Introduction
Knowledge of facial nerve microneuroanatomy is of
particular importance in the diagnosis of extratemporal facial
nerve lesions, as well as in clinical applications such as
fascicular grafting following facial nerve injuries. Animal models
are vital for establishing the microneuroanatomy of the facial
nerve. The reasons for their frequent use lie in their similarities
in gross anatomy and physiology to humans, along with economic
advantages and ethical reasons. Thus, they offer an unprecedented
opportunity to evaluate the spatial orientation of the
extratemporal facial nerve, and to preclinically ascertain the
efficacy and safety of newly developed human therapies.
It is generally accepted that the facial motor
nucleus has a somatotopic organization (1–5).
Whether this is also true for the whole trunk of the facial nerve
(WTFN) is a matter of debate, and this has been the subject of
numerous investigations utilizing a variety of methods. The use of
cadaver dissections (6) is clearly
a crude method for examining the organization of axonal
populations. In some instances, clinical observations have been
combined with neurophysiological stimulation and recording
procedures (7). These studies have
not convincingly proven the existence of a somatotopic
organization. A third way of analyzing the organization of the
facial nerve has been to make partial lesions of the facial nerve
trunk and to evaluate the resulting functional consequences. This
method has also led to different conclusions (8,9).
Other methods including radio frequency lesions, crush injuries and
various observations have met with varying degrees of success.
Following the application of horseradish peroxidase
(HRP) as a neuroanatomical tracing method (10), the question of whether the
intrinsic organization of the extratemporal tunk of the facial
nerve (ETFN) is topographically or diffusely organized remains to
be clarified (11–13).
Fluoro-Gold™ (FG) is a fluorescent tracer
that has been used successfully in numerous animal models (14–17).
Therefore, it was used in this study as a tracer to examine the
organization of the ETFN. We aimed to locate the spatial
orientation of each facial nerve branch in the distal, middle and
proximal parts of the ETFN in Sprague-Dawley albino rats, to
improve understanding of the mechanism of facial nerve regeneration
after injury of the ETFN.
Materials and methods
Facial nerve anatomy
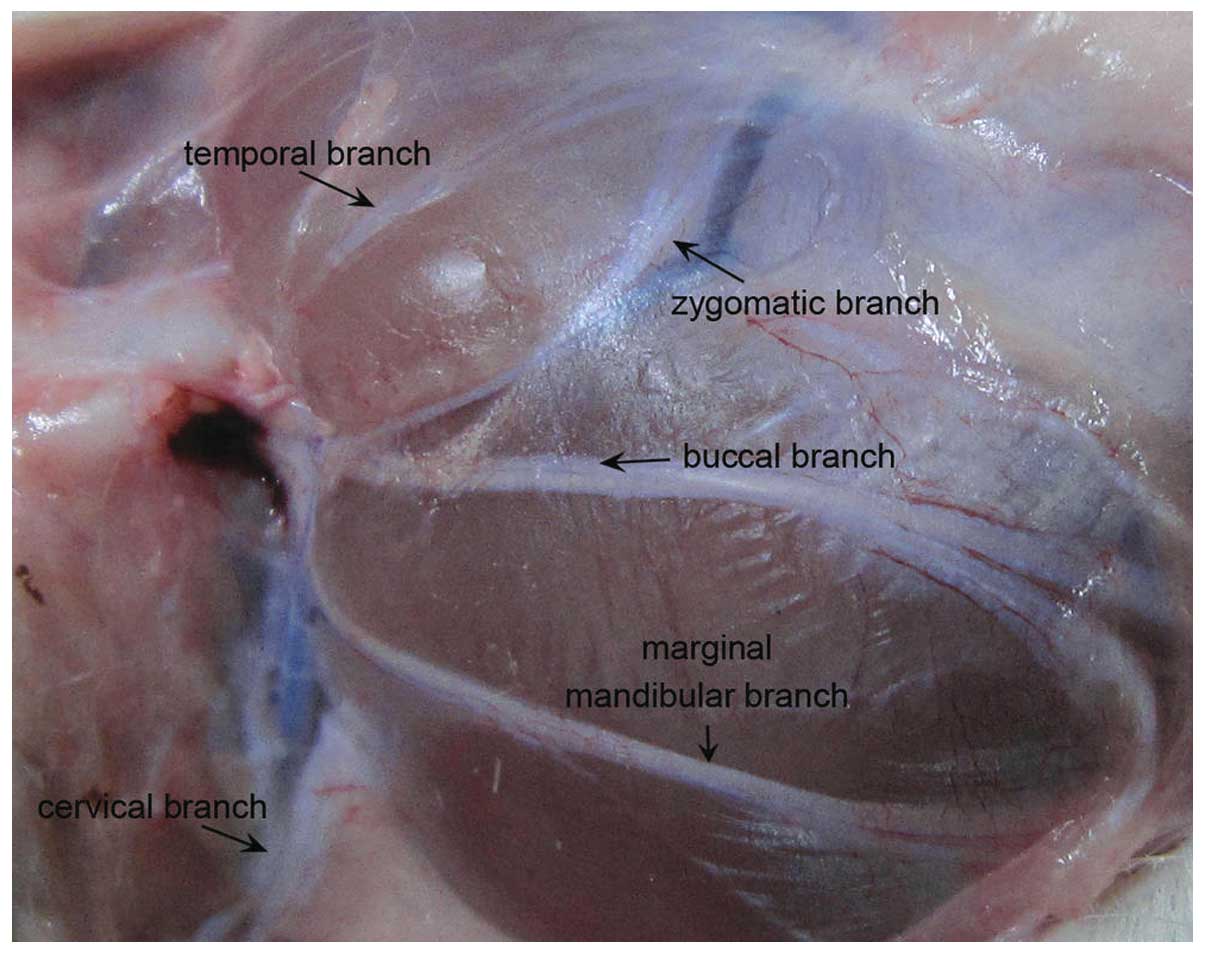
The distribution of the facial nerve and the pattern
of all the branches of the SD rats appeared similar to those of the
human (Fig. 1). The main trunk of
the rat facial nerve was divided into five main peripheral branches
(temporal, zygomatic, buccal, marginal mandibular, cervical).
Animals and surgical procedures
Fifteen adult female Sprague-Dawley albino rats,
weighing 250–300 g, were anaesthetized with an intraperitoneal
(i.p.) injection of chloral hydrate (300 mg/kg). All animals were
kept under standard laboratory conditions (artificial light cycle,
12 h on/off), with tap water and Altromin R/M standard laboratory
chow ad libitum. FG (Fluorochrome Inc., Denver, CO, USA) was
dissolved in distilled water (2% w/v). The animals were randomized
to five groups. FG was applied to one branch in each group. The
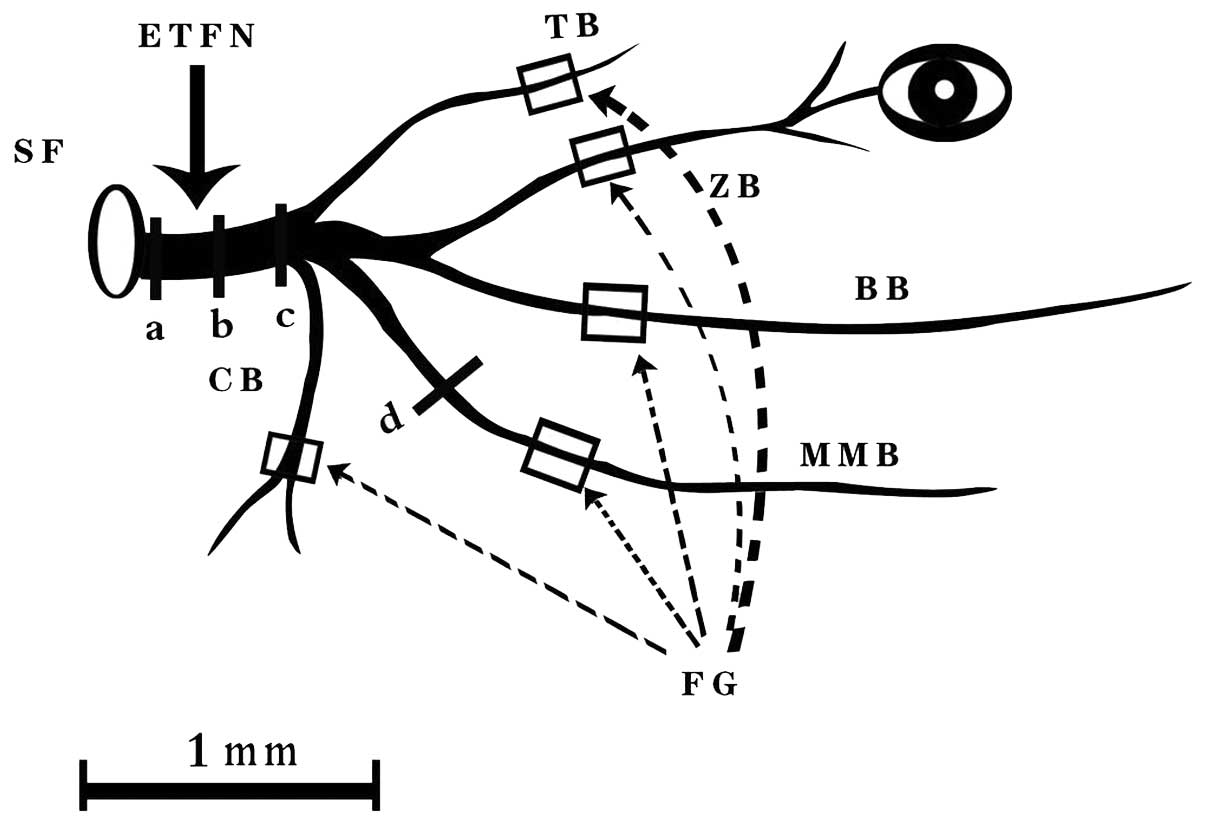
facial nerve branches were dissected carefully under an operating
microscope. A 10-μl Hamilton syringe was used to inject 5 μl 2% FG
to the proximal end of the nerve under manual pressure. The nerve
was clearly transected 10 mm distal to the trunk (Fig. 2). A pipette containing 5 μl FG was
then kept in position at the cut end of the trunk for the next 10
min to allow the tracer to penetrate into the tissue. Particular
care was taken to achieve complete immersion of the nerve stump in
the FG solution. A single 4-0 silk suture (Ethicon) was used to
close each wound. The operation was performed bilaterally. The
animals recovered from anaesthesia without side effects. After 2
days, they were perfused under deep anaesthesia by thoracotomy and
aortic cannulation using 100 ml of 0.1 M phosphate buffered saline,
followed by 500 ml of a 4% paraformaldehyde fixative solution, pH
7.4. The facial nerves were dissected from the FG injection site on
the face to the stylomastoid foramen immediately after perfusion.
The lateral aspect of the nerve was marked by opening the sheath at
the crotch of the trunk with a No. 15 Bard-Parker blade.
Subsequently, the nerves were cut serially into 10 μm-thick
cross-sections on a Leica 1900CM microtome. Care was taken to
maintain the serial order of the sections so that the location of
the labeled nerve fibers would be apparent. The specimens were
examined on three different levels [proximal, medial, and distal
parts of the extratemporal trunk of the facial nerve (Fig. 2)], using a Zeiss Axiophot
fluorescence microscope and H365 filters (band-pass 365 nm, long
pass 397 nm). The study was approved by the PLA Postgraduate
Medical School ethics board All animal experiments were carried out
in accordance with the guidelines of the Animal Care and Use
Committee of PLA Postgraduate Medical School.
Results
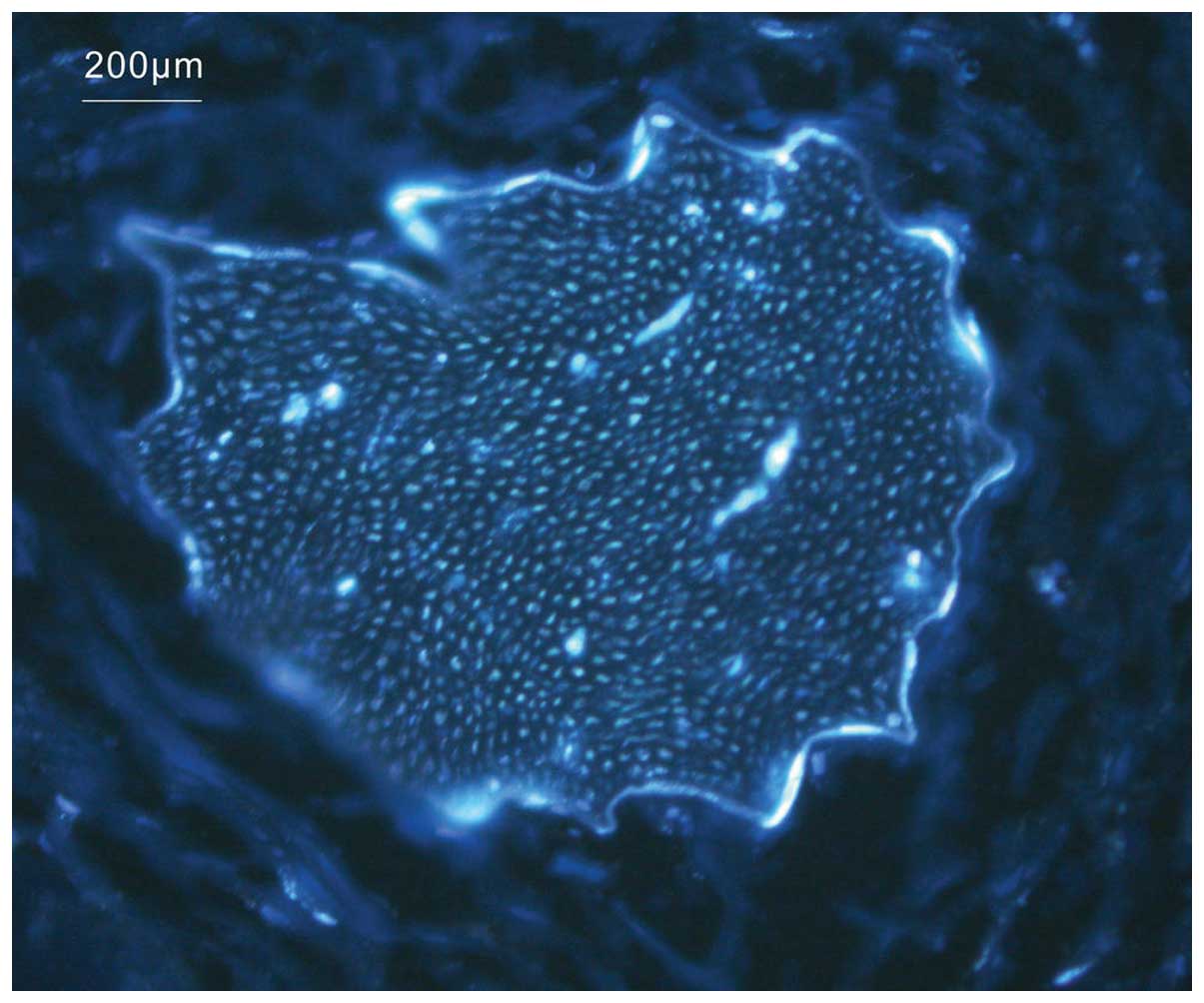
FG labeled all nerve branches. Bright white dots
representing FG-labeled fibers filled the whole cross-section of
the branch that was proximal to the injection site (Fig. 3). In general, a definite spatial
orientation was retained in the distal part of the ETFN. In the
middle part, the FG-labeled zone was partially dispersed, but the
orientation was still clear. In the proximal part, however, all
branches were diffused with blurred orientation.
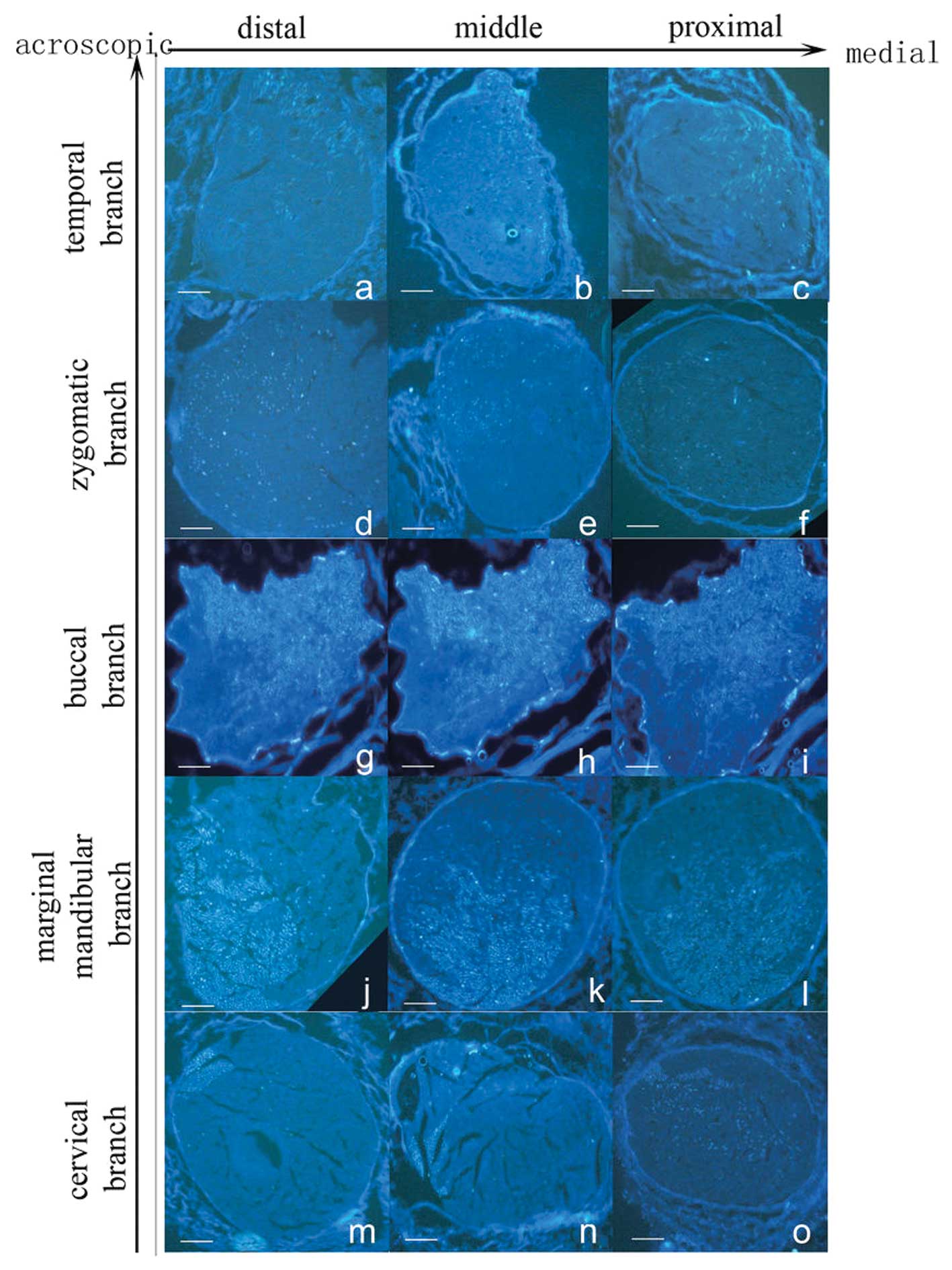
Temporal branch
In the distal part of the ETFN, a crescent-shape
labeled zone was found in the medial and acroscopic aspect of the
axonal nerve, occupying a quarter of the whole section, with a
definite border and a homogeneous distribution (Fig. 4a). The labeled zone extended to the
medial part of the ETFN, showing an oval shape (Fig. 4b). The labeled zone extended
laterally near the stylomastoid foramen, covering approximately one
third of the whole section. In the proximal part of the ETFN, the
labeled zone had a circular shape, and the labeled fibers were
sparse (Fig. 4c).
Zygomatic branch
Cross-sections of the FG-labeled zygomatic branch
occupied a quarter in the lateral and acroscopic aspect of the
nerve in the distal part of the ETFN. The labeled zone presented a
‘c’ shape, and the bright white dots in it were dense in the
lateral part (Fig. 4d). The
labeled zone expanded inward in the middle part of the ETFN. Most
of the bright white dots were concentrated in the lower part of the
zone (Fig. 4e). In the proximal
part of the ETFN, the labeled fibers were dispersed in the lateral
half of the entire section without a definite medial border
(Fig. 4f).
Buccal branch
Labeled fibers of the buccal branch were distributed
homogeneously in the upper half of the nerve in the distal part of
the ETFN. The labeled zone was much brighter than the temporal and
zygomatic branch, with a lower irregular border (Fig. 4g). The labeled area extended
inferolaterally slightly into the middle part of the ETFN (Fig. 4h). The bright white dots became
sparse in the lower part of the labeled zone in the proximal part
of the ETFN. The labeled zone occupied almost three quarters of the
whole section with an irregular lower border (Fig. 4i).
Marginal mandibular branch
When FG was applied to the marginal mandibular
branch, an oval zone was found in the inferolateral half area of
the nerve, which was slightly smaller than that found in the buccal
branch. The labeled zone almost occupied the lower half of the
whole section in the distal part of the ETFN (Fig. 4j). In the middle part of the ETFN,
the labeled area expanded upward with a moon-shaped unlabeled zone
in each lateral border. The upper border of the labeled zone was
irregular. The intensity of the labeled fibers in the expanded part
was slightly thinner than in the rest (Fig. 4k). The labeled zone dispersed
upward and occupied two thirds of the whole section in the proximal
part of the ETFN. Labeled fibers in the upper half were sparse
compared with those in the lower half (Fig. 4l).
Cervical branch
Cross-sections of the cervical branch revealed a
square zone of axonal labeling in the lateral and acroscopic aspect
of the nerve in the distal part of the ETFN, which occupied one
fifth of the first quadrant. The border of the labeled zone was
definite, which was different from the other four branches
(Fig. 4m). In the middle part of
the ETFN, the labeled zone stretched out two tapers to the center.
The bright white dots distributed homogeneously in the zone
(Fig. 4n). In the proximal part of
the ETFN, the labeled zone expanded in the center half of the
section, presenting an oval zone. Labeled fibers were distributed
non-homogeneously in the expanded part and appeared to be fewer
than in the square part (Fig.
4o).
The labeled zone of the buccal and marginal
mandibular branches were markedly larger than the other three
branches in the nerve trunk, with each branch taking over one half
of the area. Next were the zygomatic branch, and then the temporal
branch. The cervical branch showed the least occupation in the
nerve trunk. Nevertheless, among all five branches, the labeled
distribution zone of the cervical branch changed most in the
distal, middle and proximal part of the ETFN. In the distal part of
the ETFN, the labeled zone of all five branches covered the whole
cross-section of the nerve trunk, with some areas overlapping. The
labeled zone of each branch expanded in the middle part of the
nerve trunk, resulting in the corresponding expansion of the
overlapping area. In the proximal part of the ETFN, the labeled
zone of all five branches continued to expand to one half of the
nerve trunk with irregular border. Therefore, it was difficult to
distinguish the specific distribution of each branch in this
area.
Discussion
In this study, FG was applied as a tracer in the
neuroanatomical tracing method to study the spatial orientation of
the ETFN in the rat. Our findings demonstrated that each branch of
the facial motor nerve has a topographical orientation in the
distal and middle part of the ETFN, but the branches diffuse near
the stylomastoid. The question of whether the motor fibers in the
WTFN are organized somatotopically or diffusely has been the
subject of numerous investigations employing a variety of methods
(6–9). However, these methods appear to
involve considerable uncertainties. In the early years,
neurophysiological stimulation of nerves together with clinical
observations was used to detect the topographic orientation of the
facial nerve, but the result was only approximate, as the method
could only provide information on the rough spatial distribution of
nerve branches (7). It is
difficult to control the lesion area by cutting the facial nerve,
and assessment of the functional consequences is obscured
accordingly. Therefore, it is understandable that controversial
results were obtained by this method (8,9).
Until now, the neuroanatomical tracing method has
been the best way to solve this problem. With this method, axons
from different facial nerve branches can be labeled selectively
throughout the entire proximodistal length of the WTFN. The
neuroanatomical tracing method has promoted the development of
neural anatomy. It provides adequate information on the pattern of
fiber distribution from different nerve branches. It allows for
information to be obtained directly from the nerve, other than by
clinical observations or other indirect methods.
With the development of the neuroanatomical tracing
method, many commercial products have become available for such
studies. HRP was the first and most widely used tracer in the
retrograde tracing method. However, it has several disadvantages
compared to fluorescent tracers when used in the research of nerve
branch orientation in the trunk. HRP requires a series of
complicated procedures before developing color. Longer immersion
times are required for HRP to reach effective levels of labeling
and, therefore, the use of HRP prolongs the anesthesia time and
increases the surgical difficulty. Moreover, HRP can label intact,
undamaged fibers of passage, thereby interfering with the accurate
outcome of the study.
Fluorescent compounds that are used currently as
retrograde tracers have several advantages. They can be tested soon
after the specimen is obtained without the use of additional
staining techniques. This method simplifies the manipulation steps
and saves time. Furthermore, it reduces the potential variations
resulting from the staining techniques and different experimenters,
and, accordingly, improves the accuracy of the study. FG was first
introduced in 1986 (18), and
since then it has been used frequently as a retrograde tracer in
rodents (14–16). It has several advantages, such as
complete labeling of the cytoplasm without diffusion, long duration
without fading, absorption by damaged fibers only, and easy
obtainment (18). FG has been
shown to label more neurons than the fluorescent dextrans (19), and it labels more brightly and
rapidly than the other tracers (17). FG produces bright white
fluorescence under an ultraviolet filter, which is commonly found
in fluorescence microscopes.
A number of studies have focused on the spatial
relations of the peripheral branches of the intratemporal portion
of the facial nerve (ITFN) (9,12,13).
The view that facial nerve fibers are organized diffusely has been
supported by certain studies utilizing the HRP neuroanatomical
tracing method (12,13). Nevertheless, the spatial relation
of the ETFN remains unclear. Several preliminary studies have been
published in this field. Crumley (11) reported preliminary findings on the
fiber organization of the zygomatic branch, but other branches were
not included in this study. Choi and Raisman (8) combined hemisection with the
neuroanatomical tracing method, and found that 88% of the fibers
that supply the temporal branch of the facial nerve travel in the
upper half of the facial nerve trunk. However, there have been no
studies on the spatial orientation of all branches of the ETFN to
date. Therefore, this study was designed to visualize the
microanatomy of EFTN and to lay the groundwork for future repair
studies.
By dissecting the ITFN, May (9) found that the peripheral fibers in the
cat rotate as they travel from the stylomastoid foramen toward the
face. However, the result of this study showed that each branch of
the facial motor nerve had a topographical orientation in the
distal and middle part of the ETFN, but that the branches became
diffuse near the stylomastoid foramen.
In general, the results of this study also have
implications for clinical practice. Understanding the spatial
relations of the facial nerve fibers enables better understanding
of the mechanisms of certain diseases, such as Bell's palsy, a
unilateral paralysis of the peripheral facial nerve. Our study
demonstrates that the branches become diffuse near the stylomastoid
foramen. It also explains why suturing complete lesions of the ITFN
by intrafascicular repair provides little additional positive
effect on the recovery of the nerve function by trying to match the
ITFN fibers in the proximal and distal stumps. However, the results
of this study also showed that each branch of the facial motor
nerve had a topographical orientation in the distal and middle part
of the ETFN. Thus, they indicated that intrafascicular suturing may
provide a positive effect on functional recovery if the injury is
located between the distal and middle part of the ETFN. It can also
be concluded that as the injury comes near the stylomastoid
foramen, the functional recovery is poorer. The microanatomy of the
facial nerve is an important basis of the mechanism of facial nerve
regeneration following injury. Understanding the spatial
orientation of the ETFN will be beneficial to find new methods of
repairing facial nerve injuries. A particular conduit that is
designed to bridge the gap between the trunk and branches may be
advantageous. Such a conduit should consist of one trunk and
multiple branches with its shape imitating that of the facial
nerve. The inner structure of the trunk refers to the spatial
orientation of each branch. If such a conduit could be constructed,
it would be possible to achieve functional recovery.
Abbreviations:
|
FG
|
Fluoro-Gold
|
|
ETFN
|
extratemporal trunk of the facial
nerve
|
|
HRP
|
horseradish peroxidase
|
|
WTFN
|
whole trunk of the facial nerve
|
|
ITFN
|
intratemporal portion of the facial
nerve
|
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (30872898) and
Doctorial Groundbreaking Program of Chinese PLA General Hospital
(09BCZ02). We thank the International Science Editing Company for
the linguistic assistance.
References
|
1
|
Radpour S: Organization of the facial
nerve nucleus in the cat. Laryngoscope. 87:557–574. 1977.PubMed/NCBI
|
|
2
|
Radpour S and Gacek RR: Facial nerve
nucleus in the cat. Further study Laryngoscope. 90:685–692.
1980.PubMed/NCBI
|
|
3
|
Sinis N, Horn F, Genchev B, et al:
Electrical stimulation of paralyzed vibrissal muscles reduces
endplate reinnervation and does not promote motor recovery after
facial nerve repair in rats. Ann Anat. 191:356–370. 2009.
View Article : Google Scholar
|
|
4
|
Furutani R and Sugita S: Comparative
histological study of the mammalian facial nucleus. J Vet Med Sci.
70:367–372. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guntinas-Lichius O, Irintchev A, Streppel
M, et al: Factors limiting motor recovery after facial nerve
transection in the rat: combined structural and functional
analyses. Eur J Neurosci. 21:391–402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sunderland S and Cossar DF: The structure
of the facial nerve. Anat Rec. 116:147–165. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kempe LG: Topical organization of the
distal portion of the facial nerve. J Neurosurg. 52:671–673. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi D and Raisman G: After facial nerve
damage, regenerating axons become aberrant throughout the length of
the nerve and not only at the site of the lesion: an experimental
study. Br J Neurosurg. 18:45–48. 2004. View Article : Google Scholar
|
|
9
|
May M: Anatomy of the facial nerve
(spatial orientation of fibers in the temporal bone). Laryngoscope.
83:1311–1329. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kristensson K and Olsson Y: Diffusion
pathways and retrograde axonal transport of protein tracers in
peripheral nerves. Prog Neurobiol. 1:87–109. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Crumley RL: Spatial anatomy of facial
nerve fibers – a preliminary report. Laryngoscope. 90:274–280.
1980.
|
|
12
|
Lee SH, Ito J and Yamamoto E: A
horseradish peroxidase study of the fiber orientation in the facial
nerve. Eur Arch Otorhinolaryngol. 248:366–369. 1991.PubMed/NCBI
|
|
13
|
Thomander L, Aldskogius H and Grant G:
Motor fibre organization in the intratemporal portion of cat and
rat facial nerve studied with the horseradish peroxidase technique.
Acta Otolaryngol. 93:397–405. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Van Bockstaele EJ, Aston-Jones G,
Pieribone VA, Ennis M and Shipley MT: Subregions of the
periaqueductal gray topographically innervate the rostral ventral
medulla in the rat. J Comp Neurol. 309:305–327. 1991.PubMed/NCBI
|
|
15
|
Valero-Cabre A, Tsironis K, Skouras E,
Navarro X and Neiss WF: Peripheral and spinal motor reorganization
after nerve injury and repair. J Neurotrauma. 21:95–108. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kamijo Y, Koyama J, Oikawa S, et al:
Regenerative process of the facial nerve: rate of regeneration of
fibers and their bifurcations. Neurosci Res. 46:135–143. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi D, Li D and Raisman G: Fluorescent
retrograde neuronal tracers that label the rat facial nucleus: a
comparison of Fast Blue, Fluoro-ruby, Fluoro-emerald, Fluoro-Gold
and DiI. J Neurosci Methods. 117:167–172. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schmued LC and Fallon JH: Fluoro-Gold: a
new fluorescent retrograde axonal tracer with numerous unique
properties. Brain Res. 377:147–154. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Novikova L, Novikov L and Kellerth JO:
Persistent neuronal labeling by retrograde fluorescent tracers: a
comparison between Fast Blue, Fluoro-Gold and various dextran
conjugates. J Neurosci Methods. 74:9–15. 1997. View Article : Google Scholar : PubMed/NCBI
|