Introduction
Pancreatic cancer is the fourth leading cause of
cancer-related death in the United States and other Western
countries (1). Due to the frequent
delay in diagnosis, approximately 80% of patients have unresectable
disease at presentation (2).
Therefore, patients with locally advanced pancreatic cancer
predominate in clinical practice. However, no effective modality
has been identified thus far for the treatment of patients with
locally advanced pancreatic cancer, although several studies have
shown that chemoradiation offers a limited survival benefit
(3,4). The median survival time is 6–10
months for patients with locally advanced pancreatic cancer and 3–6
months for those with metastatic disease. Due to the poor prognosis
associated with late-stage and recurrent pancreatic cancer, new
treatment options are required.
HIFU appears to be a candidate for the treatment of
pancreatic cancer, where it may play a palliative role alongside
chemotherapy. During HIFU treatment, focused ultrasound waves are
emitted from a therapeutic transducer and absorbed by the target
area, thereby inducing coagulation necrosis without causing damage
to tissue in the path of the ultrasound beam (5,6).
There have been few reports on the experimental use of HIFU in
pancreatic cancer (7,8). In an effort to address this, we
present the results of a study investigating whether HIFU is an
effective treatment for pancreatic cancer in an athymic nude mouse
model.
Materials and methods
Animals
Athymic nude mice (n=44), weighing 20–25 g, were
supplied by the Shanghai Tumour Research Institute. The animals
were inoculated subcutaneously with 5–7×106 SW1990
cells. Cell line SW-1990 was established in 1978 from a pancreatic
adenocarcinoma of a 56-year-old Caucasian male. It was derived from
a pancreatic adenocarcinoma of ductal origin. Growing tumours
exhibited characteristics of a Grade II adenocarcinoma similar to
the original neoplasm (9). The
animals were monitored every 4 days for the presence and size of
tumours. Tumour volume was measured by transcutaneous ultrasound,
and the long and short dimensions of the tumour were measured
transcutaneously using a vernier calliper. As nude mice have thin
skin, no hair and little subcutaneous fat, no correction for skin
thickness was made (10,11). Tumour volume was calculated based
on the assumption that each tumour was a regular ellipsoid.
Animals that developed tumours were randomised into
three groups: HIFU treatment, sham treatment (scanned by ultrasound
only, but received no HIFU) and control (no treatment). In the HIFU
and sham groups, treatment was administered when the average tumour
volume was approximately 0.1 cm3 (approximately 0.6 cm
in diameter) after inoculation of SW1990 cells.
In the control group, tumours were allowed to grow
to a maximum of 10% of the animal’s total body weight before the
animals were euthanised.
This study received ethical and scientific approval
from the ethics board of the Affiliated No.6 Hospital, Shanghai
Jiaotong University, and complied with the Practice for Laboratory
Animals guidelines in China.
Diagnostic and therapeutic
ultrasound
Diagnostic ultrasound was carried out using an
Esaote MPX machine (Esaote S.p.A., Genova, Italy). Ultrasound
images of the tumour prior and subsequent to treatment were
recorded using a high-frequency probe whose frequency was 12.6
MHz.
HY2900 HIFU tumour therapy system (Wuxi Haiying
Techonology, Wuxi, China) was used in this study. This device,
which was designed and manufactured for clinical tumour treatments,
comprised an ultrasonic diagnostic unit under the control of a
central processing unit. The therapeutic transducer was a
self-focused 6-element transducer with a diameter of 25 cm, and a
focal length of 140 mm was fixed on the top of a water capsule
filled with degassed water. A diagnostic transducer was localized
in the center of the therapeutic transducer. The frequency of the
diagnostic transducer was set at 3.5 MHz. Thus, the tissues in the
path of therapeutic ultrasound waves could be viewed in diagnostic
ultrasonic images. Ultrasonography was used to guide HIFU radiation
and monitor therapeutic effects in real time. The maximum
electrical power from the amplifier was 1.02 kW. The spatially
averaged intensity level (ISAL) at −6dB was determined
to be 9366 W/cm2 based on radiation force measurements
and acoustic field mapping, producing a maximum acoustic power of
479.2 W. The water bag had an acoustic transparent membrane bottom
to allow HIFU to transmit without obstruction, and ultrasound
coupling gel was applied to eliminate air pockets trapped between
the membrane and the skin of the nude mice. The frequency of the
therapeutic ultrasound wave was 1.5 MHz. The focal region of the
therapeutic transducers was an ellipsoid with dimensions of 8 mm
along the beam axis and 1.15 mm in the transverse direction, which
was calibrated using a PVDF needle hydrophone with a spot diameter
of 0.5 mm in a tank filled with degassed water.
Tumour tissue ablation
The animals were anaesthetised by intravenous
injection of ketamine (2 ml/kg). After anaesthesia, the animals
were maintained in the lateral decubitus position to allow the
tumour to be viewed clearly with ultrasonography. The surface of
the skin was kept in tight contact with the water tank. Sample
images were taken under the guidance of B-mode ultrasound. The
hypoechoic tumour tissue was treated point-by-point: therapy depth
was 6 mm, and, as the tumour was small, treatment was performed in
a horizontal mode with one layer. In all cases, pulses were applied
at ISAL = 589 W/cm2 and a pulse duration of
500 msec, with an exposure separation of 5 sec between each
treatment point. The interval distance between treatment points was
1 mm. The sample images of each tumour were taken under the
guidance of B-mode ultrasound immediately after therapy in the same
manner as pretherapeutic imaging. If a hypoechoic region was
observed after treatment, treatment was repeated until the region
was hyperechoic. The sham-treated tumours were scanned by
ultrasound but received no HIFU.
Ultrasonic examination
After therapy, the tumours were examined
transcutaneously by ultrasound every 4 days for 4 weeks. The
examinations included two-dimensional ultra-sound, colour Doppler,
and power Doppler. Examination was carried out using the same
machine parameters each time. The tumour volume was measured by
vernier calliper every 4 days for 4 weeks. The volume was
calculated according to the following formula: V= 4/3 × π × ab2 =
4/3 × π × 1/2A × (1/2B)2 = 1/2AB2 (where V is volume and
A and B the long and short dimensions, respectively; with a and b
representing the half of the long and short dimensions,
respectively) of the tumour in centimetres (12). Measurement was carried out three
times, and the average value was used for tumour volume
calculation. Tumour volumes were presented as the mean ± standard
deviation of the mean (SD).
Pathology
All 44 animals were sacrificed approximately 4 weeks
after treatment, or 6 weeks after inoculation. Animals were
euthanised by placing them in a CO2 chamber for 15–20
min. Tissue samples were taken from the treatment sites in the
HIFU-treated animals, from tumours in the sham-treated and control
animals, and from the lungs, livers and kidneys of all animals. All
samples were fixed in 10% formalin, embedded in paraffin, and
stained with haematoxylin and eosin for histopathologic examination
by light microscopy and viewed at ×200 magnification. For electron
microscopy examinations, samples were fixed in glutaric
dialdehyde.
Statistical analysis
Data were processed by the statistics software SPSS
12.0. One-way ANOVA analysis was employed. The difference was
significant if the P-value was <0.05.
Results
Tumour volume and treatment time
Of the 44 animals that were inoculated, 36 developed
tumours and these animals were either HIFU-treated (n=18),
sham-treated (n=9) or received no treatment (control, n=9). After a
period of approximately 2 weeks tumour masses reached 0.1
cm3 in volume, at which point they were considered
suitable, based on the spatial dimensions of the HIFU focus, for
HIFU or sham treatment. In the HIFU- and sham-treated groups, the
average treatment times were 38±16.1 and 40±12.6 sec, respectively
(Table I).
 | Table IPre- and post-treatment tumour volume
and treatment time. |
Table I
Pre- and post-treatment tumour volume
and treatment time.
| Groups | n | Pre-treatment tumour
volume (cm3) | Post-treatment tumour
volume (cm3) | Treatment time
(sec) |
|---|
| HIFU-treated | 18 | 0.10±0.12 | 0 | 38±16.1 |
| Sham-treated | 9 | 0.12±0.06 | 0.42±0.39 | 40±12.6 |
| Control | 9 | 0.11±0.06 | 0.39±0.36 | NA |
| No tumour | 8 | NA | NA | NA |
Effects of HIFU treatment
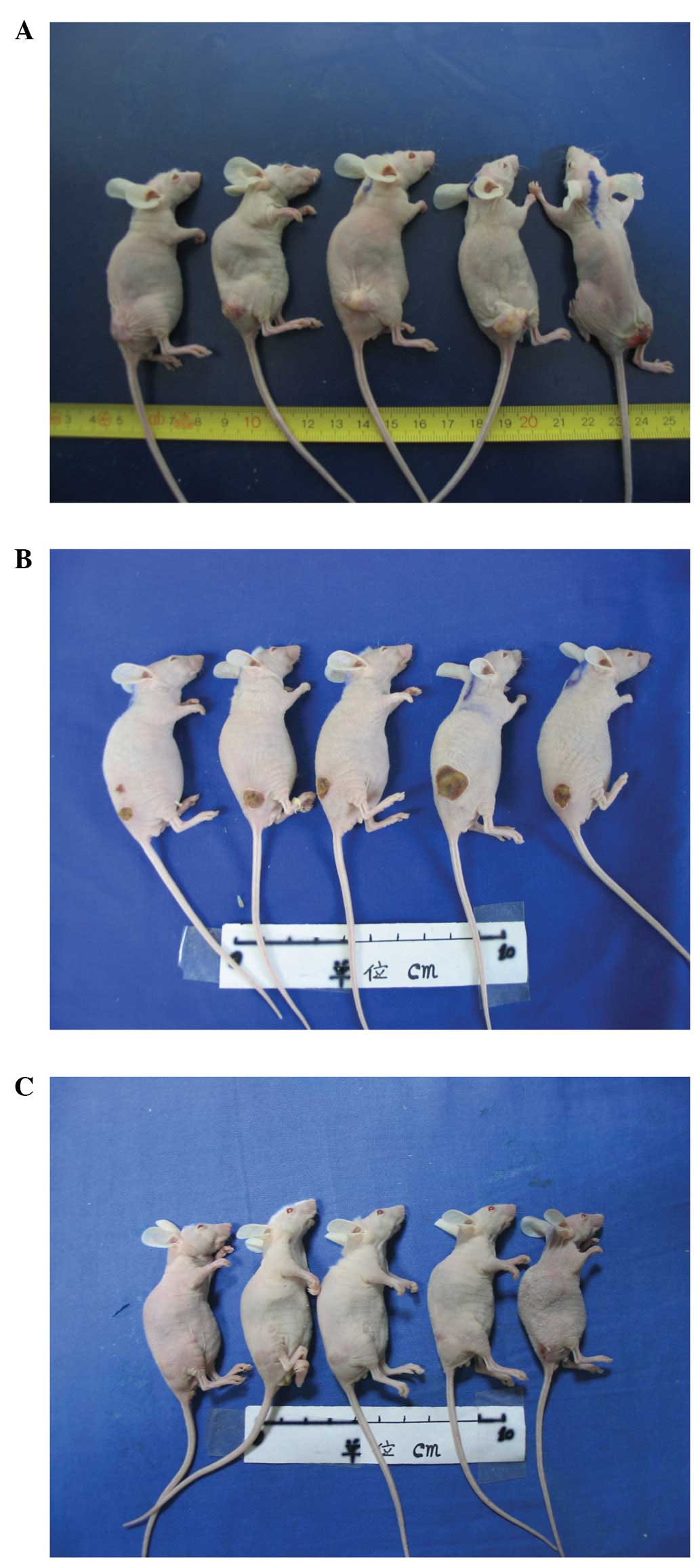
Fig. 1 shows
representative mouse tumours prior and subsequent to HIFU
treatment. The subcutaneous tumour masses are evident prior to
treatment (Fig. 1A). After the
application of HIFU, first- or second-degree skin burns were
observed (Fig. 1B). Four weeks
after HIFU no tumour mass was apparent, and the treatment site
appeared as a minor scar (Fig.
1C).
HIFU treatment to decreased tumour
volume
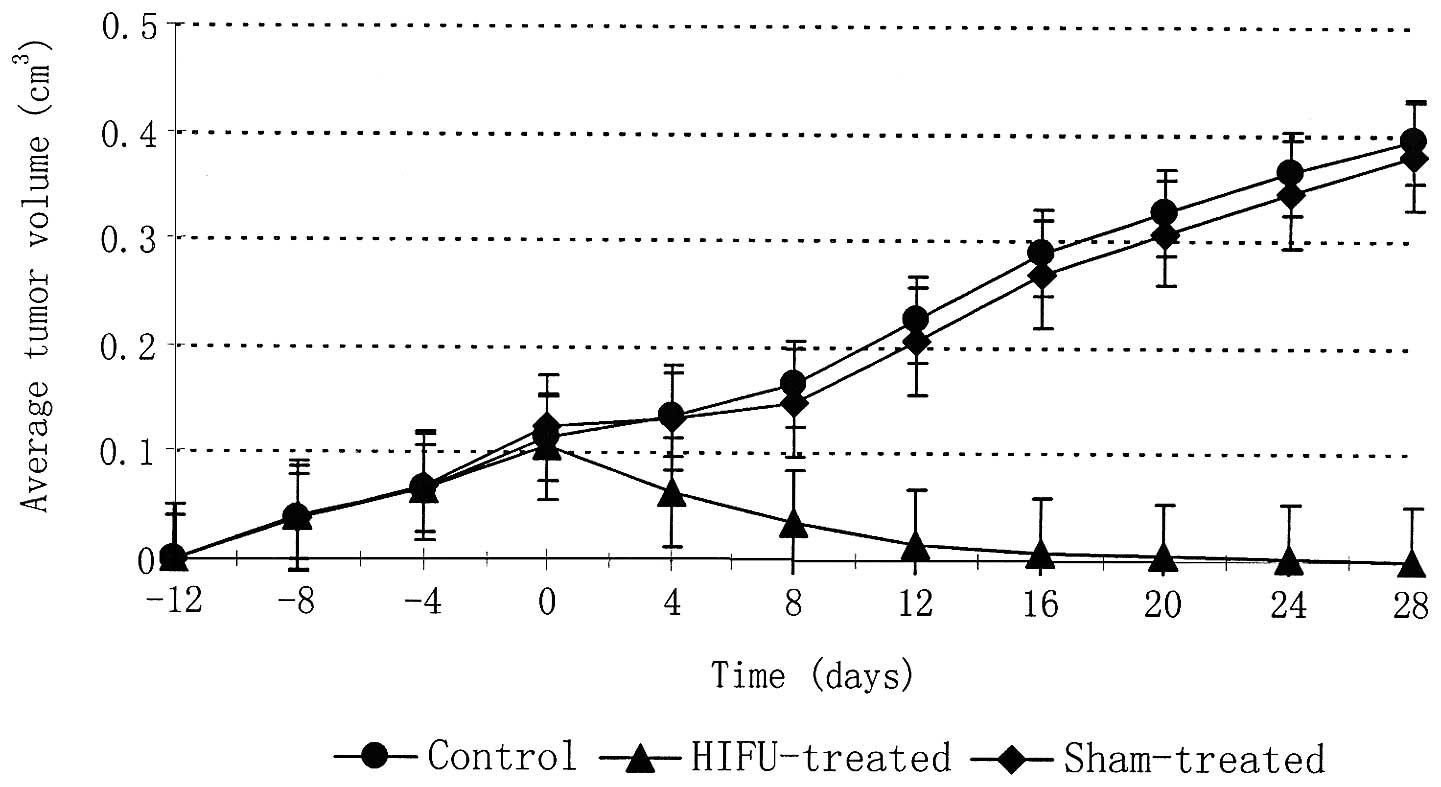
The average ± SD of tumour volumes at the time of
treatment was 0.12±0.06 and 0.10±0.12 cm3 for the sham
and HIFU treatment groups respectively (Table I). The average tumour volumes for
HIFU-treated, sham-treated, and control animals were plotted as a
function of time in days (Fig. 2).
The data were normalised in order for HIFU or sham treatment to be
applied at day 0. All tumours had similar growth rates prior to
HIFU or sham treatment. Following HIFU a 100% reduction in tumour
volume was observed in all tumours within 28 days. By contrast,
tumours of the sham-treated and control groups continued to grow,
enlarging to approximately 400% of the tumour volume at the time of
treatment. No metastases to liver, kidneys or lungs were observed
in any of the groups.
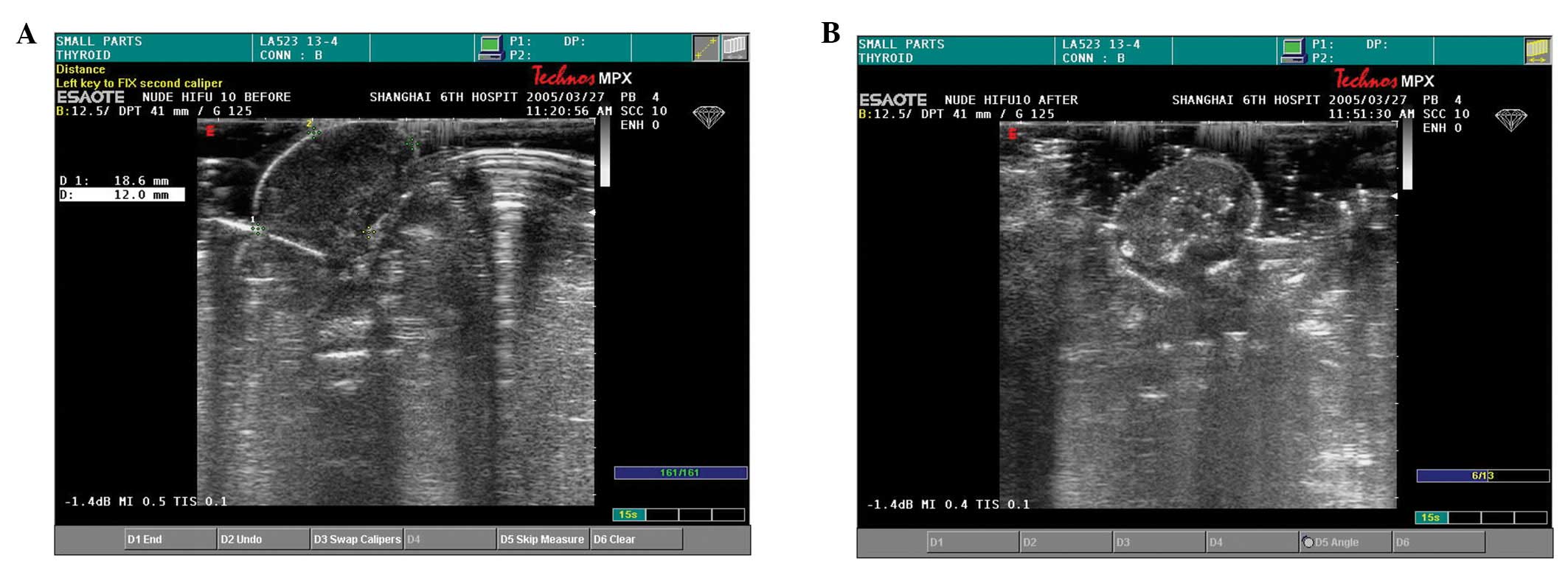
Echoicity following HIFU treatment
Before treatment, all tumours appeared hypoechoic
under B-mode ultrasonic examination (Fig. 3A). Blood flow in and around the
tumour, as detected by colour Doppler and power Doppler, was not
abundant. In reconstructed three-dimensional images, the
therapeutic region appeared hypoechoic in the horizontal plane. The
echoicity of the therapeutic region increased during HIFU
treatment, at the end of which a bright hyperechoic signal was
evident (Fig. 3B). Tumours in the
sham-treated and control groups remained hypoechoic.
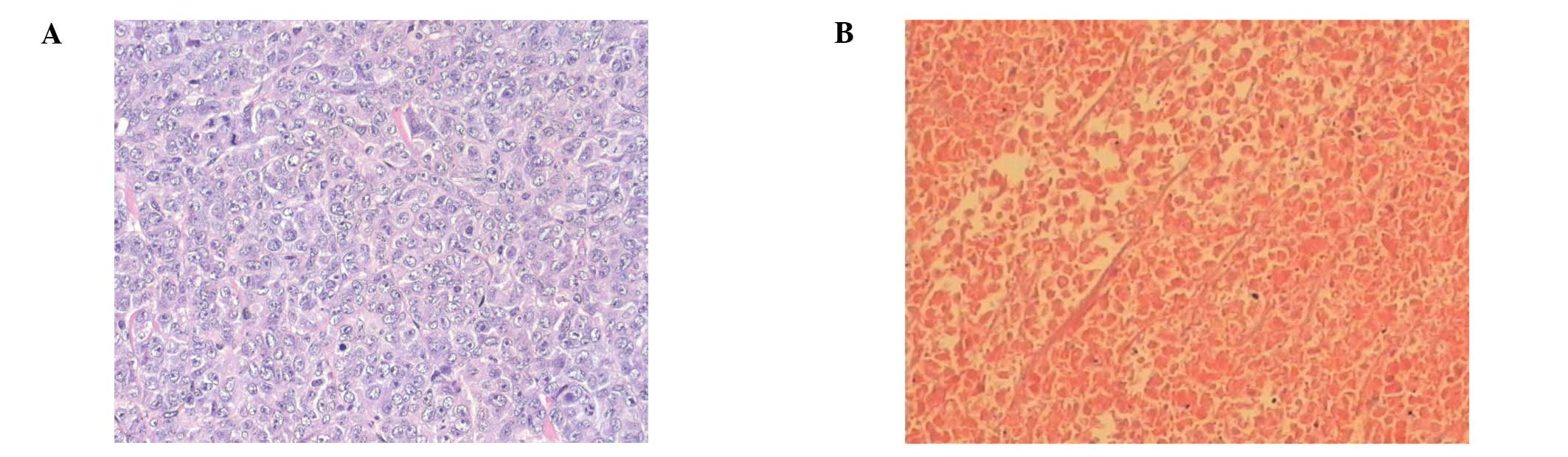
HIFU eradicated viable tumour cells
Under pathological examination, the tumours appeared
as subcutaneous masses prior to treatment. First- or second-degree
skin burns were observed in almost all of the HIFU-treated animals.
In all cases, the skin repaired itself without any intervention.
Light microscopic examination of tumour tissue taken 4 weeks after
treatment revealed tumour cells that appeared viable and had
centrally located nuclei in animals in the sham-treated and control
groups (Fig. 4A), whereas no
remaining viable tumour cells were observed in the HIFU-treated
animals (Fig. 4B).
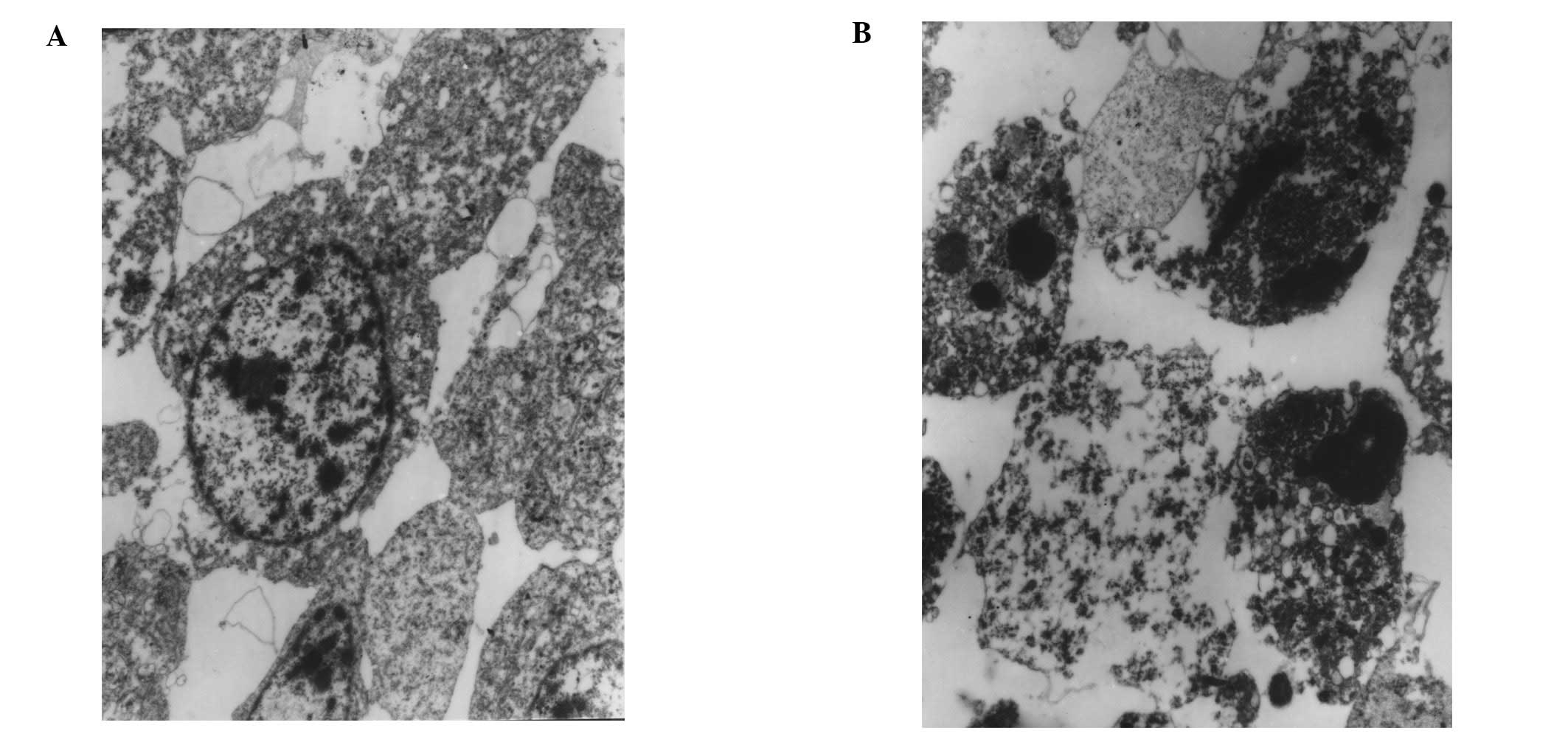
Electron microscopy of tumour tissue taken after
HIFU showed extensive disruption of cell membranes, nuclei and
cytoplasmic components (Fig. 5A).
In addition to karyorrhexis, cytoplasmic structures such as
mitochondria and rough endoplasmic reticulum had disappeared, and
chromatin was clumped at the periphery of nuclei; only some
cellular ground substance remained. Apoptotic bodies were also
observed (Fig. 5B).
Discussion
We studied the efficacy of high-intensity focused
ultrasound (HIFU) for the treatment of pancreatic cancer in a nude
mouse model. After HIFU treatment, the tumour volumes in mice were
significantly reduced with minimal side effects. Treatment using
HIFU requires an accurate visualisation method for targeting and
monitoring therapy. We found HIFU-treated tissue to have a
characteristic appearance under ultrasound: in our study, a bright
hyperechoic region appeared in the HIFU-treated region. The
appearance of a persistent hyperechoic region following HIFU
treatment has been demonstrated to be due to gas following boiling
of tissue. Boiling, that is, the growth of vapour bubbles, occurs
due to HIFU-induced temperature rise. In this sense, boiling is to
be distinguished from cavitation, which occurs due to HIFU-induced
pressure oscillations (13). Our
results also indicate that B-mode ultrasound may provide a valuable
real-time method for monitoring the HIFU treatment of these
tumours.
After HIFU treatment some malignant cell islands in
the centre of the lesion failed to show morphological changes
characteristic of cell necrosis under light microscopy. However,
electron microscopy confirmed the presence of ultrastructural
cellular damage. Electron micrographs revealed complete disruption
of the nuclei and cytoplasmic ultrastructure, indicating that the
tumour cells in question had undergone necrosis after HIFU
exposure. Although most initial cell death in tissues exposed to
HIFU fields is caused by cell necrosis due to thermal injury, HIFU
is also able to induce apoptosis. In apoptotic cells, the nucleus
of the cell self-destructs, with rapid degradation of DNA by
endonucleases. The primary mechanism of cell death by hyperthermia
is apoptosis (14).
Two weeks after treatment, a 100% reduction in
tumour volume was observed in all animals in the HIFU treatment
group, whereas tumours in the sham-treated and control groups
continued to grow. Similar findings were reported in previous
studies (10,12).
We observed that the skin overlying the treatment
area turned white immediately after HIFU application, indicating a
skin burn. We considered the burn to be due to three causes, as
follows: i) The difference in acoustic impedance between skin and
either water or air, leading to ultrasound beam reflection and
refraction, and the conversion of mechanical energy to heat at the
skin surface. ii) Air bubbles in the barrier gel during treatment,
which may have contributed to suboptimal coupling conditions.
Future studies should ensure that degassed procedure is carried
out. iii) The pancreatic tumours in our model were located
subcutaneously, reducing the focus-to-skin distance during the HIFU
tumour ablation process. In order to avoid skin injury, it would be
necessary to ensure that the focus-to-skin range is at least 1 cm;
if this is not possible, the HIFU duty factor and exposure time
should be decreased. However, the relationship between therapeutic
dose, therapeutic depth and skin burns has not been thoroughly
investigated and requires further study (15).
Cell line SW-1990 was established from a pancreatic
adenocarcinoma of a 56-year-old Caucasian male. It was derived from
a pancreatic adenocarcinoma of ductal origin. Growing tumours
exhibited characteristics of a Grade II adenocarcinoma similar to
that of the original neoplasm. Two types of mouse model of
pancreatic cancer have been used in several studies. One is the
subcutaneous transplantation model and the other is the orthotopic
transplantation model. In our study, HIFU was used to treat
subcutaneously implanted tumours. This mouse model did not exhibit
metastatic disease. However, if the tumour grew large enough, it
would indicate metastatic disease in the lung and/or liver. The
model did not simulate the situation and some clinical settings of
human pancreatic cancer. Therefore, the use of the orthotopic
transplantation mode should be investigated in future studies
(9).
Limitations of this study also included the small
number of samples and the relatively limited length of follow-up
monitoring. These limitations are to be addressed in future
studies. In the clinical setting, the application of HIFU for the
treatment of pancreatic cancer is likely to be influenced by
additional factors, such as the degree of penetration of the
abdominal wall and the possibility of interference by abdominal
gas. Further basic and clinical research is required before HIFU
becomes a safe and effective clinical therapy for pancreatic cancer
tumour. However, the results of this study indicate that HIFU holds
great promise as an alternative noninvasive method of treatment for
pancreatic cancer (16,17).
Acknowledgements
This study was financially supported
by the SJTU Medicine Engineering Interdisciplinary Research Fund
(YG2010MS39) and Shanghai Municipal Natural Science Foundation
(12ZR1422600).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics. CA Cancer J Clin. 59:225–249. 2009.
|
|
2
|
DeVita VT, Hellman S and Rosenberg SA:
Cancer: Principles and Practice of Oncology. 6th edition.
Lippincott Williams & Wilkins; Philadelphia, PA: pp. 99–104.
2001
|
|
3
|
Gastrointestinal Tumor Study Group:
Multi-institutional comparative trial of radiation therapy alone
and in combination with 5-fluorouracil for locally unresectable
pancreatic carcinoma. Ann Surg. 189:205–208. 1979.
|
|
4
|
Whittington R, Neuberg D, Tester WJ,
Benson AB III and Haller DG: Protracted intravenous fluorouracil
infusion with radiation therapy in the management of localized
pancreaticobiliary carcinoma: a phase I Eastern Cooperative
Oncology Group trial. J Clin Oncol. 13:227–232. 1995.
|
|
5
|
Van Leenders GJ, Beerlage HP, Ruijter ET,
de la Rosette JJ and van de Kaa CA: Histopathological changes
associated with high intensity focused ultrasound (HIFU) treatment
for localised adenocarcinoma of the prostate. J Clin Pathol.
53:391–394. 2000.PubMed/NCBI
|
|
6
|
Kennedy JE: High-intensity focused
ultrasound in the treatment of solid tumours. Nat Rev Cancer.
5:321–327. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu F, Wang ZB, Zhu H, Zou JZ, Bai J, Li
KQ, Jin CB, Xie FL and Su HB: Feasibility of US-guided
high-intensity focused ultrasound treatment in patients with
advanced pancreatic cancer: initial experience. Radiology.
236:1034–1040. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang RS, Liu LX, Gu YH, Lin QF, Guo RH and
Shu YQ: The effect of endostatin and gemcitabine combined with HIFU
on the animal xenograft model of human pancreatic cancer. Biomed
Pharmacother. 64:309–312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kyriazis AP, McCombs WB III, Sandberg AA,
Kyriazis AA, Sloane NH and Lepera R: Establishment and
characterization of human pancreatic adenocarcinoma cell line
SW-1990 in tissue culture and the nude mouse. Cancer Res.
43:4393–4401. 1983.PubMed/NCBI
|
|
10
|
Keshavarzi A, Vaezy S, Noble ML, Chi EY,
Walker C, Martin RW and Fujimoto VY: Treatment of uterine
leiomyosarcoma in a xenograft nude mouse model using high intensity
focused ultrasound: a potential treatment modality for recurrent
pelvic disease. Gynecol Oncol. 86:344–350. 2002. View Article : Google Scholar
|
|
11
|
Wu R, Hu B, Jiang LX, Hung Y and Kuang SL:
High-intensity focused ultrasound in ovarian cancer xenografts. Adv
Ther. 25:810–819. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vaezy S, Fujimoto VY, Walker C, Martin RW,
Chi EY and Crum LA: Treatment of uterine fibroid tumours in a nude
mouse model using high-intensity focused ultrasound. Am J Obstet
Gynecol. 183:6–11. 2000.PubMed/NCBI
|
|
13
|
Khokhlova VA, Bailey MR, Reed JA, Cunitz
BW, Kaczkowski PJ and Crum LA: Effects of nonlinear propagation,
cavitation, and boiling in lesion formation by high intensity
focused ultrasound in a gel phantom. J Acoust Soc Am.
119:1834–1848. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vykhodtseva N, McDannold N, Martin H,
Bronson RT and Hynynen K: Apoptosis in ultrasound produced
threshold lesions in the rabbit brain. Ultrasound Med Biol.
27:111–117. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leslie TA and Kennedy JE: High intensity
focused ultrasound in the treatment of abdominal and gynaecological
diseases. Int J Hyperthermia. 23:173–182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu
H, Li KQ, Xie FL, Jin CB, Su HB and Gao GW: Extracorporeal focused
ultrasound surgery for treatment of human solid carcinomas: early
Chinese clinical experience. Ultrasound Med Biol. 30:245–260. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Furukawa T, Kubota T, Watanabe M, Kitajima
M and Hoffman RM: A novel ‘patient-like’ treatment model of human
pancreatic cancer constructed using orthotopic transplantation of
histologically intact human tumor tissue in nude mice. Cancer Res.
53:3070–3072. 1993.
|