Introduction
Percutaneous nephrolithotripsy (PNL) is replacing
open surgery as the preferred treatment for kidney and upper ureter
stones due to its minimal invasiveness, high probability of
success, and low probability of mortality. However, retrospective
analysis has revealed that certain patients undergoing unilateral
PNL suffer from acute kidney injury (AKI) after surgery. AKI can
lead to a series of clinical problems; if not found and treated in
a timely manner, it can extend the length of hospital stay, affect
recovery of kidney function, increase mortality and morbidity
(1–3) and increase therapy costs (including
hemodialysis as renal replacement therapy). Laboratory results have
indicated that early treatment can effectively prevent
pathophysiological progression to AKI (4,5).
In recent years, studies have provided strong
evidence that urinary biomarkers are useful for early-stage AKI
diagnosis and therapy, including
N-acetyl-β-D-glucosaminidase (NAG) activity, neutrophil
gelatinase-associated lipocalin, interleukin-18 and kidney injury
molecule-1 (6). To date, no study
has assessed the clinical utility of NAG in PNL patients. This
study examined whether urinary NAG levels from the kidney on the
surgical side can predict AKI after unilateral PNL.
Patients and methods
Patients
This was a single-center, retrospective study. Data
were collected for patients undergoing PNL in our hospital between
September 2008 and December 2010.
The inclusion criteria were as follows: i) clear
diagnosis of a kidney stone; ii) normal results for kidney
function, routine blood parameters, electrolytes, routine urine
analysis and midstream urine culture; and iii) suitability for PNL,
with no hypertension, diabetes or coronary heart disease and no
recent intake of drugs that affect renal function. Patients with
serious hydronephrosis, a serious disease, renal insufficiency,
obesity, emaciation, renal ectopia, a horseshoe kidney or a
functional or organic solitary kidney, or for whom incomplete
clinical data or incomplete samples were available, were excluded
from this study. The research was carried out according to the
principles of the Declaration of Helsinki. Informed consent was
obtained and the Shanghai Renji Hospital Ethics Committee approved
the study. The patient data, which are contained within this
article, were obtained by a hospital-based doctor at Shanghai Renji
Hospital, Shanghai Jiao Tong University School of Medicine.
Permission to use these data in this report has been obtained from
all the subjects who participated in this study (7).
Surgical procedure
All patients underwent unilateral PNL using an F18
channel. After successful air-intravenous anesthesia with the
patient in a prone lithotomy position, a ureteroscope (Storz or
Wolf) was inserted into the bladder and advanced into the ureter on
the surgical side, led by a zebra wire. An F5 ureteral catheter was
then inserted.
A puncture was made in the pelvis guided by B
ultrasound and a 10 ml pre-operative urine sample was collected and
stored at 4°C. The channel was expanded to F18 with a fascial
dilator led by a guide wire. After the percutaneous-nephro passage
was established, the kidney stone was located using the
ureteroscope and was broken up using a holmium laser. The majority
of broken stones can be washed out by the filling pump through the
working sheath. Consistent filling pump flow and pressure were
maintained and recorded.
The stones were removed from the pelvis. When the
procedure was complete, the pressure measurement equipment was
removed, and a retrograde guide wire and double-J pipe were
inserted. A F16 nephrostomy tube was sutured in place to drain
urine from the surgical side after surgery.
At 2, 4, 6, 12, 24, 48 and 72 h after surgery, 10 ml
urine samples were collected from the kidney on the surgical
side.
Sample analysis
All urine samples were stored at 4°C and centrifuged
for 3 min at 2,000 rpm within 1 week. The supernatant was used for
NAG measurement.
Urinary NAG activity was measured using the kinetic
rate assay (8) with
2-chloro-4-nitrophenol(-yl) (CNP)-NAG (Quark Biotechnology Research
Institute, Cleveland, OH, USA) as the substrate. Reactions were
carried out at 37°C and pH 4.8. The rate of CNP production was
determined from the change in absorbance at 400 nm per minute on an
Olympus AU640 automatic analyzer. NAG activity was then calculated
as U/l. To eliminate the influence of urine amount on enzyme
activity, results are reported as the ratio of enzyme activity to
urinary creatinine (U/mmol).
Serum creatinine (Scr) levels were measured in a
single laboratory on a Hitachi 7600 analyzer using Jaffe’s kinetic
method, reference range 40–140 μmol/l.
Serum C-reactive protein (CRP) levels were measured
by latex agglutination immunoassay using the Nanopia CRP kit
(Daiichi Pure Chemicals, Tokyo, Japan). Normal values provided by
the manufacturer were ≤0.30 mg/dl, and therefore patients with a
serum CRP of ≤0.30 mg/dl were considered the ‘normal CRP’
cohort.
Statistical analysis
The SPSS 11.5 package was used for all statistical
analyses. For parametric variables with a normal distribution, the
results are presented as the means ± SD and comparisons between two
groups were carried out using a t-test. For parametric variables
with a non-normal distribution, the results are presented as the
median (interquartile range), and comparisons between groups were
carried out using a rank test. The sensitivity and specificity of
AKI diagnosis according to NAG levels were assessed by receiver
operating characteristic (ROC) analysis. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Data were collected for 115 patients. Of these, 25
were excluded due to incomplete samples or medical history. Thus,
90 patients were included in this study. They ranged in age from 35
to 72 years (52.8±9.7) and included 64 males and 26 females.
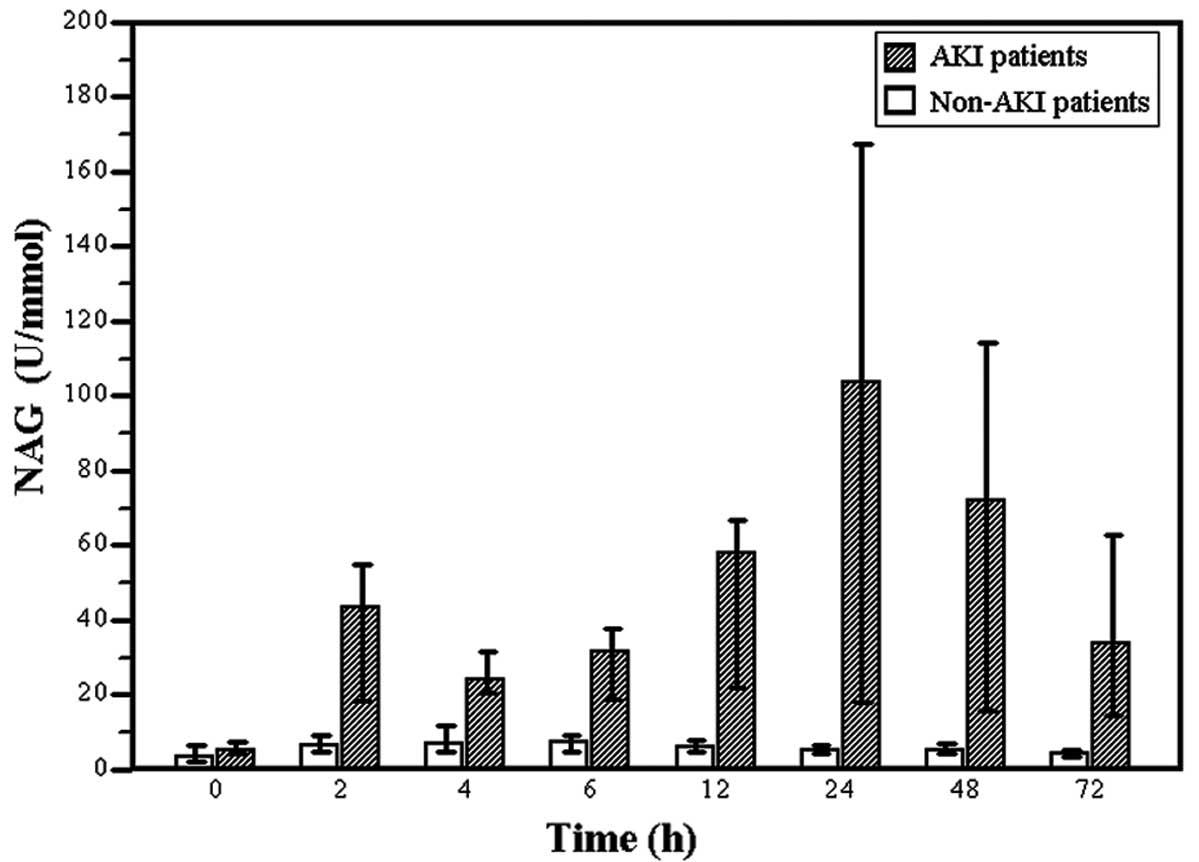
For all patients, urinary NAG increased 2 h after
surgery to reach a peak at 6 h at twice the level prior to surgery,
and then gradually decreased. Post-operative NAG levels were
significantly higher at various time-points compared to
pre-operative levels (P<0.05; Table
I).
 | Table IUrine NAG activities in the kidney on
the surgical side at various time-points (U/mmol). |
Table I
Urine NAG activities in the kidney on
the surgical side at various time-points (U/mmol).
| 0 h | 2 h | 4 h | 6 h | 12 h | 24 h | 48 h | 72 h |
|---|
| Distance between
median and interquartile range | 3.82–4.20 | 7.19–7.58 | 7.73–7.71 | 8.11–6.12 | 6.565–4.45 | 5.79–2.82 | 6.20–2.90 | 4.78–3.39 |
| P-value (compared to
0 h) | | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.013 |
Patients were categorized as AKI or non-AKI
according to the AKI diagnosis criterion of a sudden decrease in
kidney function over 48–72 h, evident as an absolute increase in
Scr levels ≥0.3 mg/dl (≥26.4 mmol/l) or a percentage increase of
≥50%. Parameters for the groups are compared in Table II.
 | Table IIComparison between AKI group and
non-AKI group. |
Table II
Comparison between AKI group and
non-AKI group.
| AKI | Non-AKI | P-value |
|---|
| No. of cases | 11 | 79 | |
| Age | 51.3±10.3 | 53.0±9.6 | >0.05 |
| Gender | 8/3 | 56/23 | >0.05 |
| Surgical duration
(min) | 113.18±14.60 | 78.27±13.80 | <0.01 |
| Urinary passage
infection rate (%) | 81.82 | 37.97 | <0.01 |
| C-reactive protein
(mg/l) | 87.82±14.11 | 47.11±15.61 | <0.01 |
| Blood creatinine
(μmmol/l) | 51.96±15.32 | 58.73±11.14 | >0.05 |
| No. of hospital
days | 5.91±0.83 | 3.80±0.88 | <0.01 |
There were no significant differences between the
groups as regards age, gender ratio or baseline creatinine levels;
however, there were significant differences in surgical duration,
post-operative infection rate, CRP levels and number of hospital
days (P<0.01; Table II). For
the AKI group, NAG levels increased at 2 h after surgery and
thereafter and were significantly higher compared to the non-AKI
group (P<0.01; Fig. 1).
To assess the utility of the extent of the increase
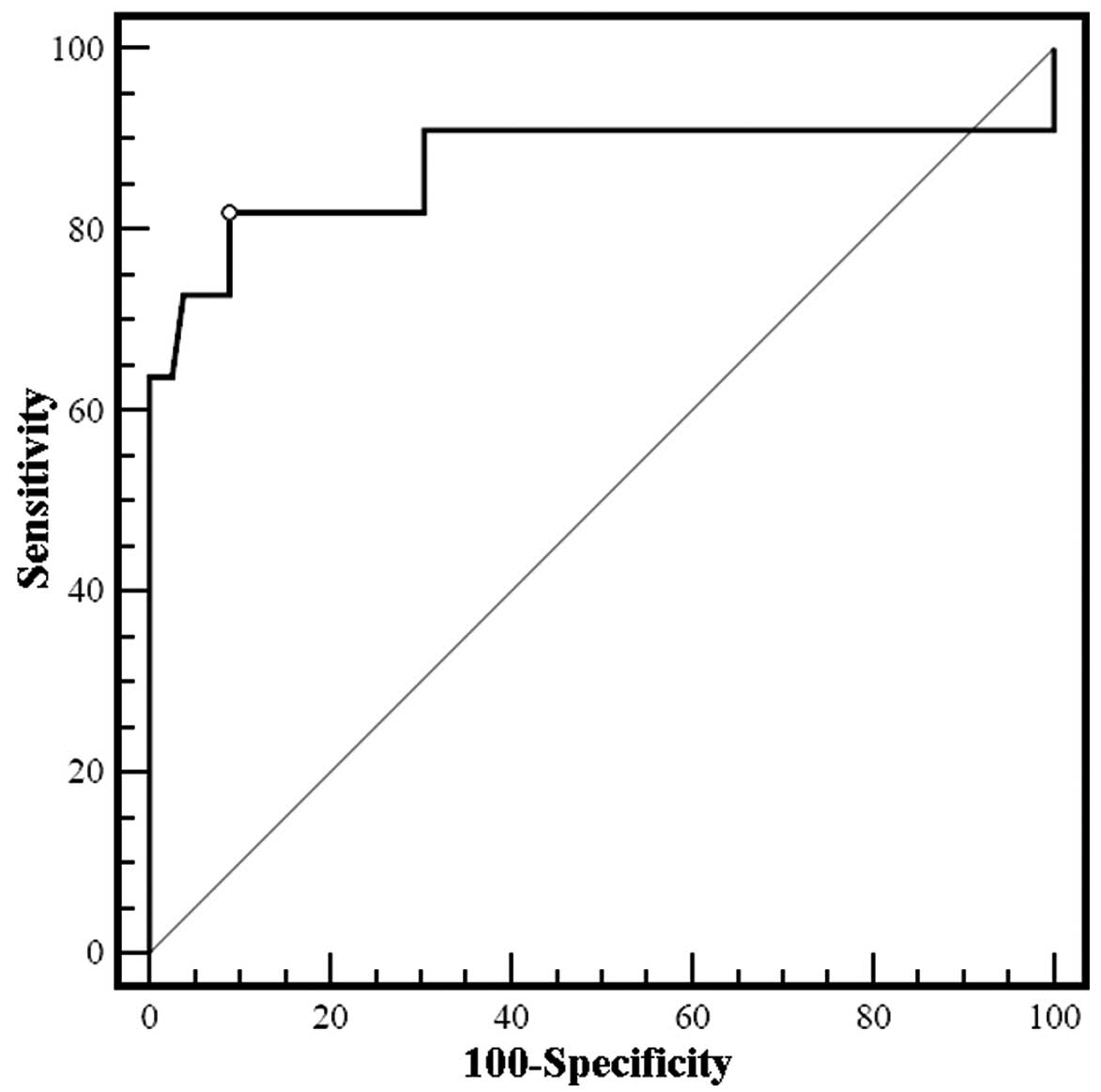
in urinary NAG at 24 h after surgery for AKI prediction, ROC
analysis was carried out. The area under the curve was 0.878 (95%
CI, 0.699–1.043, P<0.01) for a NAG increase of 235.44%, with
diagnostic sensitivity and specificity of 81.8 and 91.1%,
respectively (Fig. 2).
Discussion
PNL as a surgical technique is minimally invasive;
however, retrospective evaluation of a large amount of clinical
data revealed that AKI occurs in some patients after unilateral
PNL. Without timely adequate treatment, AKI can lead to an
unfavorable prognosis. Early diagnosis and therapy are of great
importance to reduce the risk of serious AKI complications.
The main index currently used in clinical practice
for AKI diagnosis is Scr; however, its sensitivity and specificity
are relatively low. Scr levels are only altered when renal function
decreases by approximately 50% and Scr cannot reflect changes in
renal function over time until the body reaches a stable condition,
which often takes several days. Scr levels are influenced by other
factors, such as age, race, blood volume, muscle metabolism, drugs
and nutritional condition. These limitations mean that Scr is not
an ideal index for AKI and cannot provide early clues for AKI
diagnosis, so early clinical therapy may be delayed (9–14).
Therefore, it is of clinical importance to identify biomarkers with
higher sensitivity and specificity to predict, prevent and treat
AKI after surgery.
In recent years, research on urine biomarkers has
provided strong evidence that these are suitable for the early
diagnosis and therapy of AKI (6).
NAG has attracted increasing attention as such a biomarker. Widely
distributed in various tissues and cells in the body, NAG is a
lysosome hydrolase with a molecular weight of approximately 140,000
Da that cannot normally be filtered through the glomerulus
(15). It is mainly distributed in
lysosomes in epithelial cells of nephric tubules, and small amounts
are found in the mitochondria. For patients with non-glomerulus
disease without marked albuminuria, urinary NAG mainly originates
from the nephric tubules. Since dynamic concentrations of urinary
NAG change with urine flow and the collection of 24-h urine samples
is complicated, the NAG/creatinine ratio has been used to avoid
errors caused by random measurement at a single time-point. NAG is
not easily inactivated in urine and urinary NAG output is
relatively stable under normal conditions. Moreover, urinary NAG
significantly increases during necrosis of the tubular epithelium
and this change occurs much earlier than changes in blood urea
nitrogen and Scr (16).
Consequently, urinary NAG can be used to evaluate early damage to
epithelial cells in the proximal convoluted tubules during the
progression of renal diseases and is an index that reflects renal
tubule damage (17).
Our results show that post-operative urinary NAG
levels increased to different extents in 90 PNL patients, among
whom 11 developed AKI. There were no significant differences
between the non-AKI and AKI groups as regards age, gender ratio and
baseline creatinine levels; however, the AKI group had a
significantly longer surgical duration and a greater post-operative
infection rate, CRP levels and number of hospital days. Thus, a
longer surgical duration may increase the infection rate and AKI
risk and prolong the hospital duration. If the surgical duration
can be controlled to a certain extent, the risk of post-operative
complications may be reduced.
In addition, NAG levels were higher in the AKI than
in the non-AKI group within 24 h after surgery. Thus, NAG indicates
the occurrence of AKI earlier and is a more sensitive biomarker
than Scr. ROC analysis was carried out to assess the diagnostic
utility of the increase in urinary NAG 24 h after surgery in
predicting AKI. The area under the curve was 0.878 (95% CI,
0.699–1.043, P<0.01) for a post-operative increase in NAG of
235.44%, and the sensitivity and specificity of AKI diagnosis were
81.8 and 91.1%, respectively. Thus, the extent of any increase in
urinary NAG levels may be an efficient marker for predicting
AKI.
Currently AKI is divided into 3 phases (18). In phase I or the risk phase, Scr is
>26.4 μmol/l (0.3 mg/dl) or increases by 50% and urine output
has been <0.5 ml/(kg/h) for 6 h. In phase II or the damage
phase, Scr increases by 200–300% and urine output has been <0.5
ml/(kg/h) for 12 h. In phase III or the exhaustion phase, Scr
increases by more than 300% or is >4 mg/dl and urine output has
been <0.3 ml/(kg/h) for 24 h or the patient has experienced
anuresis for 12 h. During phase I, the treatment focus is on
analysis and neutralization of risk factors, pathogen elimination,
investigation of changes in daily intake and output volumes and
body weight, evaluation of blood volume, and maintenance of
electrolytes and acid-base balance. During phase II, the focus is
on preventing or decreasing damage to target organs, to provide
out-specific nursing care (nephrostomy tube, skin, psychology and
fluid management), to identify any infection as early as possible,
and to provide nutritional support. During phase III, as it is
possible for renal function to recover completely or partially, the
illness state is complicated, and clinical manifestations are
various and unstable, dialysis should not be delayed until chronic
renal failure occurs. On the contrary, preventive dialysis should
be performed as early as possible to replace renal function,
maintain body homeostasis and provide an opportunity for recovery
of multi-organ function (19). Our
AKI patients were classified as phase I, so the focus was as
described above to prevent further deterioration of renal function.
For AKI patients without other serious complications, treatment
with drugs such as the selective dopamine receptor 1 agonist,
fenoldopam, and the free radical eliminator and antioxidant,
pentoxifylline, is feasible (20).
Liangos et al found that urinary NAG positively correlated
with the degree of acute renal failure (21). AKI detection at an early stage is
important for enhanced vigilance and to prevent further worsening
of AKI to phase III.
In conclusion, our results demonstrate that urinary
NAG activity significantly increases in AKI. This parameter is more
sensitive than Scr and can reflect impairment of renal function at
an earlier stage. The surgical duration and post-operation
infection rate are possible risk factors for AKI. Urinary NAG may
have some clinical value in the early diagnosis of AKI after
surgery.
References
|
1
|
Chertow GM, Burdick E, Honour M, Bonventre
JV and Bates DW: Acute kidney injury, mortality, length of stay,
and costs in hospitalized patients. J Am Soc Nephrol. 16:3365–3370.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoste EA, Clermont G, Kersten A, et al:
RIFLE criteria for acute kidney injury are associated with hospital
mortality in critically ill patients: a cohort analysis. Crit Care.
10:R732006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uchino S, Bellomo R, Goldsmith D, Bates S
and Ronco C: An assessment of the RIFLE criteria for acute renal
failure in hospitalized patients. Crit Care Med. 34:1913–1917.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schrier RW, Wang W, Poole B and Mitra A:
Acute renal failure: definitions, diagnosis, pathogenesis, and
therapy. J Clin Invest. 114:5–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Star RA: Treatment of acute renal failure.
Kidney Int. 54:1817–1831. 1998. View Article : Google Scholar
|
|
6
|
Sirota JC, Klawitter J and Edelstein CL:
Biomarkers of acute kidney injury. J Toxicol. 2011:3281202011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fan JY, Wang YF, Han B, Ji YR, Song HD and
Fan XQ: FOXL2 mutations in Chinese families with Blepharophimosis
syndrome (BPES). Transl Res. 157:48–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Makise J, Saito E, Obuchi M, et al:
Kinetic rate assay of urinary N-acetyl-beta-D-glucosaminidase with
2-chloro-4-nitrophenyl-N-acetyl-beta-D-glucosaminide as substrate.
Clin Chem. 34:2140–2143. 1988.
|
|
9
|
Bonventre JV and Zuk A: Ischemic acute
renal failure: an inflammatory disease? Kidney Int. 66:480–485.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han WK, Bailly V, Abichandani R, Thadhani
R and Bonventre JV: Kidney Injury Molecule-1 (KIM-1): a novel
biomarker for human renal proximal tubule injury. Kidney Int.
62:237–244. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Herget-Rosenthal S, Marggraf G, Husing J,
et al: Early detection of acute renal failure by serum cystatin C.
Kidney Int. 66:1115–1122. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herget-Rosenthal S, Pietruck F, Volbracht
L, Philipp T and Kribben A: Serum cystatin C-a superior marker of
rapidly reduced glomerular filtration after uninephrectomy in
kidney donors compared to creatinine. Clin Nephrol. 64:41–46. 2005.
View Article : Google Scholar
|
|
13
|
Hewitt SM, Dear J and Star RA: Discovery
of protein biomarkers for renal diseases. J Am Soc Nephrol.
15:1677–1689. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmidt-Ott KM, Mori K, Kalandadze A, et
al: Neutrophil gelatinase-associated lipocalin-mediated iron
traffic in kidney epithelia. Curr Opin Nephrol Hypertens.
15:442–449. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
YE RG and Xu HS: The diagnosis value of
urinary lysozyme and N-acetyl beta glucosaminidase in renal
tubulointerstitial diseases. Chin J Pract Intern Med. 19:198–199.
1999.
|
|
16
|
D’Amico G and Bazzi C: Urinary protein and
enzyme excretion as markers of tubular damage. Curr Opin Nephrol
Hypertens. 12:639–643. 2003.PubMed/NCBI
|
|
17
|
Bazzi C, Petrini C, Rizza V, et al:
Urinary N-acetyl-beta-glucosaminidase excretion is a marker of
tubular cell dysfunction and a predictor of outcome in primary
glomerulonephritis. Nephrol Dial Transplant. 17:1890–1896. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ronco C, Levin A, Warnock DG, et al:
Improving outcomes from acute kidney injury (AKI): report on an
initiative. Int J Artif Organs. 30:373–376. 2007.PubMed/NCBI
|
|
19
|
Meng J, Zhang Y, Ge Y, et al: The role of
blood purification in rhabdomyolysis complicated by acute renal
failure with excessive exercise. Chin J Blood Purif. 3:468–470.
2004.
|
|
20
|
Kim YK, Choi TR, Kwon CH, Kim JH, Woo JS
and Jung JS: Beneficial effect of pentoxifylline on
cisplatin-induced acute renal failure in rabbits. Ren Fail.
25:909–922. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liangos O, Perianayagam MC, Vaidya VS, et
al: Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney
injury molecule-1 level are associated with adverse outcomes in
acute renal failure. J Am Soc Nephrol. 18:904–912. 2007. View Article : Google Scholar : PubMed/NCBI
|