Introduction
Lung cancer was the most commonly diagnosed cancer
as well as the leading cause of cancer-related death in males and
the second cause of cancer-related death in females globally in
2008 (1). To date, no effective
treatment is available and the five-year survival rate remains less
than 15%, despite advances in diagnosis and treatment. Numerous
patients suffer from recurrence and metastasis following surgery,
chemotherapy and radiotherapy (2).
Angiogenesis is crucial for tumor growth and
metastasis, and can be stimulated by several regulators including
vascular endothelial growth factor (VEGF), basic fibroblast growth
factor (bFGF), transforming growth factor-β (TGF-β),
platelet-derived growth factor (PDGF), interleukin-8, and
angiogenin. Among these, VEGF has the greatest potential for
research (3,4). The level of VEGF is significantly
increased in NSCLC and is intimately associated with tumor
metastasis as well as poor prognosis (5–10).
Cyclooxygenase (COX) is an enzyme that catalyzes the
rate-limiting step in prostaglandin synthesis. To date, two
isoforms, COX-1 and COX-2, have been identified (11). COX-1 is constitutively expressed in
virtually all cells, whereas COX-2 is only expressed in regions of
inflammation and in the cancer microenvironment. COX-2
overexpression has been observed in the majority of human
malignancies, including lung cancer, and its expression level
correlates with VEGF (12–17). Matrix metalloproteinases (MMPs) are
a diverse family of enzymes capable of degrading various components
of the ECM. MMP expression has been found to be upregulated in
several human tumors and correlates with advanced stage, invasion,
angiogenesis, metastatic properties and poor prognosis (18). Among secreted MMPs, MMP2 and MMP9
(gelatinase A and gelatinase B) are known to play a key role in
tumor invasion and metastasis development (19). The expression of large amounts of
MMP2 and MMP9 has been documented in NSCLC (20). Wild-type (WT)p53 is one of the most
intensively studied anti-oncogenes. It acts as a nuclear
transcription factor that transactivates genes involved in
apoptosis, cell cycle regulation, and other vital processes
(21). A previous study revealed
that WTp53 is significantly inversely associated with VEGF
expression (22). On the basis of
these studies, it is conceivable that VEGF expression may correlate
with COX-2, MMP and WTp53 expression. However, the relationships
between VEGF expression and tumor growth and cell cycle status have
not been examined previously.
Therefore, the present study aimed to elucidate the
relationship between VEGF expression and COX-2, MMP2, MMP9 and
WTp53 expression and cell growth and cell cycle progression in
Lewis lung cancer (LLC) cells.
Materials and methods
Reagents
Lipofectamine 2000™, G418 and propidium iodide were
obtained from Invitrogen (Carlsbad, CA, USA). Plasmid mini kit and
DNA Gel extraction kit were purchased from Omega Bio-Tek (Norcross,
GA, USA). Rabbit anti-mouse VEGF polyclonal antibodies and mouse
anti-human COX-2, MMP2, MMP9, phospho-MMP2 and phospho-MMP9, WTp53
and β-actin monoclonal primary antibodies, sheep anti-mouse and
sheep anti-rabbit secondary antibodies labeled horseradish
peroxidase were all purchased from Santa Cruz Biotechnology Inc.
(Santa Cruz, CA, USA). The recombinant plasmids of pIRES2-VEGF-GFP
(VEGF overexpression plasmid), pIRES2-GFP and pSUPER-VEGF-GFP
(microRNA expression of VEGF plasmid) were kindly provided by Dr
Yuan Su.
Cell culture
LLC, a mouse lung cancer cell line, was purchased
from the cell bank of the Chinese Academy of Science, Shanghai,
China. Cells were stored in a humidified 5% CO2
atmosphere and cultured in 1640 medium supplemented with 10% fetal
calf serum, glutamine (2 mM), penicillin (100 U/ml) and
streptomycin (100 U/ml).
Cell transfection and stable cell line
establishment
LLC cells were transfected with pIRES2-VEGF-GFP
(LLC-VEGF), pSUPER-VEGF-GFP (LLC-RNAi), pIRES2-GFP (LLCGFP)
plasmids using Lipofectamine 2000. The stable cells were selected
following 2 months in 1 mg/ml of G418-containing medium. The
resistant colonies were removed and VEGF protein expression was
examined. The selected colonies were separately maintained in 1640
medium supplemented with 10% fetal calf serum, glutamine (2 mM),
penicillin (100 U/ml), streptomycin (100 U/ml) and 100 μg/ml
G418. After stable expression was achieved, the cells were observed
under fluorescence microscopy. Parent cells (LLC) were used as the
control group.
In vitro cell growth assay
To compare the cell growth in vitro, cells
were seeded into a 96-well plate at a density of 1×104
cells/ml (200 μl per well), and the number of cells of each
cell line was counted directly every day. All experiments were
repeated three times.
Flow cytometric analysis of the cell
cycle
Cultured cells were harvested after 48 h and washed
with phosphate-buffered saline (PBS). The cell cycle status was
then analyzed by flow cytometry as described previously. Briefly,
1×106 cells were washed twice with PBS, re-suspended in
a buffer (500 μl; containing 0.5% Triton X-100, PBS, 0.05%
RNaseA) and incubated for 30 min. Finally, 400 μl of
propidium iodide solution (50 mg/ml) and 400 μl Annexin
V-FITC were added. Cells were then left on ice for 30 min.
Fluorescence emitted from propidium iodide-DNA complexes was
quantified after laser excitation of the fluorescent dye by
fluorescence-activated cell sorting flow cytometry (Becton
Dickinson, Mountain View, CA, USA). The cell cycle distribution and
the percentage of apoptotic cells were determined by measuring the
DNA content of the cells.
Western blotting
To prepare the whole cell extracts for western
blotting, cells were harvested 48 hours after the culture and
washed three times with PBS, and lysed in radio-immunoprecipitation
lysis buffer [50 mM Tris-Cl (pH 7.4), 1% Nonidet P-40, 40 mM NaF,
10 mM NaCl, 10 mM Na3VO4, 1 mM
phenylmethylsulfonyl fluoride, 10 mM dithiothreitol, and 1
μg/ml each of leupeptin and aprotinin]. The cell lysates (50
μg of protein) were separated by SDS-PAGE followed by
transfer to polyvinylidene difluoride membranes. Size
approximations were made by comparing the stained bands with those
of the marker or ladder loaded during electrophoresis. After
blocking with 10% non-fat dry milk or bovine serum albumin in
Tris-buffered saline containing 0.1% Tween-20, the membrane was
incubated with the aforementioned primary antibody, and then the
corresponding specific horseradish peroxidase-conjugated secondary
antibody was added. The blot was exposed to Hypofilm ECL (GE
healthcare, Buckinghamshire, UK), developed, and signal intensity
values of the bands were obtained by photodenstometric analysis
(Bio-Rad, Laboratories Ltd., Hemel Hempstead, UK).
Gelatin zymography
Cells were incubated at 37°C in a 5% CO2
atmosphere and cultured in 1640 medium without fetal calf serum.
The supernatant was collected the next day and centrifuged and
stored at −80°C for later use. Then, 10% running gels were prepared
with gelatin stock solution (10 mg/ml in H2O) added to
obtain a concentration of 0.1% (1 mg/ml). The samples (typically
10–25 μl) were applied and the gel was run with 1X
Tris-glycine SDS running buffer under the standard running
conditions (∼125 V constant voltage). The running was completed
when the bromophenol blue tracking dye reached the bottom of the
gel. After running, the gel was incubated in the zymogram
renaturing buffer and then in zymogram developing buffer. The gel
was equilibrated for 30 min at room temperature then put in fresh
developing buffer and incubated at 37°C for at least 4 h. The
optimal result could be determined empirically by varying the
sample load or incubation time. Gels were stained with Coomassie
Blue R-250 for 30 min and destained with washing solution (50%
methanol, 10% acetic acid). Areas with protease activity appeared
as clear bands against a dark blue background where the protease
had digested the substrate.
Statistical analysis
Data are presented as the means ± SD. Parametric
testing between two groups was performed by Student’s t-test, and
comparison among three or more groups was carried out using one-way
ANOVA. For all analyses, P-values <0.05 were regarded as
indicative of statistical significance. The statistical analysis
was performed using SPSS13.0 for Microsoft Windows (SPSS Inc,
Chicago, IL, USA).
Results
Establishment and characterization of the
VEGF-transfected cells
To evaluate the biological effect of VEGF on LLC
cells, we generated the VEGF gene expression vector. After G418
stable selection, the fluorescence was observed under fluorescence
microscopy. Green fluorescence in the LLC-VEGF and LLC-RNAi groups
was mainly observed in the cytoplasm, whereas the green
fluorescence was scattered in cells in the LLC-GFP group (data not
shown). Subsequently, we confirmed the VEGF production by western
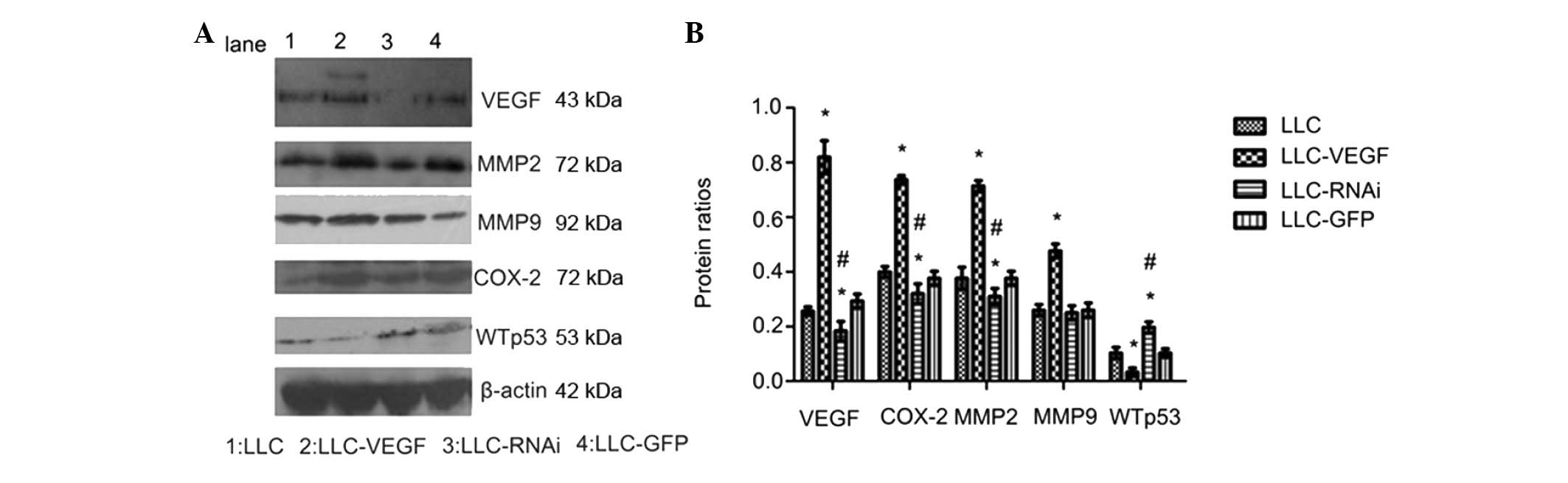
blotting (Fig. 1A). The expression
level of VEGF in the LLC-VEGF group was significant higher
(2.19-fold) than that in the control; the VEGF expression was
decreased in the LLC-RNAi group, indicating that the VEGF
expression was successfully inhibited. This indicated that the
cells were transfected successfully.
Effects of VEGF expression on the protein
levels of COX-2, MMPs and WTp53
To further investigate the role of VEGF in the
production of MMP2, MMP9 and COX-2, Western blot analysis was used
to assess the protein levels. The expression of MMP2, MMP9 and
COX-2 was increased 1.89-, 1.83- and 1.84-fold, respectively, in
the LLC-VEGF group compared to levels in the control (Fig. 1B), and the expression of MMP2, MMP9
and COX-2 was downregulated 0.82-, 0.96- and 0.8-fold,
respectively, in the LLC-RNAi group. Conversely, GFP alone did not
influence the protein expression compared with the control. These
results demonstrated that the VEGF expression correlated with MMP
and COX-2 expression. We also found that VEGF overexpression
decreased the expression of wild-type P53, which indicates that
VEGF may promote tumor progression via decrease in WTp53
expression.
VEGF expression correlates with COX-2,
MMP2, MMP9 and WTp53 expression
To estimate the correlation between VEGF expression
and COX-2, MMP2, MMP9 and WTp53 expression, the correlation
coefficient was used to confirm the relationship. There was a
significant correlation of VEGF and COX-2, MMP2, MMP9 and WTp53
expression. COX-2, MMP2 and MMP9 were positively correlated with
VEGF expression (r=0.984, 0.978 and 0.969, respectively,
p<0.01), and WTp53 was negatively correlated with VEGF
expression. (r=−0.809, p<0.01)
Effects of overexpression or
underexpression on the growth of Lewis lung cancer cells
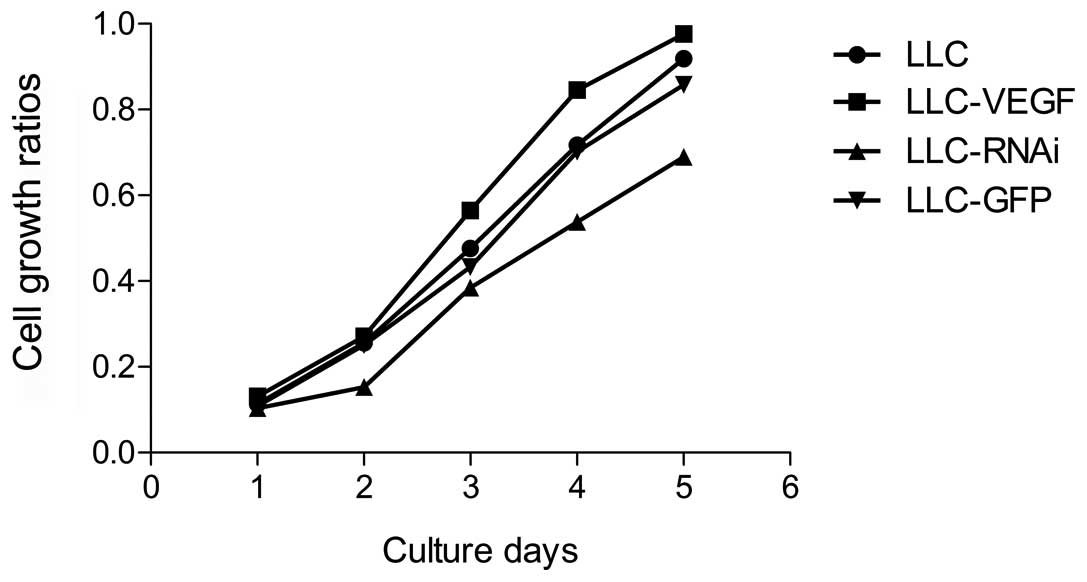
In order to estimate the effect of VEGF on LLC cell
growth, the cell numbers were counted directly each day. The
results revealed that there were no changes in the VEGF
overexpression group when compared with the control or GFP
transfection group, but the growth rate was significantly
suppressed on the 4th and 5th day in the LLC-RNAi group as compared
with the control (P<0.05) (Fig.
2).
VEGF promotes LLC cell entry into S
phase
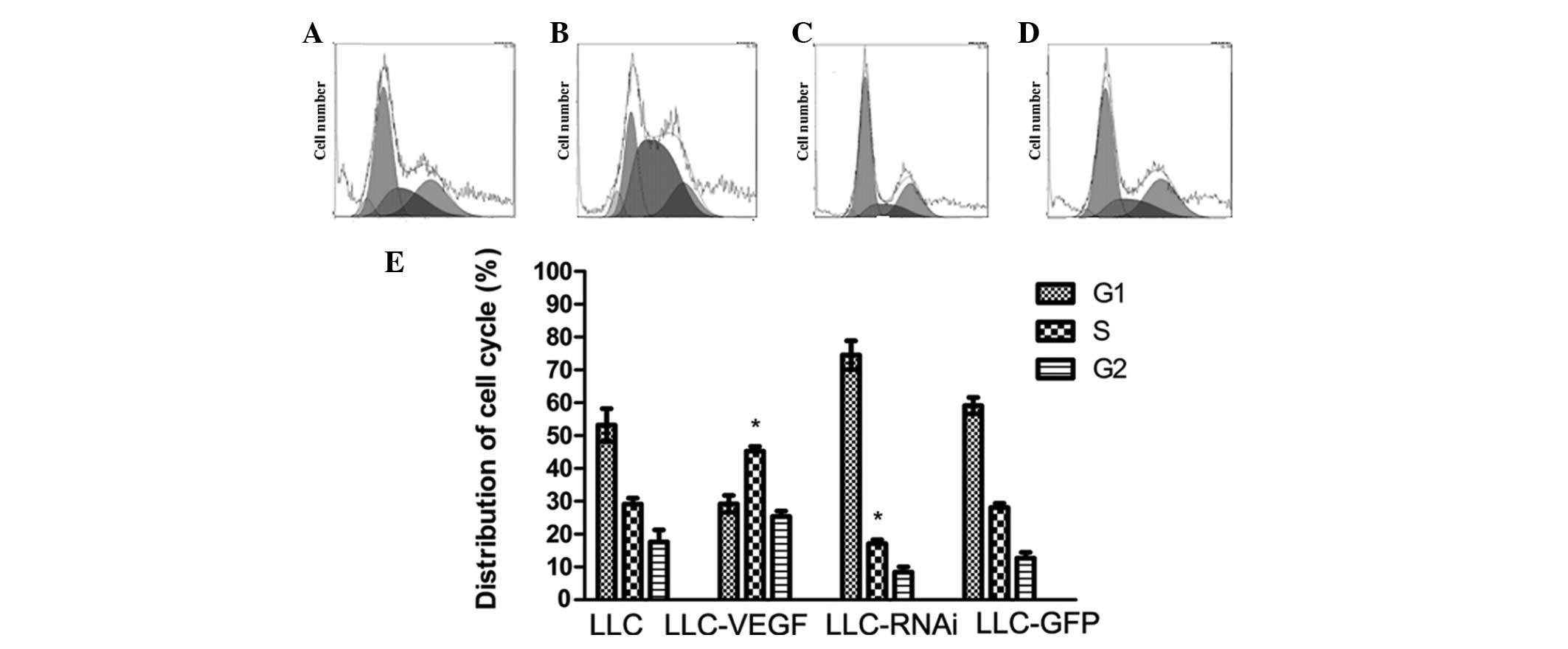
To determine the effect of VEGF on the cell cycle of
LLC cells, flow cytometry was performed to determine the cell cycle
status. The results showed that there were more cells in the S
stage in the LLC-VEGF group (45.3%) compared to the number in the
control (29.1%). By contrast, the cell cycle in the LLC-RNAi group
was blocked and approximately 74.5% of the cells remained in the
G0/G1 stage, and DNA content under the G0/G1 peak was higher,
indicating that more cells were undergoing apoptosis. Transfection
of GFP alone did not affect cell cycle status (Fig. 3).
Zymographic detection of MMP
activity
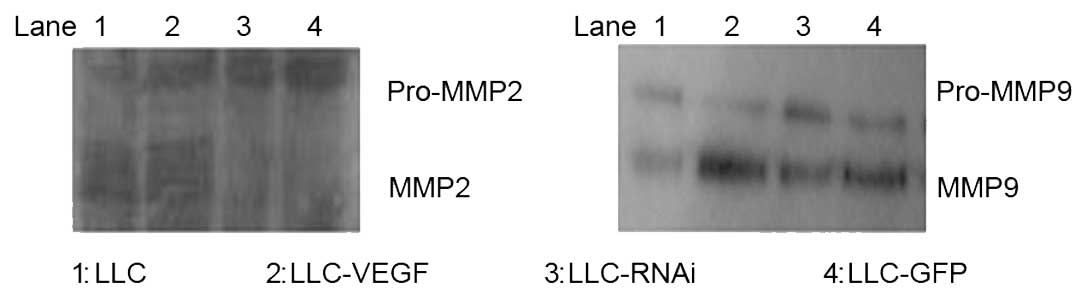
Zymography was performed to determine the effects of
VEGF on MMP2, MMP9 activities. The activities of MMP2 and MMP9 were
significantly higher in the LLC-VEGF group than the activities in
the other groups, while decreased activities were noted in the
LLC-RNAi group (Fig. 4).
Discussion
This study demonstrated that a certain level of VEGF
expression is essential for the growth of LLC cells, and that
downregulation of VEGF expression slows the growth of the cells and
causes more cells to undergo apoptosis instead of entering the
normal cell cycle. Moreover, COX-2, MMP2 and MMP9 was positively
correlated to VEGF expression, and WTp53 was negatively correlated
to VEGF expression. These findings support our hypothesis.
VEGF, a potent mitogenic and angiogenetic factor, is
capable of inducing endothelial cell proliferation, increasing
vascular permeability and modifying the status of the
extra-cellular matrix or altering gene expression (23–26).
Unlike the previous study on endothelial cells, we found that
overexpression of VEGF did not exert a marked growth-promoting
effect on the LLC cells, whereas downregulation of VEGF slowed the
cell growth. Tumor tissue is composed of cancer cells, vascular
endothelial cells and infiltrating inflammatory cells (mainly
lymphocytes). The discrepancies between our findings and their
results may be explained by the fact that they used tumor tissue,
whereas we employed cancer cells.
COX-2 is implicated in tumor cell proliferation,
resistance to apoptosis, angiogenesis and tumor invasiveness
(27). The COX-2-VEGF correlation
has been investigated by several groups. Several studies found that
VEGF and COX-2 are co-expressed in tumor tissues, and that COX-2
modulates VEGF expression (12–17).
An additional study demonstrated that VEGF stimulated the release
of COX-2 in endothelial cells by increasing COX-2 transcription and
prolonging the COX-2 mRNA half-time (28). However, few data are available on
the potential association between VEGF and COX-2 in Lewis lung
carcinoma. In the present study, we revealed that elevated VEGF
expression induces higher expression of COX-2, whereas
downregulation of VEGF leads to lower COX-2 expression. Although
the mechanism by which VEGF promotes COX-2 in NSCLC cells is
unknown, VEGF increases COX-2 expression in endothelial cells
through the P38 MAPK and JNK signal pathways or involves the PKC
and NOS pathways (28,29).
Gelatinases (MMP2 and MMP9), two important isoforms
in the MMP family, are considered to be closely correlated with
tumor invasion and metastasis. Some studies have indicated that
there is a close relationship between VEGF and MMP expression. It
was demonstrated that VEGF increased the release of MMP2 in brain
and ovarian tumor cells (19,30).
Moreover, Lee et al, using a murine model of asthma, found
that inhibition of VEGF receptor downregulated the expression of
MMP9 (20). In smooth muscle
cells, VEGF promotes MMP9 mRNA transcription and protein activities
(31). However, there is a small
amount of evidence demonstrating the association among VEGF and
MMP2 and MMP9 in Lewis lung carcinoma cells. Our results revealed
that VEGF overexpression may increase the production and activity
of MMP2 and MMP9. VEGF overexpression-elevated MMP2 and MMP9
expression may explain, in part, the mechanism by which VEGF
promotes the invasion of Lewis lung cancer cells.
WTp53 is one of the most intensively studied
anti-oncogenes. It acts as a nuclear transcription factor that
transactivates genes involved in apoptosis, cell cycle regulation
and other important processes (21). It is clear that the wild-type p53
can inhibit angiogenesis, whereas the mutant p53 promotes
neovascularization. An in vitro study demonstrated that
WTp53 downregulated endogenous VEGF mRNA levels and VEGF promoter
activity in a dose-dependent manner, whereas mutant forms of p53
did not (22). To date, the
association between WTp53 and VEGF remains to be elucidated. The
majority of previous studies found that WTp53 expression was
negatively associated with VEGF (32), although some studies have yielded
discrepant findings (33,34). By examining the tumor samples
surgically obtained from 116 esophageal adenocarcinoma patients,
Cavazzola et al (34) found
no correlation between WTp53 protein and VEGF expression. However,
it is unclear as to whether VEGF exerts any effect on WTp53
expression. To confirm the definite relation between WTP53 and
VEGF, we examined WTp53 protein expression in LLC-VEGF cells. Our
results revealed that WTp53 protein levels were negatively
correlated with VEGF expression.
In our study, we hypothesized that VEGF did not only
promote angiogenesis and metastasis and suppress cell apoptosis
independently, but also that it was involved in the production of
COX-2 and MMPs and inhibition of WTP53 expression. COX-2, MMP2 and
MMP9 were positively correlated with VEGF expression, and WTp53 was
negatively correlated with VEGF expression.
In conclusion, our results indicate that
overexpression of VEGF enhances the growth of Lewis lung cancer
cells, stimulates the production of COX-2, MMP2, MMP9 and enhances
the functional activities of MMP2 and MMP9 in vitro. VEGF
expression, however, suppresses the WTp53. These findings may, at
least in part, elucidate the manner by which VEGF is implicated in
angiogenesis, invasion and metastasis in lung cancer, and provides
experimental basis for anti-angiogenic therapy for cancer.
Nevertheless, the exact mechanism of the effect of VEGF on COX-2,
MMP2, MMP 9 and WTp53 requires further investigation.
Acknowledgements
This work was supported by a grant
from the Scientific Research Foundation of Hubei Health Department
(2005JX2B18), National Natural Science Foundation of China
(81072400) and the Scientific Research Foundation of Hubei Health
Department (2005JX2B18). We thank Dr Du Yimei for providing
linguistic advice.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Brundage MD, Davies D and Mackillop WJ:
Prognostic factors in non-small cell lung cancer: a decade of
progress. Chest. 122:1037–1057. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Folkman J: Clinical applications of
research on angiogenesis. N Engl J Med. 333:1757–1763. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saphir A: Angiogenesis the unifying
concept in cancer. J Natl Cancer Inst. 89:1658–1659.
1995.PubMed/NCBI
|
|
5
|
Chen HJ, Treweeke AT, Ke YQ, West DC and
Toh CH: Angiogenically active vascular endothelial growth factor is
over-expressed in malignant human and rat prostate carcinoma cells.
Br J Cancer. 82:1694–1701. 2000.PubMed/NCBI
|
|
6
|
George ML, Tutton MG, Janssen F, Arnaout
A, Abulafi AM, Eccles SA and Swift RI: VEGF-A, VEGF-C, and VEGF-D
in colorectal cancer progression. Neoplasia. 3:420–427. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishida N, Yano H, Komai K, Nishida T,
Kamura T and Kojiro M: Vascular endothelial growth factor C and
vascular endothelial growth factor receptor 2 are related closely
to the prognosis of patients with ovarian carcinoma. Cancer.
101:1364–1374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohta Y, Endo Y, Tanaka M, Shimizu J, Oda
M, Hayashi Y, Watanabe Y and Sasaki T: Significance of vascular
endothelial growth factor messenger RNA expression in primary lung
cancer. Clin Cancer Res. 2:1411–1416. 1996.PubMed/NCBI
|
|
9
|
Jin Y, Xiong X, Su Y, Hu J and Tao X:
Serum vascular endothelial growth factor levels in patients with
non-small cell lung cancer and its relations to the micrometastasis
in peripheral blood. J Huazhong Univ Sci Technolog Med Sci.
29:462–465. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Niki T, Iba S, Tokunou M, Yamada T,
Matsuno Y and Hirohashi S: Expression of vascular endothelial
growth factors A, B, C, and D and their relationships to lymph node
status in lung adenocarcinoma. Clin Cancer Res. 6:2431–2439.
2000.PubMed/NCBI
|
|
11
|
Smith WL and Langenbach R: Why there are
two cyclooxygenase isozymes. J Clin Invest. 107:1491–1495. 2011.
View Article : Google Scholar
|
|
12
|
Lim SC, Park SY and Do NY: Correlation of
cyclooxygenase-2 pathway and VEGF expression in head and neck
squamous cell carcinoma. Oncol Rep. 10:1073–1079. 2003.PubMed/NCBI
|
|
13
|
Von Rahden BH, Stein HJ, Pühringer F, Koch
I, Langer R, Piontek G, Siewert JR, Höfler H and Sarbia M:
Coexpression of cyclooxygenases (COX-1, COX-2) and vascular
endothelial growth factors (VEGF-A, VEGF-C) in esophageal
adenocarcinoma. Cancer Res. 65:5038–5044. 2005.PubMed/NCBI
|
|
14
|
Cianchi F, Cortesini C, Bechi P, Fantappiè
O, Messerini L, Vannacci A, Sardi I, Baroni G, Boddi V, Mazzanti R
and Masini E: Up-regulation of cyclooxygenase-2 gene expression
correlates with tumour angiogenesis in human colorectal cancer.
Gastroenterology. 121:1339–1347. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang SP, Wu MS, Shun CT, Wang HP, Hsieh
CY, Kuo ML and Lin JT: Cyclooxygenase-2 increases hypoxia inducible
factor-1 and vascular endothelial growth factor to promote
angiogenesis in gastric carcinoma. J Biomed Sci. 12:229–241. 2005.
View Article : Google Scholar
|
|
16
|
Dirim A, Haberal AN, Goren MR, Tekin MI,
Peskircioglu L, Demirhan B and Ozkardes H: VEGF, COX-2, and PCNA
expression in renal cell carcinoma subtypes and their prognostic
value. Int Urol Nephrol. 40:861–868. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim HS, Youm HR, Lee JS, Min KW, Chung JH
and Park CS: Correlation between cyclooxygenase-2 and tumor
angiogenesis in non-small cell lung cancer. Lung Cancer.
42:163–170. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rundhaug JE: Matrix metalloproteinases and
angiogenesis. J Cell Mol Med. 9:267–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rooprai HK, Rucklidge GJ, Panou C and
Pilkington GJ: The effects of exogenous growth factors on matrix
metalloproteinase secretion by human brain tumor cells. Br J
Cancer. 82:52–55. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee KS, Min KH, Kim SR, Park SJ, Park HS,
Jin GY and Lee YC: Vascular endothelial growth factor modulates
matrix metalloproteinase-9 expression in asthma. Am J Respir Crit
Care Med. 174:161–170. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zilfou JT and Lowe SW: Tumor suppressive
functions of p53. Cold Spring Harb Perspect Biol1. a001883
View Article : Google Scholar : 2009.PubMed/NCBI
|
|
22
|
Mukhopadhyay D, Tsiokas L and Sukhatme VP:
Wild-type p53 and v-Src exert opposing influences on human vascular
endothelial growth factor gene expression. Cancer Res.
55:6161–6165. 1995.PubMed/NCBI
|
|
23
|
Birk DM, Barbato J, Mureebe L and Chaer
RA: Current insights on the biology and clinical aspects of VEGF
regulation. Vasc Endovascular Surg. 42:517–530. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dvorak HF, Brown LF, Detmar M and Dvorak
AM: Vascular permeability factor/vascular endothelial growth
factor, micro-vascular hyperpermeability, and angiogenesis. Am J
Pathol. 146:1029–1039. 1995.PubMed/NCBI
|
|
25
|
Fujio Y and Walsh K: Akt mediates
cytoprotection of endothelial cells by vascular endothelial growth
factor in an anchorage-dependent manner. J Biol Chem.
274:16349–16354. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Claffey KP, Brown LF, del Aguila LF,
Tognazzi K, Yeo KT, Manseau EJ and Dvorak HF: Expression of
vascular permeability factor/vascular endothelial growth factor by
melanoma cells increases tumor growth, angiogenesis, and
experimental metastasis. Cancer Res. 56:172–181. 1996.
|
|
27
|
Miyata Y, Koga S, Kanda S, Nishikido M,
Hayashi T and Kanetake H: Expression of cyclooxygenase-2 in renal
cell carcinoma: correlation with tumor cell proliferation,
apoptosis, angiogenesis, expression of matrix metalloproteinase-2,
and survival. Clin Cancer Res. 9:1741–1749. 2003.
|
|
28
|
Wu G, Mannam AP, Wu J, Kirbis S, Shie JL,
Chen C, Laham RJ, Sellke FW and Li J: Hypoxia induces
myocyte-dependent COX-2 regulation in endothelial cells: role of
VEGF. Am J Physiol Heart Circ Physiol. 285:2420–2429. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu G, Luo J, Rana JS, Laham R, Sellke FW
and Li J: Involvement of COX-2 in VEGF-induced angiogenesis via P38
and JNK pathways in vascular endothelial cells. Cardiovasc Res.
69:512–519. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang A, Meng L, Wang Q, Xi L, Chen G,
Wang S, Zhou J, Lu Y and Ma D: Enhanced in vitro invasiveness of
ovarian cancer cells through up-regulation of VEGF and induction of
MMP-2. Oncol Rep. 15:831–836. 2006.PubMed/NCBI
|
|
31
|
Yao JS, Chen Y, Zhai W, Xu K, Young WL and
Yang GY: Minocycline exerts multiple inhibitory effects on vascular
endothelial growth factor-induced smooth muscle cell migration.
Circ Res. 95:364–371. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Giatromanolaki A, Koukourakis MI,
Kakolyris S, Turley H, O’Byrne K, Scott PA, Pezzella F, Georgoulias
V, Harris AL and Gatter KC: Vascular endothelial growth factor,
wild-type p53, and angiogenesis in early operable non-small cell
lung cancer. Clin Cancer Res. 4:3017–3024. 1998.PubMed/NCBI
|
|
33
|
Ambs S, Bennett WP, Merriam WG, Ogunfusika
MO, Oser SM, Khan MA, Jones RT and Harris CC: Vascular endothelial
growth factor and nitric oxide synthase expression in human lung
cancer and the relation to p53. Br J Cancer. 78:233–239. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cavazzola LT, Rosa AR, Schirmer CC, Gurski
RR, Telles JP, Mielke F, Meurer L, Edelweiss MI and Kruel CD:
Immunohistochemical evaluation for P53 and VEGF (Vascular
Endothelial Growth Factor) is not prognostic for long term survival
in end stage esophageal adenocarcinoma. Rev Col Bras Cir. 36:24–34.
2009. View Article : Google Scholar
|