As-CD133 (Fig.
3B). It is likely that the downregulation of CD133 in
transfected B16F10 cells is correlated with a loss of DNA integrity
and p53 activation, causing the cells to enter apoptosis.
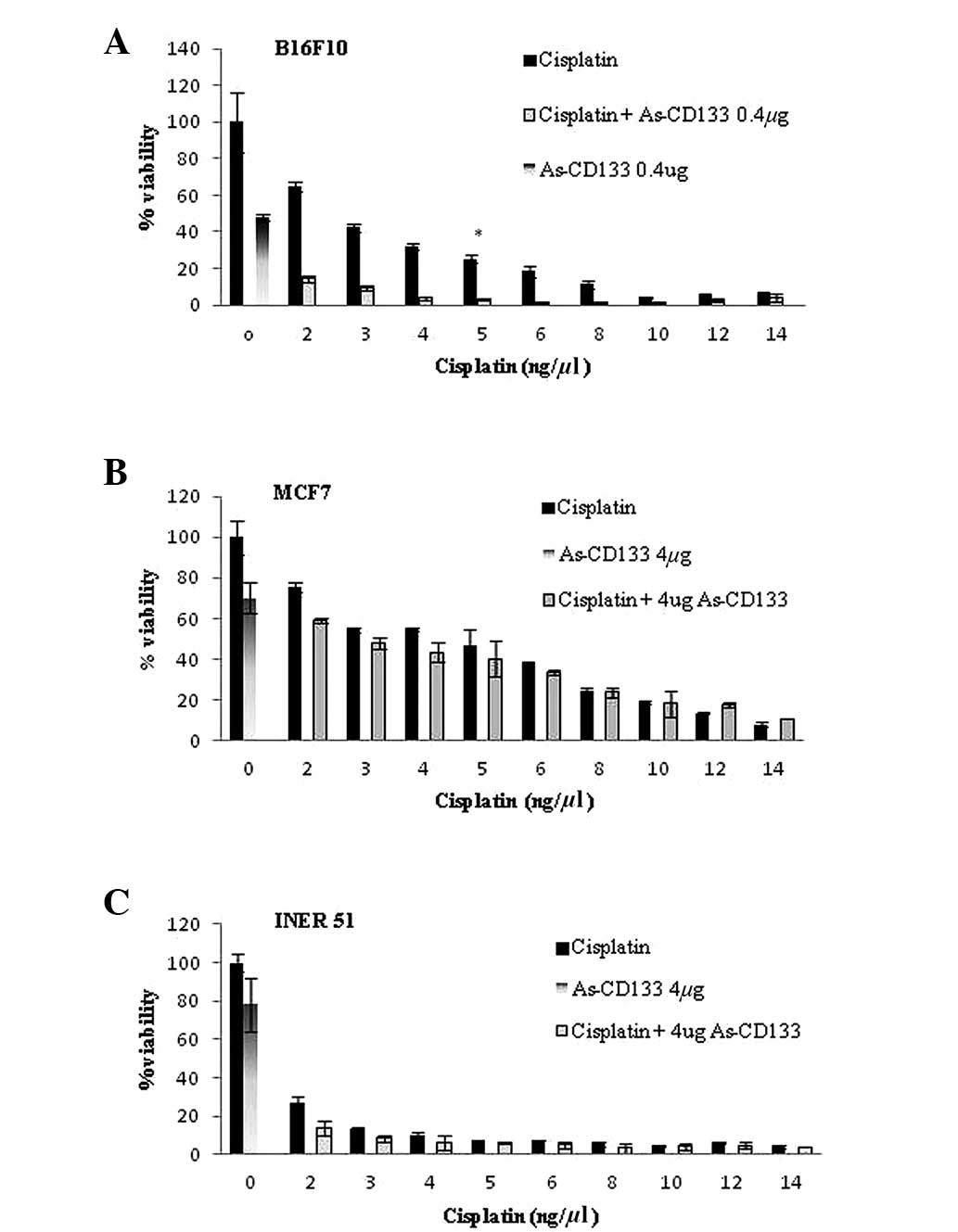
Chemosensitization by As-CD133 in
combination with cisplatin
To determine whether the inhibition of CD133
expression by As-CD133 has a chemosensitizing effect in B16F10,
MCF7 and INER51 cells, the cells were co-treated with a median
lethal dose (LD50) of As-CD133 (0.4 μg) and various
concentrations of cisplatin (2–14 ng/μl). This combination produced
a synergistic effect in B16F10 cells, since the cell viability
decreased significantly with the combination treatment compared
with individual treatments (3.1% viability for the combination
compared with 48% viability for 0.4 μg As-CD133 and 25% for 5 ng/μl
cisplatin; P<0.05). However, MCF7 and INER51 cells did not
exhibit the same effect, and there was no statistical difference in
cell viability between the individual and combined treatments in
these cell lines (Fig. 4).
Discussion
Our results indicate the presence of a high
percentage (≥70%) of CD133+ cells in the three cancer
cell lines analyzed (B16F10 murine melanoma, MCF7 breast cancer and
INER51 lung cancer cells) as assessed by immunocytochemistry. The
results obtained in this study are not in agreement with those
reported by Wright et al (12), who analyzed the breast cancer cell
line RP.1 by flow cytometry and observed that only a small
percentage (2.0–5.9%) of the cells expressed CD133, or with the
findings of Dou et al (13), who analyzed CD133 expression in the
B16F10 murine melanoma cell line and reported a low expression of
CD133+ (3.40%) using the Magnetic Activated Cell Sorting
(MACS) technique. It is possible that we obtained a greater
percentage of positive cells since we used a polyclonal antibody,
compared with the monoclonal antibody used in the other
studies.
The CD133 molecule is crucial in the survival of
cancer cells, and our results showed that downregulation of the
CD133 protein by an antisense construct resulted in a decrease in
cancer cell viability. These results support the findings of other
authors such as Immervoll et al (24), whose data indicated that CD133 is
involved in cellular polarity and is required for cellular movement
as well as the processes of chemotaxis, embryonic development,
invasive growth and metastasis. In addition, Yang et al
(25) reported CD133 involvement
in glucose metabolism and cytoskeleton alteration. Additionally,
Rappa et al (7) showed that
the downregulation of CD133 resulted in retarded cell growth,
reduced cell motility and a decreased ability to form spheroids
under stem cell-like growth conditions.
Findings of the present study also showed that the
decrease in cancer cell viability following transfection with
As-CD133 was most likely the result of increased cell death through
an apoptotic mechanism. This pathway was likely activated via the
pro-apoptotic gene p53, which was itself most likely activated by a
member of the MAP kinase family, which responds to various types of
stress resulting in the upregulation of p53 expression being
triggered (26). However,
additional studies are required to confirm this pathway.
The synergistic effect of an antisense sequence and
an anticancer drug are likely to provide a good alternative
treatment against CD133+ cancer since downregulation of
the CD133 protein may result in chemosensitization of cancer cell
lines, as observed in the B16F10 cell line used in this study. This
finding presents a potentially effective and promising approach to
cancer therapy, which may decrease the required drug dose, thereby
reducing the secondary effects in patients. In a previous study,
Tirino et al (19)
mentioned that CD133+ cells represent a small population
of cells that possess stem features and are potentially resistant
to drugs, and thus may effectively drive cancer progression.
Dell’Albani (16) and Liu et
al (20) reported that
CD133+ cells express high levels of apoptotic
suppressors (Bcl2, FLIP, BCL-XL) and several apoptotic protein
inhibitors (XIAP, cIAP1, cIAP2, NAIP), which are linked to caspases
3, 7 and 9 to prevent apoptosis and modulate cellular division, as
well as progression of the cell cycle and signal transduction
pathways (16,20).
In the present study, the synergistic effect of
antisense and cisplatin was not observed in the INER51 and MCF7
cell lines. With respect to the INER 51 cell line, it is necessary
to identify a more effective transfection method than
polyethylenimine since improved transfection efficiency may lead to
results similar to, or even better than, those obtained with the
B16F10 cells. Additionally, various drugs should be tested to
obtain improved results with the MCF7 line.
In conclusion, findings of this study have provided
evidence that CD133 is important in the viability of cancer cells
and suggest that CD133 downregulation by antisense, alone and in
combination with cisplatin, is potentially a new and powerful
therapeutic strategy for CD133+ cancers.
References
|
1
|
Greenwald P and Dunn BK: Landmarks in the
history of cancer epidemiology. Cancer Res. 69:2151–2162. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gray-Schopfer V, Wellbrock C and Marais R:
Melanoma biology and new targeted therapy. Nature. 445:851–857.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Murray T, Ward E, et al: Cancer
statistics. CA Cancer J Clin. 55:10–30. 2005.
|
|
4
|
Organización Mundial de la Salud [OMS]:
2011.Cáncer. Nota descriptiva No. 297. http://www.who.int/mediacentre/factsheets/fs297/es/index.htmluri.
Accessed April 16, 2012.
|
|
5
|
MacKie RM, Hauschild A and Eggermont AMM:
Epidemiology of invasive cutaneous melanoma. Ann Oncol. 20(Suppl
6): vi1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bongiorno MR, Doukaki S, Malleo F and
Aricò M: Identification of progenitor cancer stem cell in lentigo
maligno melanoma. Dermatol Ther. 21(Suppl 1): S1–S5. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rappa G, Fodstad O and Lorico A: The stem
cell-associated antigen CD133 (Prominin-1) is a molecular
therapeutic target for metastatic melanoma. Stem Cells.
26:3008–3017. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shmelkov SV, Jun L, St Clair R, et al:
Alternative promoters regulate transcription of the gene that
encodes stem cell surface protein AC133. Blood. 103:2055–2061.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beier D, Hau P, Proescholdt M, et al:
CD133+ and CD133− glioblastoma-derived cancer
stem cells show differential growth characteristics and molecular
profiles. Cancer Res. 67:4010–4015. 2007.PubMed/NCBI
|
|
10
|
Bruno S, Bussolati B, Grange C, Collino F,
Graziano ME, Fernando U and Camussi G: CD133+ renal
progenitor cell contribute to tumor angiogenesis. Am J Pathol.
169:2223–2236. 2006.
|
|
11
|
Sims AH, Howell A, Howell SJ and Clarke
RB: Origins of breast cancer subtypes and therapeutic implications.
Nat Clin Pract Oncol. 4:516–525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wright MH, Calcagno AM, Salcido CD,
Carlson MD, Ambudkar SV and Varticovski L: Brca1 breast tumor
contain distinct CD44+/CD24− and
CD133+ cell with cancer stem cell characteristic. Breast
Cancer Res. 10:R102008. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dou J, Pan M, Wen P, et al: Isolation and
identification of cancer stem-like cells from murine melanoma cell
lines. Cell Mol Immunol. 4:467–472. 2007.PubMed/NCBI
|
|
14
|
Huang EH, Heidt DG, Li CW and Simeone DM:
Cancer stem cells: a new paradigm for understanding tumor
progression and therapeutic resistance. Surgery. 141:415–419. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song W, Li H, Tao K, Li R, Song Z, Zhao Q,
Zhang F and Duo K: Expression and clinical significance of the stem
cell marker CD133 in hepatocellular carcinoma. Int J Clin Pract.
62:1212–1218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dell’Albani P: Stem cell marker in
gliomas. Neurochem Res. 33:2407–2415. 2008.
|
|
17
|
Abbott A: Cancer: the root of the problem.
Nature. 442:742–743. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maeda S, Shinchi H, Kurahara H, et al:
CD133 expression is correlated with lymph node metastasis and
vascular endothelial growth factor-C expression in pancreatic
cancer. Br J Cancer. 98:1389–1397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tirino V, Desiderio V, d’Aquino R, et al:
Detection and characterization of CD133+ cancer stem
cells in human solid tumours. PLoS One. 3:e34692008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu G, Yuan X, Zeng Z, et al: Analysis of
gene expression and chemoresistence of CD133+ cancer
stem cells in glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shmelkov SV, Butler JM, Hooper AT, et al:
CD133 expression is not restricted to stem cells, and both
CD133+ and CD133− metastatic colon cancer
cells initiate tumors. J Clin Invest. 118:2111–2120.
2008.PubMed/NCBI
|
|
22
|
Al Dhaybi R, Sartelet H, Powell J and
Kokta V: Expression of CD133+ cancer stem cells in
childhood malignant melanoma and its correlation with metastasis.
Mod Pathol. 23:376–380. 2010.
|
|
23
|
Klein WM, Wu BP, Zhao S, Wu H,
Klein-Szanto AJ and Tahan SR: Increased expression of stem cell
markers in malignant melanoma. Mod Pathol. 20:102–107. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Immervoll H, Hoem D, Sakariassen PØ,
Steffensen OJ and Molven A: Expression of the stem cell marker
CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC
Cancer. 8:482008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang C, Yang Y, Gupta N, et al: Pentaspan
membrane glycoprotein, prominin 1, is involved in glucose
metabolism and cytoskeleton alteration. Biochemistry (Mosc).
72:854–862. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo F, Li Y, Liu Y, Wang J and Li G:
ARL6IP1 mediates cisplatin-induced apoptosis in CaSki cervical
cancer cells. Oncol Rep. 23:1449–1455. 2010.PubMed/NCBI
|