Introduction
Malignant gliomas are the most common primary
malignant tumors of the central nervous system. Despite the
improvement in conventional treatment such as surgery, radiotherapy
and chemotherapy, the prognosis for patients with high-grade
gliomas is still very poor (1). In
recent years, more and more researchers have shown interest in
targeted gene therapies against gliomas (2,3) and
there is a great need to develop appropriate carriers for these
treatment programs to deliver therapeutic agents efficiently into
glioma tissues. As a type of adult multipotent stem cell, bone
marrow-derived mesenchymal stem cells (BMSCs) are characterized by
their easy isolation and high ex vivo expansion potential
(4,5). Previous studies have proven that
BMSCs can migrate towards gliomas after intracerebral or systemic
injection, which has made these cells an attractive delivery
vehicle in targeted gene therapy of gliomas (2,3).
Elucidating the molecular signaling pathway of this migratory
behavior will help us to further improve the efficiency of targeted
gene therapy mediated by BMSCs.
Vascular endothelial growth factor (VEGF) is one of
the most important angiogenic cytokines expressed in glioma tissues
and is involved in the progression of malignant brain tumors
(6,7). Previous studies have proven the
correlation of VEGF expression and glioma grade (8). In addition, the chemotactic effects
of VEGF on various cell types have been widely reported (9,10).
As a cell surface glycoprotein, vascular cell adhesion molecule-1
(VCAM-1) mediates the adhesion and migration of leukocytes and T
lymphocytes through brain microvascular endothelial cells,
according to binding to its receptors, the α4β1 and α4β7 integrins
(11,12). In a previous study, we proved that
VCAM-1 plays an important role in the migration of BMSCs induced by
C6 and U87 glioma cells (13).
Analyzing the role of VEGF in regulating the glioma-induced
migration and VCAM-1 expression of BMSCs may help us understand the
mechanism of BMSCs migrating towards gliomas after intravascular
delivery or intracerebral transplantation. Furthermore, previous
data have demonstrated that phosphoinositide-3-kinase (PI3K) plays
a crucial role in the signaling pathways linking extracellular
signals to crucial cellular processes and contributes to growth
factor stimulating signal transduction from cell membrane to
cytoplasm. It has also been confirmed that PI3K participates in the
intracellular signal transduction of VEGF-induced cell migration
(14,15).
Therefore, in this study, we attempted to evaluate
the role of VEGF in the C6 glioma-induced migration of BMSCs,
whether VEGF upregulates the VCAM-1 expression of BMSCs, the
relation between VCAM-1 and the VEGF-induced migration of BMSCs and
whether PI3K is involved in the signal transduction of VEGF-induced
migration and VCAM-1 upregulation of BMSCs.
Materials and methods
Isolation and culture of BMSCs
Four-week-old male Wistar rats (80–100 g) were used
in our study. The rats were purchased from the Laboratory Animal
Center of China Medical University. All experiments using animals
were approved by the Animal Care and Use Committee of China Medical
University and in accordance with the National Institute of Health
Guide for the Care and Use of Laboratory Animals. BMSCs were
prepared as previously described with some modifications, according
to their adherence to plastic (16,17).
In brief, the rats were sacrificed by cervical dislocation after
they were anesthetized with 10% chloral hydrate (0.3 ml/100 g) by
intraperitoneal injection. Bilateral tibias and femurs were
dissected aseptically, and bone marrow was collected and suspended
in low glucose Dulbecco’s modified Eagle’s medium (L-DMEM; Gibco,
Invitrogen Corp., Grand Island, NY, USA) containing 10% fetal
bovine serum (FBS; Gibco). The cell suspension was then transferred
into 25-cm2 culture flasks. After 48 h of cultivation,
the culture medium was replaced to remove the non-adherent hemato
poietic lineage cells, and adherent cells were further cultured and
expanded. BMSCs at passage 3 were used for the studies.
Culture of C6 glioma cells
Rat C6 glioma cells (American Type Culture
Collection, Rockville, MD) were maintained in L-DMEM supplemented
with 10% FBS.
In vitro migration assay
In our experiments, 24-well Transwell inserts with
an 8-μm pore size (Corning Costar) were used to evaluate the
migration of BMSCs under different conditions. C6 glioma cells and
BMSCs were trypsinized and resuspended in serum-free L-DMEM at
5x105/ml and 1x106/ml, respectively. To
explore the migration of BMSCs toward C6 glioma cells, 1 ml of C6
glioma cell suspension was added into the lower chambers and 6 h
later, a 200-μl BMSC suspension was added into the upper
chambers. Moreover, to ascertain whether VEGF promotes the
migration of BMSCs towards C6 glioma, we examined the migration of
BMSCs in response to a suspension of C6 glioma cells supplemented
with or without a VEGF neutralizing antibody (1 μg/ml,
Abcam, Cambridge, UK), which were placed in the lower chambers,
respectively. In addition, we applied VEGF164, one major
isoform of rat VEGF, to test the chemotactic effect of VEGF on
BMSCs. Serum-free L-DMEM with recombinant rat VEGF164
(R&D Systems, Minneapolis, MN, USA) at concentrations of 20
ng/ml was added into the lower wells. VCAM-1 blocking antibody
(Covance, Princeton, NJ, USA) was added to the suspension of BMSCs
at 10 μg/ml to evaluate the role of VCAM-1 in the
VEGF164-induced migration of BMSCs. Furthermore, we used
the PI3K selective inhibitor, LY294002, to assess the role of PI3K
in the VEGF-induced migration of BMSCs. BMSCs were treated with
LY294002 (20 µM; Cell Signaling Technology, Inc., Danvers, MA, USA)
for 30 min prior to, and for the duration of, the stimulation of 20
ng/ml VEGF164. The contents of the upper and lower wells
were separated by a polycarbonate membrane (8-μm pore size).
Cell migration was allowed for 36 h at 37°C. Following incubation,
the media were aspirated, and the cells remaining on the upper
surface of the polycarbonate membrane were removed with a cotton
swab. The cells migrating to the lower surface were stained with
Giemsa stain for 15 min. Cell counting was performed under an
inverted microscope by two researchers separately. The average
numbers of migrated cells were determined by counting the cells in
6 random high-power fields (x400). Serum-free L-DMEM alone served
as negative controls.
RT-PCR
To investigate the change in VEGF-induced VCAM-1
expression of BMSCs and the relevance of PI3K to this process, the
cells were incubated with L-DMEM containing 10% FBS in the presence
of VEGF164 (20 ng/ml) with or without LY294002 (20
μmol/l) for 12 h. The expression of VCAM-1 mRNA was
evaluated by reverse transcriptase-polymerase chain reaction
analysis (RT-PCR). The cells incubated with L-DMEM containing 10%
FBS served as a negative control. Total RNA was extracted from the
cultured cells using TRIzol reagent (Invitrogen) and cDNA was
generated from 1 μg of total RNA from each sample. The
primers used were as follows: VCAM-1, 5′-ACACCTCCCCCAAGAATACAG-3′
(forward) and 5′-GCT CATCCTCAACACCCACAG-3′ (reverse) (18); β-actin, 5′-TCA
GGTCATCACTATCGGCAAT-3′ (forward) and 5′-AAAGA AAGGGTGTAAAACGCA-3′
(reverse). PCR conditions for both were as follows: 32 cycles of
denaturation at 94°C for 30 sec, annealing at 55°C for 30 sec and
extension at 72°C for 2 min. The PCR products were subjected to
electrophoresis on 1.5% agarose gels. All of the cDNA bands were
scanned using Chemi Imager 5500 V2.03 software, and the integrated
density values (IDV) were calculated by computerized image analysis
system (Fluor Chen 2.0) and normalized with that of β-actin.
Immunofluorescence assays
For immunofluorescence assays, BMSCs were cultured
on glass coverslips coated with 0.1% gelatin. After incubation was
performed as mentioned above, the cells were fixed with acetone and
immunostained with mouse monoclonal anti-VCAM-1 antibody (diluted
1:100; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at 4°C
overnight. Subsequent visualization was performed with anti-mouse
IgG conjugated with rhodamine (TRITC) (diluted 1:200; Santa Cruz
Biotechnology, Inc.) for 1 h at 37°C in darkness. Coverslips were
mounted in mounting media, and images were captured with an Olympus
BX60 upright fluorescence microscope with appropriate filters and
objectives, using identical acquisition parameters per experiment.
L-DMEM containing 10% FBS served as a control.
Statistical analysis
All results are described as mean ± SD for each
group. A Student’s t-test was used to assess a significant
difference between two groups. One-way analysis of variance (ANOVA)
was performed to determine significant differences between multiple
groups. P<0.05 was considered to indicate a statistically
significant result.
Results
BMSCs exhibit the capacity to migrate
towards C6 glioma in vitro
We used an in vitro migration asssay to
evaluate the migratory ability of BMSCs towards C6 glioma cells.
Consistent with previous studies, in our experiment, we found that
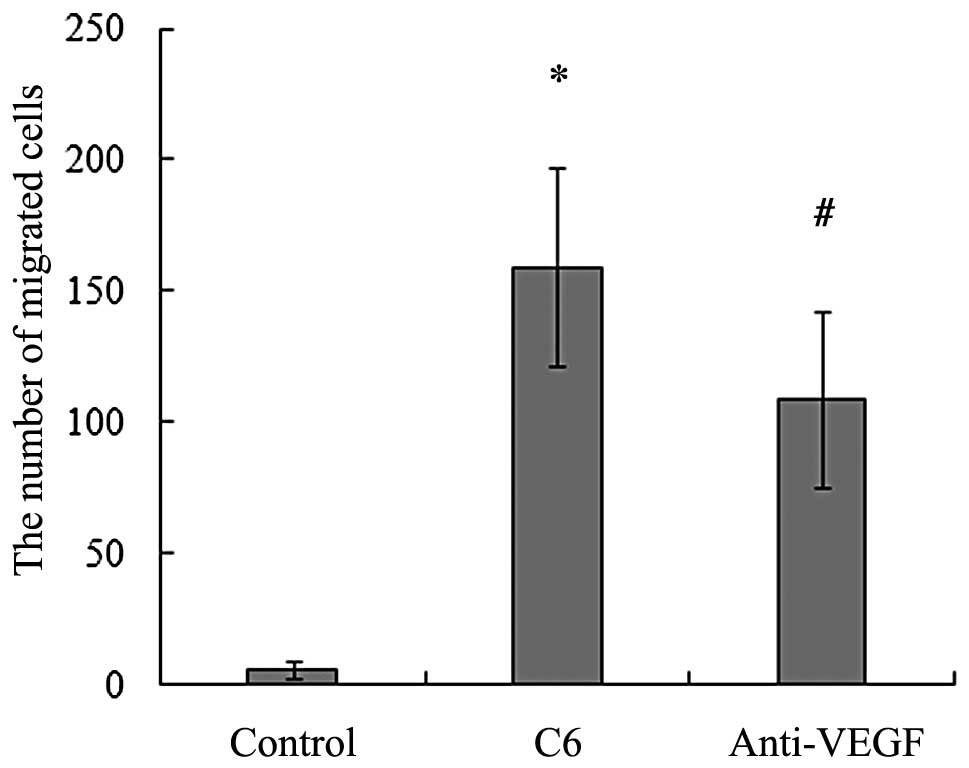
BMSCs migrated directionally towards glioma (2). As shown in Fig. 1, the average number of migrating
cells towards C6 glioma cells was significantly higher than that in
the control.
Neutralization of VEGF decreases the
number of BMSCs migrating towards C6 glioma
To determine whether VEGF has a role in the BMSC
migration toward gliomas, a VEGF neutralizing antibody was added
into the lower chamber together with the C6 glioma cells. We found
that the neutralization of VEGF significantly reduced the
migration-enhancing effect of C6 glioma cells and the number of
migrating cells decreased compared with the control (Fig. 1). These results suggest that VEGF
participates in mediating the migration of BMSCs toward C6 glioma
in vitro.
Recombinant rat VEGF164
promotes the migration of BMSCs
To evaluate the chemotactic effect of VEGF on rat
BMSCs, we analyzed the effect of recombinant rat VEGF164
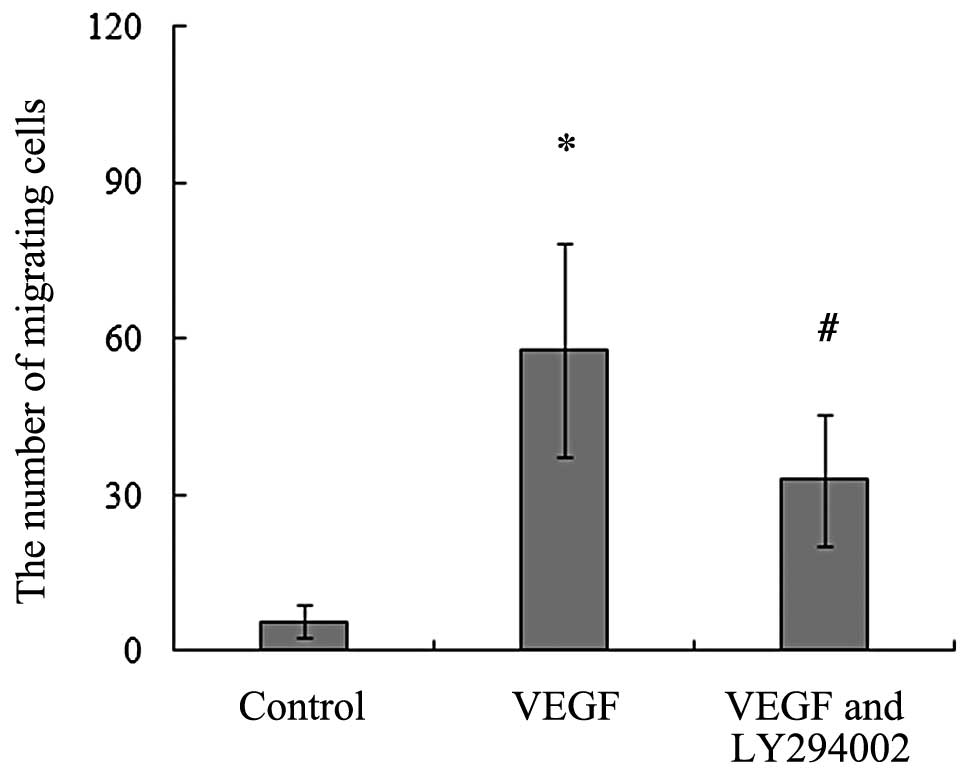
on the migration of BMSCs. Our data showed that the addition of
recombinant rat VEGF164 caused chemotaxis activity of
BMSCs and induced them to migrate towards the lower chambers. When
compared to the control, VEGF164, at a concentration of
20 ng/ml, led to a significant increase in the number of migrating
BMSCs (Fig. 2).
Blocking of VCAM-1 decreases the
migration of BMSCs induced by VEGF164
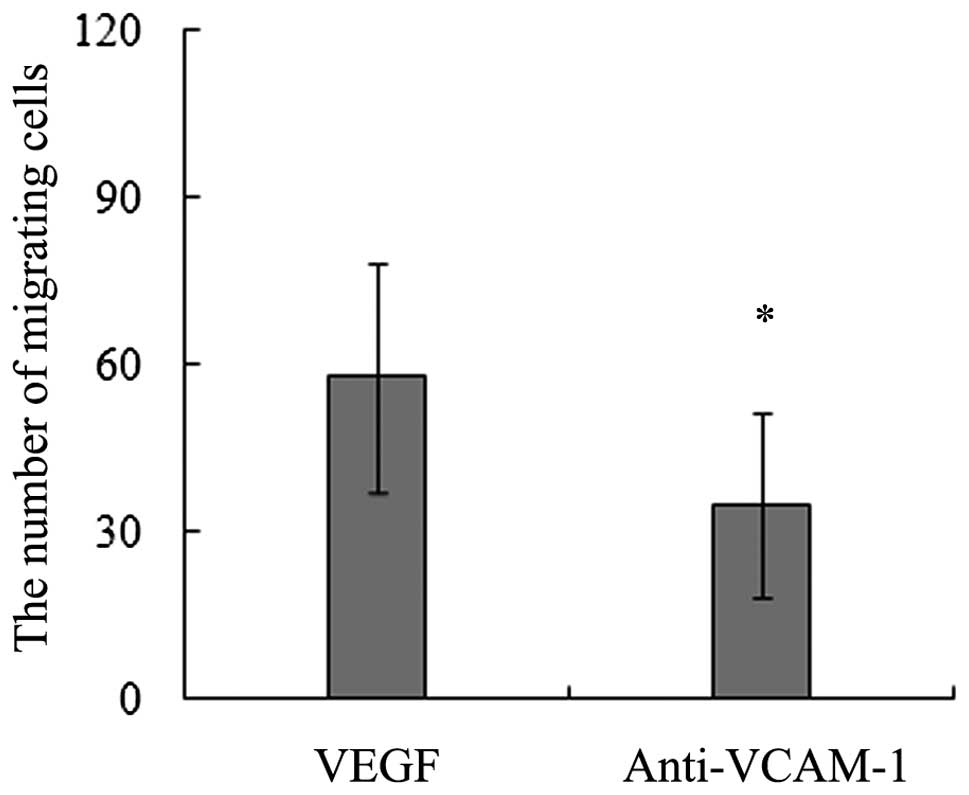
Our data of the in vitro migration assays
demonstrated that the addition of a VCAM-1 neutralizing antibody
significiantly decreased the number of migrating BMSCs towards
VEGF164 (Fig. 3). These
results imply that VCAM-1 is a key adhesion molecule mediating the
migration of BMSCs induced by VEGF164.
Recombinant rat VEGF164
upregulates the VCAM-1 expression of BMSCs
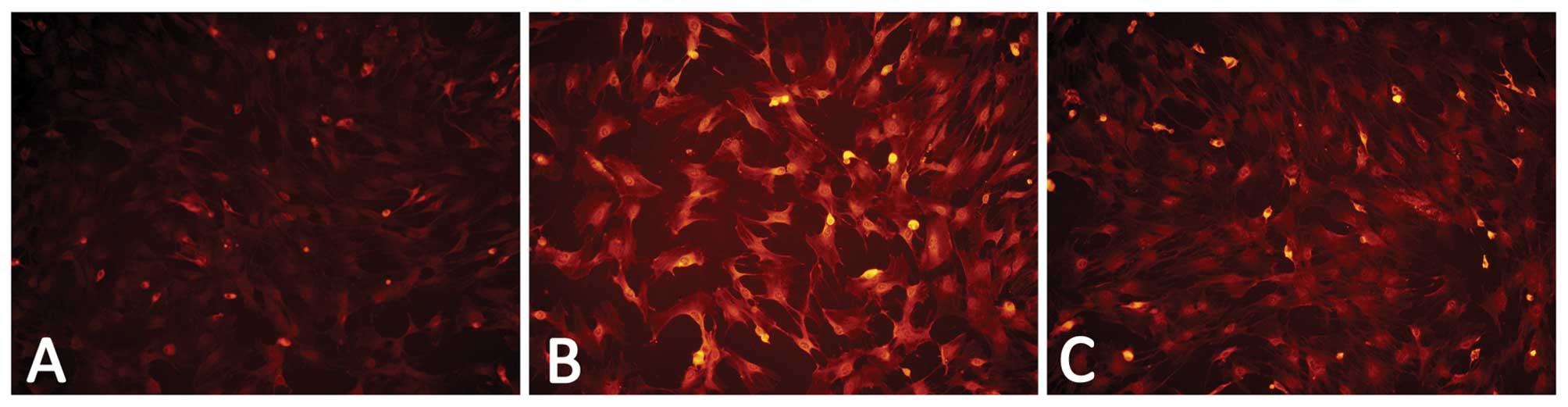
The results of RT-PCR and immunofluorescence assays
revealed that the BMSCs expressed a low level of VCAM-1 in the
culture without VEGF164, while the cells treated with 20
ng/ml VEGF164 for 12 h exhibited a higher VCAM-1
expression than the cells without the stimulation of
VEGF164 (Figs. 4 and
5).
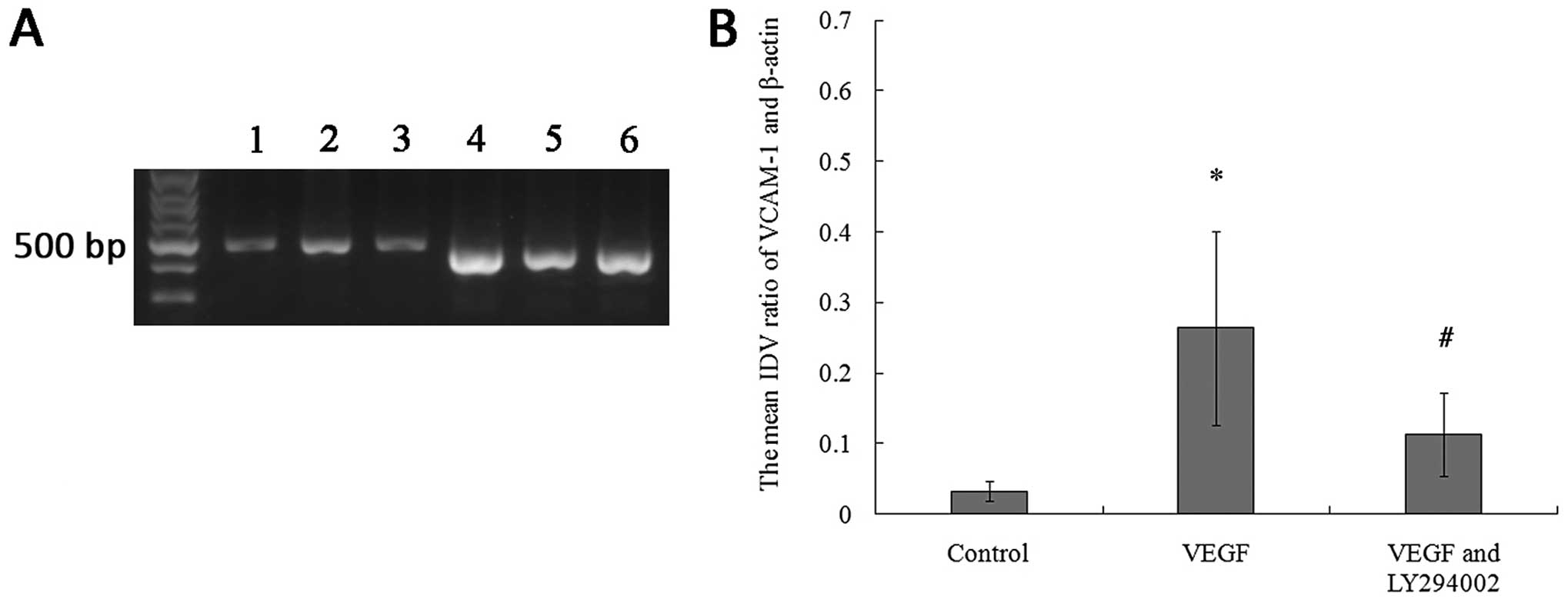 | Figure 4RT-PCR analysis reveals that VCAM-1
mRNA expression of the BMSCs is upregulated by the incubation with
20 ng/ml VEGF164, which was inhibited by LY294002. (A)
Representative results of the RT-PCR illustrating differences in
the 477-bp band of VCAM-1; (Lane 1, VCAM-1 in the control group;
Lane 2, VCAM-1 in the VEGF group; Lane 3, VCAM-1 in the VEGF and
LY294002 group; Lane 4, β-actin in the control group; Lane 5,
β-actin in the VEGF group; Lane 6, β-actin in the VEGF and LY294002
group). (B) Relative integrated density value (IDV) analysis of
VCAM-1 mRNA. VCAM-1 mRNA expression of BMSCs (n=8, each;
*P<0.05 vs. control group and #P<0.05
vs. VEGF group). Control group, the cells were incubated with
L-DMEM containing 10% FBS for 12 h; VEGF group, the cells were
incubated with L-DMEM containing 10% FBS in the presence of VEGF
(20 ng/ml) for 12 h; VEGF and LY294002 group, the cells were
incubated with L-DMEM containing 10% FBS in the presence of VEGF
(20 ng/ml) and LY294002 (20 μM) for 12 h. |
Inhibition of PI3K reduces the migratory
response and VCAM-1 expression of BMSCs to recombinant rat
VEGF164
The results of the in vitro migration assays
showed that the migration of BMSCs toward recombinant rat
VEGF164 was found to be partially blocked and the number
of migrating BMSCs significantly decreased with the addition of
LY294002. This indicates that VEGF164-mediated PI3K
activation may correlate with the migration of BMSCs induced by
VEGF164 (Fig. 2). We
also found that the upregulation of VCAM-1 mRNA and protein
expression decreased following treatment with LY294002, which
suggests that PI3K activation may play an active role in VCAM-1
upregulation of BMSCs stimulated by VEGF164 (Figs. 4 and 5).
Discussion
In the present study, we demonstrated that VEGF
participated in modulating C6 glioma-induced migration and in
promoting VCAM-1 expression of BMSCs, VCAM-1 plays an important
role in VEGF-induced migration of BMSCs and that PI3K was involved
in the signal transduction of this regulatory process.
It has been reported that BMSCs have the capacity of
migrating to gliomas (2,19). Consistent with these findings, our
in vitro migration results demonstrated that rat BMSCs
migrated directionally to C6 glioma cells. After being co-cultured
36 h with C6 glioma cells, the average number of migrating cells
was significantly higher than that in the control. This directional
migratory behavior may be due to the soluble factors released from
C6 glioma cells.
VEGF is one of the strongest and most specific
angiogenesis cytokines. VEGF is not only closely related to the
invasiveness of glioma, but is also proportional to the grade of
malignancy of glioma (20,21). The expression of VEGF mRNA and
protein has been reported to increase in C6 glioma (22). Moreover, it has been shown that
VEGF is expressed in glioma tissues and acts as an angiogenic
factor for tumor vessels (6,23).
Therefore, we added a VEGF neutralizing antibody into the lower
wells to block the effect of VEGF on the C6 glioma-induced
migration of BMSCs. We found that the number of migrating BMSCs
decreased obviously, which suggests the important role of VEGF in
this process. Our results also showed that the number of migrating
BMSCs was still higher than the control after blocking VEGF, which
indicates that there may be other soluble active factors released
from C6 glioma cells which participate in inducing the migration of
BMSCs. These results were further supported by analyzing the effect
of recombinant rat VEGF164, one major isoform of rat
VEGF, on the migration of BMSCs using an in vitro migration
assay. The data demonstrated that recombinant rat
VEGF164 at a concentration of 20 ng/ml in the lower
wells led to a significant increase in migrating BMSCs.
As a transmembrane glycoprotein, VCAM-1 is a member
of the immunoglobulin superfamily. VCAM-1 and its receptor
intergrin α4β1 are not only expressed on BMSCs, but are also
expressed on glioma microvascular endothelia (24,25).
Previous data showed that the interaction of VCAM-1 and integrin
α4β1 enhances the migration of human melanoma cells across
activated endothelial cell layers (26). In addition, VCAM-1 contributes to
neutrophil trafficking into the central nervous system (27). Our previous data also demonstrated
the important role of VCAM-1 in the migration towards gliomas
(13). Since VEGF164 is
one of the most abundant forms of VEGF found in C6 glioma cells and
rat brain, we investigated the effect of recombinant rat
VEGF164 on the VCAM-1 expression of BMSCs (28). The RT-PCR and immunofluorescence
assay revealed that the incubation with 20 ng/ml VEGF164
elevated the expression of VCAM-1 mRNA and protein in BMSCs. Our
data also showed that the neutralization of VCAM-1 decreased the
number of migrating BMSCs towards VEGF164, which
indicates that VCAM-1 plays an important role in the
VEGF164-induced migration of BMSCs. Collectively we
conclude that VEGF induces the migration of BMSCs by increasing the
VCAM-1 expression in BMSCs.
Initially defined as an important intracellular
signal transduction pathway, PI3K extensively takes part in a
series of intracellular physiological and pathological responses
induced by VEGF, including migration (29,30).
We utilized LY294002, a PI3K-specific inhibitor, to explore the
effect of PI3K on the VEGF-induced migration of BMSCs. The data
showed that after blocking PI3K, the migration of BMSCs induced by
VEGF164 was inhibited. We also found that the
upregulated expression of VCAM-1 mRNA and protein decreased
following the treatment with LY294002. These results provide
evidence that the PI3K signaling pathway is correlated with the
intracellular signal transduction of this directional migration.
Since LY294002 cannot completely block VEGF-induced migration of
BMSCs, there may be other signaling transduction pathways
participating in the regulation of the migratory capacity of BMSCs
and VCAM-1 upregulation.
In summary, our results demonstrate that VEGF plays
an important role in C6 glioma-induced migration, and recombinant
rat VEGF164 promotes the migration of BMSCs by elevating
the VCAM-1 expression of BMSCs, and that PI3K is one of the
important signaling molecules mediating the signal transduction of
VEGF164-induced migration and VCAM-1 expression of
BMSCs.
Acknowledgements
This study was supported by the
Natural Science Foundation of China (under contract nos. 30901781,
81171131, 81172197, 30973079 and 81072056) and the Doctoral
Start-Up Foundation of Liaoning Province (no. 20091107).
References
|
1
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Doucette T, Rao G, Yang Y, Gumin J,
Shinojima N, Bekele BN, Qiao W, Zhang W and Lang FF: Mesenchymal
stem cells display tumor-specific tropism in an RCAS/Ntv-a glioma
model. Neoplasia. 13:716–725. 2011.PubMed/NCBI
|
|
3
|
Lee DH, Ahn Y, Kim SU, Wang KC, Cho BK,
Phi JH, Park IH, Black PM, Carroll RS, Lee J and Kim SK: Targeting
rat brainstem glioma using human neural stem cells and human
mesenchymal stem cells. Clin Cancer Res. 15:4925–4934. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chamberlain G, Fox J, Ashton B and
Middleton J: Concise review: mesenchymal stem cells: their
phenotype, differentiation capacity, immunological features, and
potential for homing. Stem Cells. 25:2739–2749. 2007. View Article : Google Scholar
|
|
6
|
Keunen O, Johansson M, Oudin A, Sanzey M,
Rahim SA, Fack F, Thorsen F, Taxt T, Bartos M, Jirik R, et al:
Anti-VEGF treatment reduces blood supply and increases tumor cell
invasion in glioblastoma. Proc Natl Acad Sci USA. 108:3749–3754.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Knizetova P, Ehrmann J, Hlobilkova A,
Vancova I, Kalita O, Kolar Z and Bartek J: Autocrine regulation of
glioblastoma cell cycle progression, viability and radioresistance
through the VEGF-VEGFR2 (KDR) interplay. Cell Cycle. 7:2553–2561.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rahmah NN, Sakai K, Sano K and Hongo K:
Expression of RECK in endothelial cells of glioma: comparison with
CD34 and VEGF expressions. J Neurooncol. 107:559–564. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herzog B, Pellet-Many C, Britton G,
Hartzoulakis B and Zachary IC: VEGF binding to NRP1 is essential
for VEGF stimulation of endothelial cell migration, complex
formation between NRP1 and VEGFR2, and signaling via FAK Tyr407
phosphorylation. Mol Biol Cell. 22:2766–2776. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schmidt NO, Koeder D, Messing M, Mueller
FJ, Aboody KS, Kim SU, Black PM, Carroll RS, Westphal M and Lamszus
K: Vascular endothelial growth factor-stimulated cerebral
micro-vascular endothelial cells mediate the recruitment of neural
stem cells to the neurovascular niche. Brain Res. 1268:24–37. 2009.
View Article : Google Scholar
|
|
11
|
Yilmaz G and Granger DN: Leukocyte
recruitment and ischemic brain injury. Neuromolecular Med.
12:193–204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hyun YM, Chung HL, McGrath JL, Waugh RE
and Kim M: Activated integrin VLA-4 localizes to the lamellipodia
and mediates T cell migration on VCAM-1. J Immunol. 183:359–369.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu Y, Cheng P, Xue YX and Liu YH: Glioma
cells promote the expression of vascular cell adhesion molecule-1
on bone marrow-derived mesenchymal stem cells: a possible mechanism
for their tropism toward gliomas. J Mol Neurosci. 48:127–135. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Wei Y, Chen Y, Xu X and Zhang H:
Differentiation of neural stem cells influences their chemotactic
responses to vascular endothelial growth factor. J Neurosci Res.
89:1173–1184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bekhite MM, Finkensieper A, Binas S,
Müller J, Wetzker R, Figulla HR, Sauer H and Wartenberg M:
VEGF-mediated PI3K class IA and PKC signaling in cardiomyogenesis
and vasculo-genesis of mouse embryonic stem cells. J Cell Sci.
124:1819–1830. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mias C, Lairez O, Trouche E, Roncalli J,
Calise D, Seguelas MH, Ordener C, Piercecchi-Marti MD, Auge N,
Salvayre AN, et al: Mesenchymal stem cells promote matrix
metalloproteinase secretion by cardiac fibroblasts and reduce
cardiac ventricular fibrosis after myocardial infarction. Stem
Cells. 27:2734–2743. 2009. View
Article : Google Scholar
|
|
17
|
Xu F, Shi J, Yu B, Ni W, Wu X and Gu Z:
Chemokines mediate mesenchymal stem cell migration toward gliomas
in vitro. Oncol Rep. 23:1561–1567. 2010.PubMed/NCBI
|
|
18
|
Jiang B, Xu S, Hou X, Pimentel DR and
Cohen RA: Angiotensin II differentially regulates
interleukin-1-beta-inducible NO synthase (iNOS) and vascular cell
adhesion molecule-1 (VCAM-1) expression: role of p38 MAPK. J Biol
Chem. 279:20363–20368. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamizo A, Marini F, Amano T, Khan A,
Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, et
al: Human bone marrow-derived mesenchymal stem cells in the
treatment of gliomas. Cancer Res. 65:3307–3318. 2005.PubMed/NCBI
|
|
20
|
Hlobilkova A, Ehrmann J, Knizetova P,
Krejci V, Kalita O and Kolar Z: Analysis of VEGF, Flt-1, Flk-1,
nestin and MMP-9 in relation to astrocytoma pathogenesis and
progression. Neoplasma. 56:284–290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Korkolopoulou P, Patsouris E,
Konstantinidou AE, Pavlopoulos PM, Kavantzas N, Boviatsis E,
Thymara I, Perdiki M, Thomas-Tsagli E, Angelidakis D, et al:
Hypoxia-inducible factor 1alpha/vascular endothelial growth factor
axis in astrocytomas. Associations with microvessel morphometry,
proliferation and prognosis. Neuropathol Appl Neurobiol.
30:267–278. 2004. View Article : Google Scholar
|
|
22
|
Boveri M, Berezowski V, Price A, Slupek S,
Lenfant AM, Benaud C, Hartung T, Cecchelli R, Prieto P and Dehouck
MP: Induction of blood-brain barrier properties in cultured brain
capillary endothelial cells: comparison between primary glial cells
and C6 cell line. Glia. 51:187–198. 2005. View Article : Google Scholar
|
|
23
|
Miletic H, Niclou SP, Johansson M and
Bjerkvig R: Anti-VEGF therapies for malignant glioma: treatment
effects and escape mechanisms. Expert Opin Ther Targets.
13:455–468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Segers VF, Van Riet I, Andries LJ, Lemmens
K, Demolder MJ, De Becker AJ, Kockx MM and De Keulenaer GW:
Mesenchymal stem cell adhesion to cardiac microvascular
endothelium: activators and mechanisms. Am J Physiol Heart Circ
Physiol. 290:H1370–H1377. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rosenman SJ, Shrikant P, Dubb L,
Benveniste EN and Ransohoff RM: Cytokine-induced expression of
vascular cell adhesion molecule-1 (VCAM-1) by astrocytes and
astrocytoma cell lines. J Immunol. 154:1888–1899. 1995.PubMed/NCBI
|
|
26
|
Klemke M, Weschenfelder T, Konstandin MH
and Samstag Y: High affinity interaction of integrin alpha4beta1
(VLA-4) and vascular cell adhesion molecule 1 (VCAM-1) enhances
migration of human melanoma cells across activated endothelial cell
layers. J Cell Physiol. 212:368–374. 2007. View Article : Google Scholar
|
|
27
|
Wewer C, Seibt A, Wolburg H, Greune L,
Schmidt MA, Berger J, Galla HJ, Quitsch U, Schwerk C, Schroten H
and Tenenbaum T: Transcellular migration of neutrophil granulocytes
through the blood-cerebrospinal fluid barrier after infection with
Streptococcus suis. J Neuroinflammation. 8:512011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bacic M, Edwards NA and Merrill MJ:
Differential expression of vascular endothelial growth factor
(vascular permeability factor) forms in rat tissues. Growth
Factors. 12:11–15. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang F, Yamauchi M, Muramatsu M, Osawa T,
Tsuchida R and Shibuya M: RACK1 regulates VEGF/Flt1-mediated cell
migration via activation of a PI3K/Akt pathway. J Biol Chem.
286:9097–9106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Graupera M, Guillermet-Guibert J, Foukas
LC, Phng LK, Cain RJ, Salpekar A, Pearce W, Meek S, Millan J,
Cutillas PR, Smith AJ, Ridley AJ, Ruhrberg C, Gerhardt H and
Vanhaesebroeck B: Angiogenesis selectively requires the p110alpha
isoform of PI3K to control endothelial cell migration. Nature.
453:662–666. 2008. View Article : Google Scholar : PubMed/NCBI
|