Introduction
Stroke is one of the most serious events that
affects the elderly population. Stroke patients suffer impairment,
including the loss of memory, vision, speech, motor function and
voiding control (1). A number of
stroke patients suffer from lower urinary tract symptoms (LUTS)
that include frequency, urgency, urge incontinence, dysuria and
urinary retention (2). Untreated
voiding symptoms may have a major negative impact on the quality of
life of stroke patients and their relatives and are associated with
a high mortality risk (3–5). LUTS are important to urologists and
it is essential to evaluate detrusor function to manage LUTS in
stroke patients (6).
The majority of common urinary symptoms in stroke
patients are storage symptoms, such as nocturia, urgency, frequency
and urge incontinence. However, certain patients complain of
voiding symptoms such as strain, weak stream and tenesmus (6). Stroke patients may experience storage
and voiding symptoms together (7).
When these symptoms occur together it is termed detrusor
overactivity (DO) with impaired contractility (DOIC). DOIC is
complicated to manage and presents a challenging clinical problem
(8). Stroke patients with LUTS
should undergo urodynamic study to evaluate their detrusor
function, particularly when the patient has urgency, urge
incontinence, weak stream or tenesmus. However, urodynamic
examination of stroke patients is a difficult task due to their
poor condition or inability to cooperate.
In the present study, the urodynamic characteristics
of patients with detrusor abnormalities and stroke were
retrospectively analyzed to ascertain how total bladder capacity
(TBC) and post-void residual urine volume (PVR) were reflected in
diagnosing DOIC. The aim of the present study was to develop a
simple method for detecting DOIC.
Patients and methods
Patients
Between August 2003 and November 2010, 178 stroke
patients underwent urodynamic study due to LUTS. A total of 51
patients were excluded due to prostate volumes >35 ml on
transrectal ultrasonography, poorly controlled diabetes, bladder
outlet obstruction, detrusor-external urethra sphincter dyssynergia
or normal findings on urodynamic study. Informed consent was
obtained from all patients.
Urodynamic studies
The urodynamic studies were performed using the
Libra urodynamic test system (Medical Measurement Systems,
Enschede, The Netherlands) (6).
All patients were cooperative and free of any urological
medications, such as antimuscarinic agents or α-blockers, which may
have affected the test. Each patient was supine for a check that
the bladder was empty prior to performing the tests. An 8-French
double lumen catheter was inserted into the bladder via the urethra
to fill the bladder and measure the bladder pressure. The bladder
was filled with room temperature sterile saline at a rate of 30
ml/min. Intravesical pressures were checked during all the tests.
As saline flushing of the bladder proceeded, each patient was asked
if they had a desire to urinate. The points of the patients' first,
normal and strong desire to urinate were recorded. Filling of the
bladder was stopped when the desire to urinate was strong and
represented the cystometric capacity. When filling was complete,
the patients were requested to urinate. Urodynamic parameters,
including TBC, PVR, voided volume (VV), peak flow rate (Qmax),
average flow rate (Qavg), compliance and detrusor pressure at peak
flow rate (PdetQmax) were measured.
Patients were divided into three groups according to
the results. The detrusor underactivity (DU) group was defined as
voiding with abdominal pressure instead of detrusor contraction,
residual urine volume >50 ml or an inability to void (9,10).
The DO group included patients who exhibited involuntary detrusor
contraction during the filling phase (2). The third group was the DOIC group.
Since the diagnostic criteria of DOIC have not been confirmed by
the International Continence Society (ICS), DOIC was defined as the
presence of involuntary contraction during the filling phase and
under-active detrusor function during voiding phase, voiding with
abdominal pressure only or residual urine >50 ml (2,9,10).
Statistical analysis
The significance of differences between the three
groups was analyzed using the Kruskal-Wallis test. The baseline
characteristics and urodynamic parameters of each group were
compared. Receiver operating characteristic (ROC) curves were drawn
to clarify which parameters best aided in determining the status of
DOIC. P<0.05 was considered to indicate a statistically
significant result.
Results
Patients
Of the 127 patients, 57 were male and 70 were
female. The basic characteristics of each group are shown in
Table I. The patient numbers of
the DO, DOIC and DU groups were 58 (45.6%), 16 (12.5%) and 53
(41.7%), respectively, and the mean ages were 64.13, 67.13 and
67.90 years, in the same respective order. Comparing male and
female patients did not reveal any significant differences.
Furthermore, there was no correlation between a patient's gender
and neurogenic bladder type. No significant differences were
observed among the three groups in the mean interval between the
stroke event and the test.
 | Table IBaseline characteristics of study
population. |
Table I
Baseline characteristics of study
population.
| Characteristic | DO | DOIC | DU | P-value |
|---|
| Number of patients
(%) | 58 (45.6) | 16 (12.5) | 53 (41.7) | |
| Age (years), mean
(range) | 64.13 (23–87) | 67.31 (51–79) | 67.90 (45–83) | 0.420 |
| Interval (months),
mean (±SD) | 13.95±27.84 | 9.31±16.03 | 21.32±47.30 | 0.506 |
Urodynamic studies
When DOIC was compared with DO, the two groups
demonstrated significant differences in TBC and PVR. The urodynamic
factors of the two groups are shown in Table II. The average TBCs of the DO and
DOIC groups were 219.15±98.30 and 330.25±115.75 ml, respectively.
The average PVRs of the DO and DOIC groups were 22.64±20.85 and
146.87±95.09 ml, respectively. The differences between the two
groups were significant (P<0.001). However, no significant
differences were observed for the other factors. The average VV of
each group was 196.67±101.46 (DO) and 185.87±108.48 ml (DOC;
P=0.724). The other urodynamic factors, including Qmax, Qavg,
compliance and PdetQmax, were not observed to be significantly
different between the two groups.
 | Table IIUrodynamic factors of the DO and DOIC
groups. TBC and PVR were the only factors which had significant
differences between the two groups. |
Table II
Urodynamic factors of the DO and DOIC
groups. TBC and PVR were the only factors which had significant
differences between the two groups.
| Factor | DO, mean ± SD | DOIC, mean ± SD | P-value |
|---|
| TBC (ml) | 219.15±98.30 | 330.25±115.75 | 0.002 |
| VV (ml) | 196.67±101.46 | 185.87±108.48 | 0.724 |
| PVR (ml) | 22.64±20.85 | 146.87±95.09 | <0.001 |
| Qmax (ml/sec) | 15.12±9.98 | 11.95±6.66 | 0.152 |
| Qavg (ml/sec) | 6.74±3.80 | 5.28±3.19 | 0.142 |
| Compliance | 25.18±72.43 | 16.98±23.46 | 0.498 |
| PdetQmax
(ml/sec) | 41.05±24.12 | 30.00±10.59 | 0.011 |
Similar results were revealed by the ROC curve of
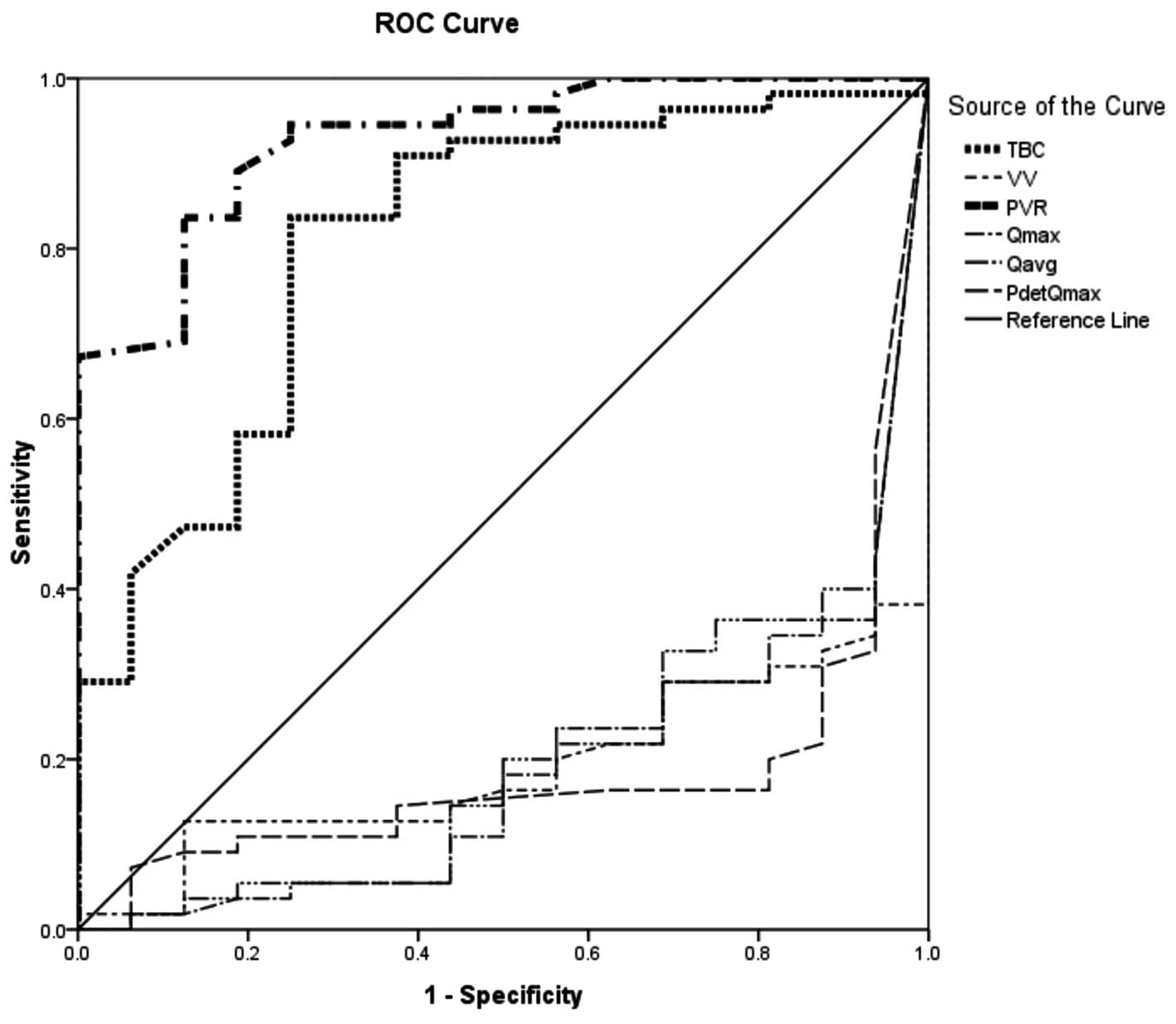
DOIC and DO. Fig. 1 shows a ROC
curve of DOIC and DO. The area under the curve (AUC) of TBC was
0.812 (P<0.001) and that of PVR was 0.987 (P<0.001). However,
the AUC of VV was 0.474 and those of Qmax, Qavg and PdetQmax were
0.421, 0.591 and 0.369, respectively. Only TBC and PVR were
significantly different and clinically useful factors for
distinguishing DOIC from DO. Compared with the DO group, if the
cut-off value of TBC in the DOIC group was set to >262.50 ml,
the sensitivity was 90.9% and the specificity was 68.4%. If the
cut-off value of PVR in the DOIC group was set to >67.50 ml then
the sensitivity was 90.9% and the specificity was 94.7%.
By contrast, the DOIC and DU groups demonstrated
significant differences in all urodynamic factors. The factors of
the two groups are presented in Table
III. The average TBCs of the DOIC and DU groups were
330.25±115.75 and 486.00±111.48 ml (P<0.001), respectively. The
average PVRs of the DOIC and DU groups were 146.87±95.09 and
425.33±136.70 ml, respectively, and the difference between the two
groups was significant (P<0.001). The average VVs of the two
groups were 185.87±108.48 (DOIC) and 63.94±109.30 ml (DU) and were
significantly different (P= 0.001). The other urodynamic factors,
including Qmax, Qavg, compliance and PdetQmax also exhibited
significant differences between the two groups.
 | Table IIIUrodynamic factors of the DU and DOIC
groups. All urodynamic factors had significant differences between
the two groups. |
Table III
Urodynamic factors of the DU and DOIC
groups. All urodynamic factors had significant differences between
the two groups.
| Factor | DU, mean ± SD | DOIC, mean ± SD | P-value |
|---|
| TBC (ml) | 486.00±111.48 | 330.25±115.75 | <0.001 |
| VV (ml) | 63.94±109.30 | 185.87±108.48 | 0.001 |
| PVR (ml) | 425.33±136.70 | 146.87±95.09 | <0.001 |
| Qmax (ml/sec) | 3.43±5.23 | 11.95±6.66 | 0.003 |
| Qavg (ml/sec) | 1.66±2.51 | 5.28±3.19 | 0.010 |
| Compliance | 139.04±206.60 | 16.98±23.46 | 0.002 |
| PdetQmax
(ml/sec) | 10.56±15.07 | 30.00±10.59 | 0.001 |
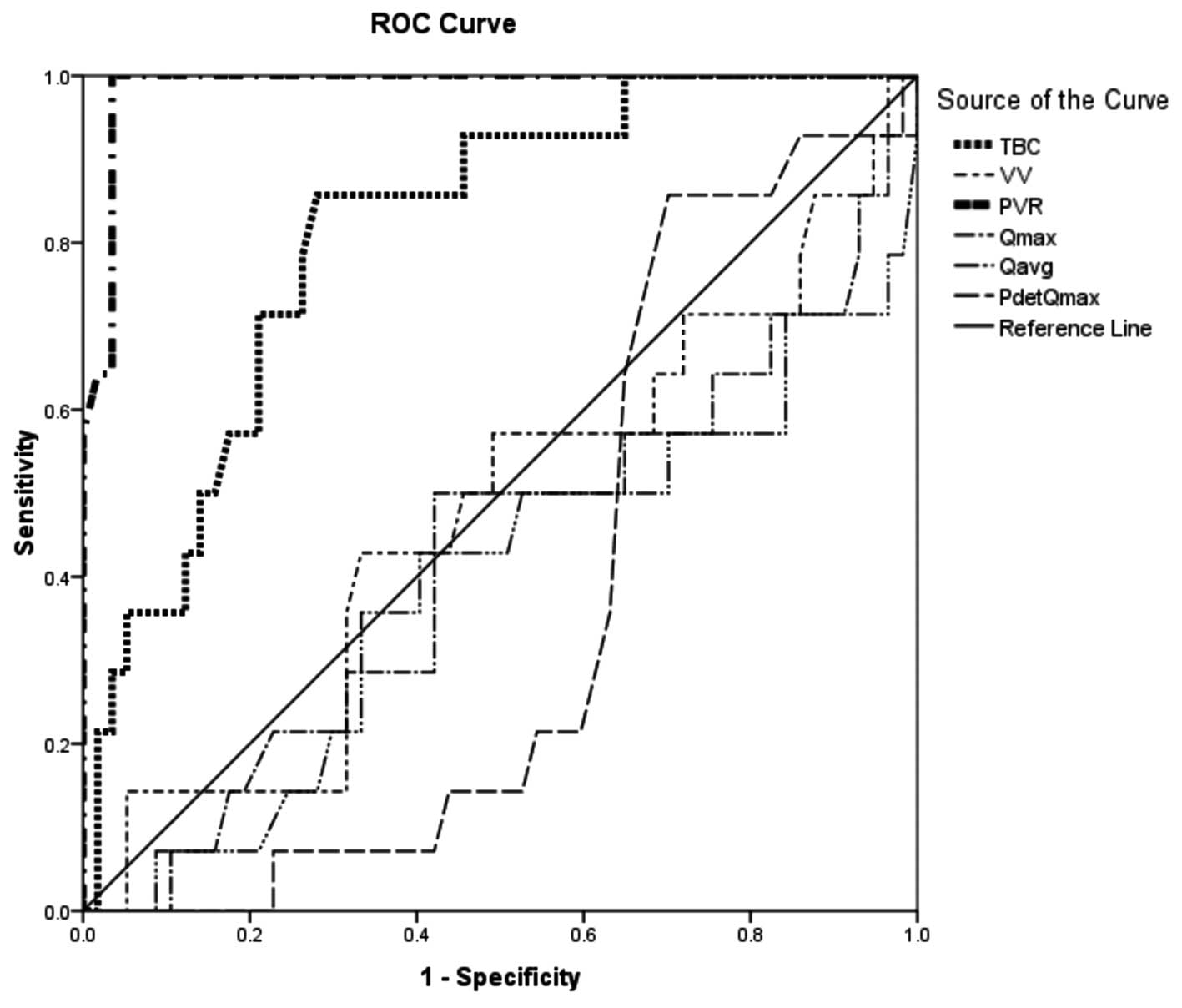
Fig. 2 presents a
ROC curve of DOIC and DU. TBC and PVR were significantly different
between the DOIC and DU groups. The AUC of TBC was 0.813
(P<0.001) and that of PVR was 0.929 (P<0.001). The cut-off
value was also determined. Compared with the DU group, if the
cut-off value of TBC in the DOIC group was set to <335 ml, the
sensitivity was 90.3% and the specificity was 72.7%. If the cut-off
value of PVR in the DOIC group was set to <220 ml then the
sensitivity was 90.3% and the specificity was 81.8%.
TBC and PVR were the only factors that were able to
distinguish DOIC from DO and DU. The TBC and PVR of the DOIC group
were greater than those of the DO group but smaller than those of
the DU group. Therefore, the ranges of TBC and PVR were useful for
diagnosing DOIC using the ROC curves. If the TBC and PVR of
patients with LUTS were 262.5–335 and 67.5–220 ml, respectively,
DOIC may be predicted.
Discussion
Since the first description of patients with DOIC,
numerous studies concerning DOIC have been published (8,11).
The reported criteria for DOIC are a combination of urodynamically
proven urgency or urge incontinence and a high PVR. In the present
study, DOIC was defined more strictly urodynamically in terms of
using abdominal pressure instead of detrusor contraction in voiding
or a PVR of over 50 ml, indicating impaired contractile function
(9,10). The present urodynamic criteria for
DOIC yielded clinical results that did not differ from those of
previous studies (8,11).
The high prevalence of LUTS, including urinary
incontinence, in stroke patients has been reported previously
(12,13). Incontinence may persist for months
following the event (3). Stroke
patients with urinary incontinence have a higher risk of mortality
than stroke patients who are not incontinent. Urinary incontinence
in stroke patients has a significant impact on their lives,
frequently including greater morbidity and mortality (14,15).
However, the previously mentioned studies noted that not only
urinary incontinence but also other urinary tract symptoms, such as
nocturia, frequency, urgency, pain and straining affect quality of
life. Two studies reported all the possible urinary symptoms of
stroke patients (14,15). These studies focused not only on
urge incontinence but also frequency, nocturia and straining
(3,14,15).
Tibaek et al reported that at least one LUTS may bother
stroke patients (16). In the
present study, the most frequent symptom in females and males was
nocturia (76% for both) followed by urgency (70 and 61%,
respectively). Furthermore, 60% of all patients (51% females, 67%
males) suffered from voiding symptoms such as weak stream,
hesitancy, incomplete emptying or straining.
Few studies have mentioned the association between
stroke and DOIC. Yamamoto et al suggested that numerous
neurological diseases cause DOIC and that multiple cerebral
infarction (MCI) is one cause of DOIC (7). The authors reported that 9 of 70
(12%) MCI patients had DOIC. However, the authors noted that the
study did not consider and examine a combination of multiple
diseases, such as MCI and diabetic cystopathy. Tibaek et al
reported that stroke may affect the patients' voiding symptoms,
precluding the development of a variety of LUTS (16). The authors surveyed 482 stroke
patients and noted both storage and voiding symptoms in 43% of this
population. Although the authors did not perform a urodynamic study
and instead gathered information solely by a questionnaire, it
appears likely that at least some of these patients with both
symptoms had DOIC. The design of the present study enabled the
determination of the presence of storage and voiding symptoms in
DOIC patients.
Previously, we were able to evaluate the bladder
function of stroke patients by the type of stroke (6) and concluded that an evaluation of the
stroke type is likely to aid the determination of the type of
urinary dysfunction and in deciding the appropriate bladder
management. However, we observed no significant differences in LUTS
between dominant, non-dominant and bilateral hemispheric ischemic
stroke patients (17). It has been
argued that despite problems in elderly patients, it is reasonable
to perform complex urodynamic studies (18). The collective results favor the
suggestion that urodynamic examination is necessary for stroke
patients with LUTS to understand detrusor function and manage
voiding symptoms.
Diagnosis and management of DOIC are difficult.
Empirical prescription of anticholinergic agents may lead to acute
urinary retention and α-blockers may aggravate incontinent urgency
(8). To manage DOIC, precise
knowledge of detrusor function is necessary. The common criteria
for DOIC are involuntary contraction during the filling phase and
no detrusor contraction during the voiding phase or a high PVR.
Studies that have urodynamically defined DOIC have concluded that
this strategy is necessary for a diagnosis of DOIC. However, this
strategy is challenging for stroke patients with DOIC who cannot
cooperate with the examiner in expressing their intention or whose
urine analysis reveals consistent pyuria. Problems unique to the
evaluation of elderly patients with stroke include technical
difficulties in interpreting urodynamic results (8). Consequently, if there was an easy
method for predicting DOIC, clinicians may choose this method for
stroke patients whose cooperation is difficult to achieve.
In the present study, the DU, DOIC and DO groups
revealed significant differences in all urodynamic factors which
may reflect different characteristics of the groups. In ROC curve
analysis, TBC and PVR were significant factors for distinguishing
DOIC from DU or DO. Checking TBC and PVR may aid clinicians in the
diagnosis of DOIC. By determining the cut-off value, DOIC may be
predicted when TBC is 262.5–335 ml and PVR is 67.5–220 ml.
To clarify the criteria and definition of DOIC,
further functional studies are required. Since there is no easy
method to diagnose DOIC, urodynamic examination is an inevitable
test for patients with storage and emptying symptoms. However, in
patients for whom urodynamic information cannot be easily obtained,
the evaluation of TBC and PVR may be useful for the determination
of DOIC and bladder management. TBC and PVR data may be used to
predict whether stroke patients in poor condition have DOIC.
In conclusion, evaluation of TBC and PVR may be
useful for diagnosing DOIC in stroke patients who cannot undergo
urodynamic study. DOIC may be predicted when TBC and PVR are within
target ranges.
References
|
1
|
Marinkovic SP and Badlani G: Voiding and
sexual dysfunction after cerebrovascular accidents. J Urol.
165:359–370. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abrams P, Cardozo L, Fall M, et al:
Standardisation Sub-committee of the International Continence
Society: The standardisation of terminology of lower urinary tract
function: report from the Standardisation Sub-committee of the
International Continence Society. Neurourol Urodyn. 21:167–178.
2002. View Article : Google Scholar
|
|
3
|
Brittain KR, Perry SI, Peet SM, et al:
Prevalence and impact of urinary symptoms among community-dwelling
stroke survivors. Stroke. 31:886–891. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tilvis RS, Hakala SM, Valvanne J and
Erkinjuntti T: Urinary incontinence as a predictor of death and
institutionalization in a general aged population. Arch Gerontol
Geriatr. 21:307–315. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Campbell AJ, Reinken J and McCosh L:
Incontinence in the elderly: prevalence and prognosis. Age Ageing.
14:65–70. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han KS, Heo SH, Lee SJ, et al: Comparison
of urodynamics between ischemic and hemorrhagic stroke patients;
can we suggest the category of urinary dysfunction in patients with
cerebrovascular accident according to type of stroke? Neurourol
Urodyn. 29:387–390. 2010.
|
|
7
|
Yamamoto T, Sakakibara R, Uchiyama T, et
al: Neurological diseases that cause detrusor hyperactivity with
impaired contractile function. Neurourol Urodyn. 25:356–360. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Griffiths DJ, McCracken PN, Harrison GM,
et al: Urge incontinence and impaired detrusor contractility in the
elderly. Neurourol Urodyn. 21:126–131. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abdel-Rahman M, Coulombe A, Devroede G, et
al: Urorecto-dynamic evaluation of healthy volunteers. Urology.
19:559–564. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Robertson AS, Griffiths CJ, Ramsden PD and
Neal DE: Bladder function in healthy volunteers: ambulatory
monitoring and conventional urodynamic studies. Br J Urol.
73:242–249. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Resnick NM and Yalla SV: Detrusor
hyperactivity with impaired contractile function. An unrecognized
but common cause of incontinence in elderly patients. JAMA.
257:3076–3081. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brittain KR, Peet SM and Castleden CM:
Stroke and incontinence. Stroke. 29:524–528. 1998. View Article : Google Scholar
|
|
13
|
Gelber DA, Good DC, Laven LJ and Verhulst
SJ: Causes of urinary incontinence after acute hemispheric stroke.
Stroke. 24:378–382. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barer DH: Continence after stroke: useful
predictor or goal of therapy? Age Ageing. 18:183–191. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gariballa SE: Potentially treatable causes
of poor outcome in acute stroke patients with urinary incontinence.
Acta Neurol Scand. 107:336–340. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tibaek S, Gard G, Klarskov P, et al:
Prevalence of lower urinary tract symptoms (LUTS) in stroke
patients: a cross-sectional, clinical survey. Neurourol Urodyn.
27:763–771. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim TG, Yoo KH, Jeon SH, et al: Effect of
dominant hemispheric stroke on detrusor function in patients with
lower urinary tract symptoms. Int J Urol. 17:656–660. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Resnick NM, Yalla SV and Laurino E: The
pathophysiology of urinary incontinence among institutionalized
elderly persons. N Engl J Med. 320:1–7. 1989. View Article : Google Scholar : PubMed/NCBI
|