Introduction
Spinal cord injury (SCI) is a severe health problem
worldwide, and it often causes enormous physical and mental pain to
the patient and family (1–3). Normally, the primary injury is the
mechanical impact afflicted directly on the spine, while the
secondary injury to the spinal cord involves a number of
self-destructive processes that occur by a variety of factors based
on disturbances in ionic homeostasis, local edema, focal
hemorrhage, excitotoxicity, presence of free radicals and free
fatty acids (4–6). Previous studies have indicated that
the TUNEL-positive glial cells and neurons within the lesion area
are present at all stages studied between 4 h and 14 days, with a
maximum presence between 8 h to 24 h, and spread to at least a few
millimeters around the center of the lesion (7). However, the exact mechanism of cell
death has not been fully clarified in nervous system injury
(8). Therefore, identifying the
specific molecular pathway mediating the apoptosis after SCI would
be of great value to the patients concerned.
Certain studies have indicated that endoplasmic
reticulum (ER) dysfunction leads to the activation of unfolded
protein response under any destructive stimulus (9,10).
When stress is present, the expression of chaperones is activated,
protein translation is attenuated and ER-associated degradation is
activated (11,12). Eventually, the cell may undergo
apoptosis under a prolonged ER stress environment (13). Growth arrest and DNA damage
153/C/EBP homologous protein (CHOP) is a prominent protein involved
in the pathway of ER stress-mediated cell apoptosis (14,15).
CHOP-mediated ER stress induces cell death and is involved in
several neurodegenerative diseases (16,17).
Therefore, we hypothesized that increasing the expression of CHOP
can prolong injury after SCI.
Materials and methods
Animal model induction
Twenty Sprague-Dawley (SD) male rats (Experimental
Animal Center of Zhejiang University Hangzhou, China) were divided
into two groups at random, the sham-operated group and the SCI
group. Ten rats were assigned to the SCI group and spinal cord
contusion injuries were inflicted by modified Allen’s method (using
a weight of 10 g dropped from a height of 50 mm on the exposed
spinal cord to produce a moderate contusion) after the T10 spinous
process and the corresponding vertebral lamina were removed. The
remaining ten rats received only T10 laminectomies and were not
injured, and served as the sham-operated group. Following SCI, the
rats had their bladders expressed at least twice a day. A test of
the locomotor activities of both groups was carried out using an
open-field locomotor scale, described by Basso, Beattie and
Bresnahan (BBB) from complete paralysis (score 0) to normal
locomotion (score 21) 12 h after SCI (18). All procedures were carried out
according to the National Institutes of Health Guide for the Care
and Use of Laboratory Animals (NIH Publications). Furthermore, all
efforts were made to minimize the number of animals used and their
suffering.
Histological staining, TUNEL staining and
immunohistochemistry
Five rats per group were anaesthetized 12 h after
SCI by intraperitoneal injection of a lethal dose of Nembutal. The
animals were perfused with 100 ml of normal saline and 250 ml of 4%
formaldehyde by aortic cannulation for 20–30 min. The injured
spinal cords of each rat were embedded in paraffin, and transverse
paraffin sections (8-μm thick) were mounted on silane-coated
slides.
TUNEL staining was performed to visualize apoptotic
cells in the injured spinal cord according to the manufacturer’s
instructions. The cells labeled with trypan blue were counted under
a microscope.
For immunohistochemistry, the sections were washed
in 0.01 M PBS containing 0.3% Triton X-100 (pH 7.4, PBS-T),
immersed in 2% normal horse serum in PBS for 120 min at 37°C,
incubated overnight at 4°C with polyclone CHOP antibody (1:100,
Santa Cruz Biotechnology, Santa Cruz, CA, USA) in PBS containing 1%
bovine serum albumin and washed in PBS (3×5 min). The sections were
incubated in biotinylated IgG (1:200; Boster Biotechnology, Wuhan,
China) in PBS for 2 h at room temperature and washed in PBS-T (3×5
min). The sections were then incubated in avidin-biotin-peroxidase
complex solution (1:100; Boster Biotechnology) for 2 h at room
temperature and then rinsed in PBS-T (3×5 min). Visualization was
achieved by incubating the tissue for 10 min in 0.04%
3-diaminobenzidine containing 0.01% H2O2. Rat
immunoglobulin G (IgG) (1:200; Biomeda Corp., Foster City, CA, USA)
was used instead of a primary antibody as the negative control.
Quantitative real-time reverse
transcription-polymerase chain reaction (qRT-PCR) analysis
The remaining five rats per group were
re-anesthetized and decapitated 12 h after SCI. Total RNA of the
lesion epicenter around the segment at T8 was obtained using the
RNA extraction kit (Qiagen, Hilden, Germany) following the
manufacturer’s instructions. For reverse transcription, RNA
concentration was measured spectrophotometrically and 2 mg total
RNA was added to the cDNA synthesis reaction system (20 ml). The
reaction mixture consisted of 4 μl 5X RT buffer, 2.5
μmol/l oligo(dT), 5 mmol/l deoxyribo-nucleotide
triphosphates and 20 U RNAasin (RNase inhibitor). The hexamers were
annealed by incubating the samples at 70°C for 5 min. M-MLV reverse
transcriptase of 200 U was added and then incubated at 42°C for 60
min and 72°C for 10 min. For qRT-PCR analysis, the reaction mixture
(40 ml) consisted of 4 ml cDNA, 35.2 μl SYBR-Green PCR mix,
0.5 μl 5 U Taq DNA polymerase and 0.3 μl 20 pmol/ml
CHOP primer. The cDNA was denatured to 94°C for 3 min. The template
was amplified for 40 cycles (denaturation at 94°C for 10 sec,
annealing at 57°C for 30 sec and extension at 72°C for 30 sec),
before collecting fluorescence at 72°C. Primers were used for the
housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase
(GAPDH), in qRT-PCR to amplify GAPDH (forward,
5′-GGTGGACCTCATGGCCTACAT-3′; reverse, 5′-GCCTCT
CTCTTGCTCTCAGTATCCT-3′), as an internal control, and CHOP (forward,
5′-CGGAGTGTACCCAGCACCATCA-3′; reverse,
5′-CCCTCTCCTTTGGTCTACCCTCA-3′).
Statistical analysis
The sections were examined at ×400 magnification
with UTHSCSA Image Tools 3.0 (University of Texas Medical School,
San Antonio, TX, USA). The number of the positive cells was
determined. Values are expressed as the means ± SD. The significant
difference was calculated by a two-tailed Student’s t-test.
P<0.05 was considered to indicate a statistically significant
result. The expression of mRNA were calculated by fold change and
estimated using the comparative CT method (2−ΔΔCT)
normalizing to GAPDH CT values and relative to control samples.
Results
Successful induction of SCI animal
model
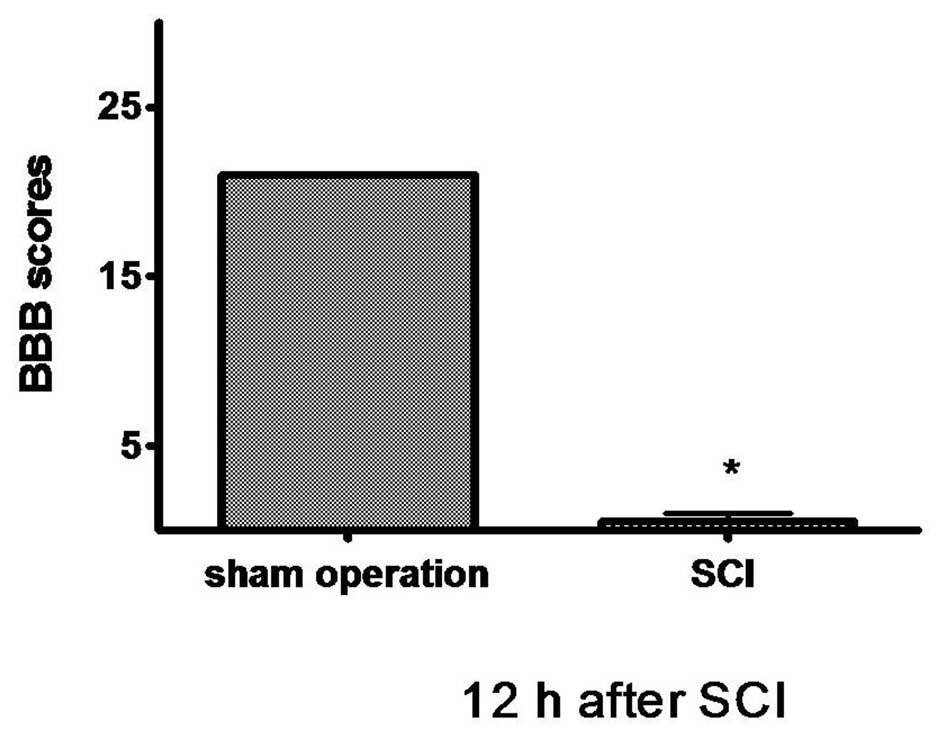
The animals in the SCI group demonstrated extreme
and bilateral hind limb paralysis with no movement (score 0) or
only slight movements of a joint (score 1) from the first hours
post-injury when observed during open-field experimentation. The
sham-operated group animals walked normally after recovery from the
anesthesia. We scored animal locomotor activity according to the
BBB scale 12 h after SCI, and the BBB score revealed that rats with
SCI could no longer move (score 0–1) while the sham-operated rats
walked normally (score 21). There were significant differences
between both groups (P<0.01; Fig.
1).
Pathological changes and TUNEL assay of
injured spinal cord
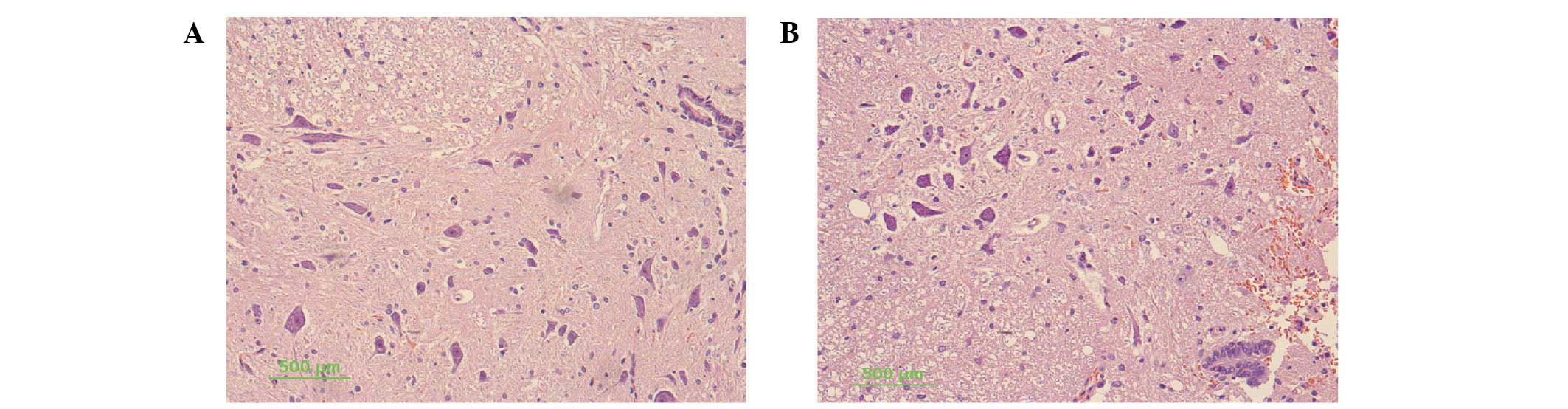
For histological evaluation, transverse sections of
injured spinal cords were examined. Blood cells, dead neurons and
an increased number of gliocytes were observed 12 h after injury in
the injured spinal cord. The lesion center was characterized by the
destruction of gray and white matter. A large cavity involving the
dorsal and partially the lateral funiculus extended for at least 2
mm while the tissue was integrated. The neurons in the spinal cord
of the sham-operated group had normal morphology, with a clear
cytoplasm, and uniform and clear nuclei (Fig. 2).
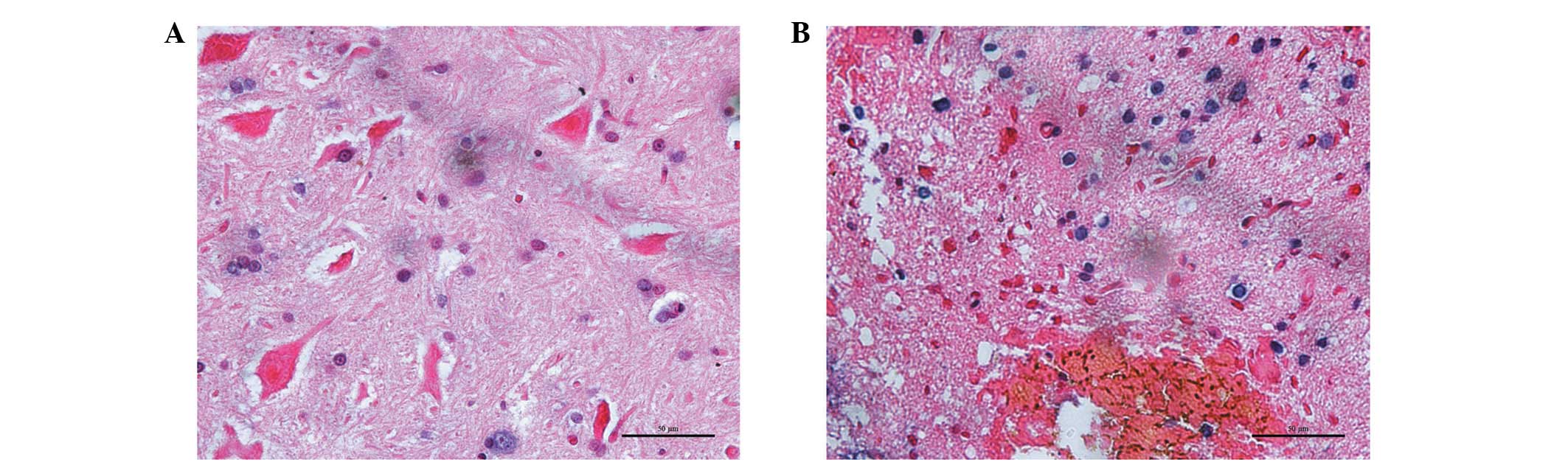
A few TUNEL-positive trypan blue cells were observed
12 h after injury in the sham-operated rats. Numerous
TUNEL-positive trypan blue cells were present in the gray matter,
confined to the lesion area surrounding the cavity. Morphological
analysis indicated that a number of these TUNEL-positive cells were
neurons and others were glial cells. These TUNEL-positive neurons
typically exhibited a reduction of the cytoplasm and nucleus,
creating pericellular space and nuclear fragmentation. Apoptotic
changes in presumptive glial cells were also observed after injury.
The majority of these TUNEL-positive glial cells were located
within the lesion area, although several were present in the
neighboring white matter. The cells typically exhibited small,
fragmented nuclei with scarce visible cytoplasm surrounding them
(Fig. 3). The percentage of
TUNEL-positive cells within the lesion area in the rats with SCI
was increased by 72.3±12.6% while compared with the normal rats
3.2±1.5% (P<0.01).
Expression of CHOP in the spinal
cord
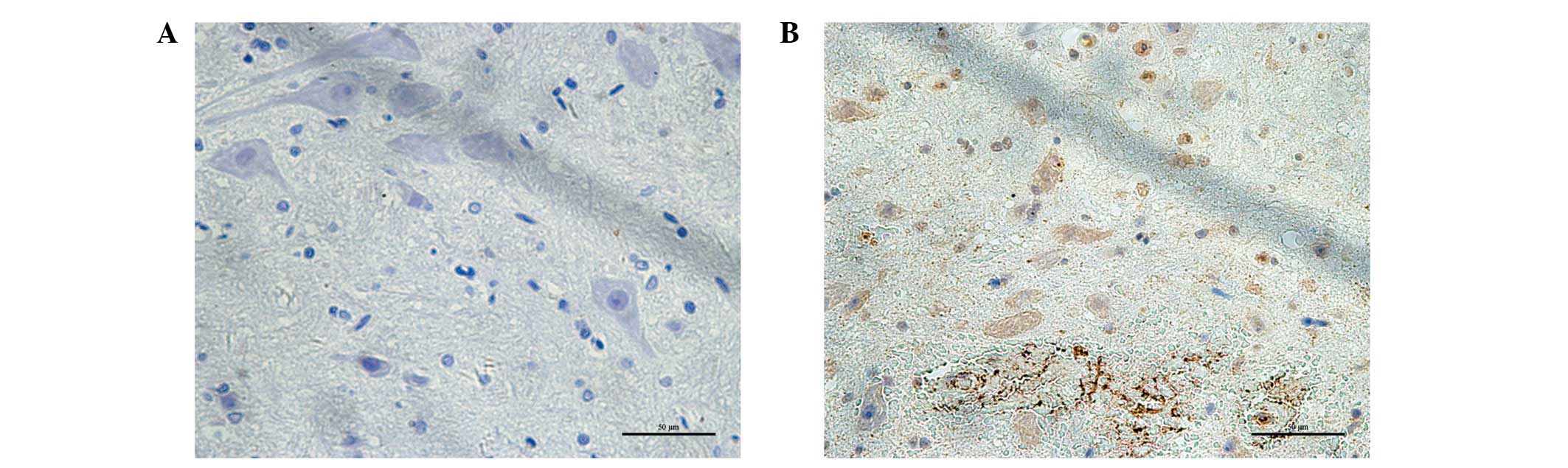
The immunoreactivity of the CHOP protein was
visualized as a granular immunostaining pattern. CHOP protein was
expressed predominantly in the nucleus. Compared with the control
group, CHOP-positive neurons were significantly increased in the
injured spinal cord (Fig. 4).
Quantitative analysis of the number and optical density of
CHOP-positive neurons was increased compared with the sham-operated
rats (P<0.01; Table I). The
levels of CHOP mRNA in the injured spinal cord of rats with SCI
were also increased 3.3±0.17 fold compared with the sham-operated
group by qRT-PCR assay (P<0.01).
 | Table ICHOP-positive cells in the spinal cord
of the two groups. |
Table I
CHOP-positive cells in the spinal cord
of the two groups.
| CHOP-positive neurons
|
|---|
| Group | Number
(/mm2) | Optical density |
|---|
| Sham-operated
group | 1.3±0.7 | 132.5±18.8 |
| SCI group | 8.9±2.8a | 186.2±21.6a |
Discussion
Previous studies have demonstrated that SCI is a
process involving various self-destructive processes that occur by
a variety of factors based on disturbances in ionic homeostasis,
local edema, focal hemorrhage, excitotoxicity, presence of free
radicals and free fatty acids (5,6).
Certain studies have indicated that neuronal and glial cell
apoptosis plays a role in SCI and that the inhibition of neuronal
and oligodendroglial apoptosis may be a therapeutic strategy
(19,20). The results from the present study
demonstrated that the pathological changes of the neurons and
TUNEL-positive cells increased in the injured spinal cord, which
led to neuronal and glial cell loss, and finally induced the
impairment of locomotor activity according to the BBB scale.
Two major pathways are involved in apoptosis
induction: the extrinsic pathway which is activated by the plasma
membrane death receptor ligation, and the intrinsic pathway which
is activated by the mitochondria and other organelles, including
the ER, Golgi apparatus and lysosomes (21–23).
Experimental results provide evidence that the ER is the site of
complex processes, including calcium storage, synthesis and folding
of proteins and cell response to stress. ER function is impaired in
numerous acute and chronic diseases of the brain, which in turn
induce calcium store depletion and conserved stress responses
(24). ER stress is present in
physiological and pathological conditions, cellular injuries,
tissue ischemia and amyotrophic lateral sclerosis (25–27).
Certain studies, however, have suggested that the ER stress-signal
may have a direct role in promoting cell death in neuronal injury
diseases (26,28). CHOP plays a critical role in ER
stress-induced apoptosis, and it is believed to play a central role
in ER stress-induced cell death (29). CHOP has been implicated in
mediating neurode-generation in animals with Alzheimer’s disease
(30). CHOP activation has been
observed in neurons undergoing apoptosis due to perturbations in ER
calcium levels in an in vivo neurotoxin model of
parkinsonism (17). Our results
suggest a crucial role for CHOP in SCI-induced apoptosis of injured
SCI. SCI may induce ER stress by the generation of free radicals,
breakdown ionic homeostasis and excitotoxicity. If these effects
were not counterbalanced, then the ER would be overwhelmed and
initiate apoptosis as the ultimate ER stress. The present results
provide a comprehensive view of the activation of ER stress
pathways following SCI.
In conclusion, this study demonstrates that SCI may
damage the ultrastructure of the spinal cord and cause locomotor
activity impairment, and that CHOP plays a role in ER
stress-mediated apoptosis in the injured spinal cord. These results
may aid the design of potential therapeutic interventions for
SCI.
Acknowledgements
The study was supported by the Health
Bureau of Zhejiang Province, China (No. 2010KYB121).
References
|
1
|
Lidal IB, Veenstra M, Hjeltnes N and
Biering-Sørensen F: Health-related quality of life in persons with
long-standing spinal cord injury. Spinal Cord. 46:710–715. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Müller R, Peter C, Cieza A and Geyh S: The
role of social support and social skills in people with spinal cord
injury - a systematic review of the literature. Spinal Cord.
50:94–106. 2012.PubMed/NCBI
|
|
3
|
Zou W, Guo Q, Chen C, Yang Y and Wang E:
Intrathecal herpes simplex virus type 1 amplicon vector-mediated
human proenkephalin reduces chronic constriction injury-induced
neuropathic pain in rats. Mol Med Report. 4:529–533. 2011.
|
|
4
|
Liu WM, Wu JY, Li FC and Chen QX: Ion
channel blockers and spinal cord injury. J Neurosci Res.
89:791–801. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dumont RJ, Okonkwo DO, Verma S, et al:
Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin
Neuropharmacol. 24:254–264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park E, Velumian AA and Fehlings MG: The
role of excitotoxicity in secondary mechanisms of spinal cord
injury: a review with an emphasis on the implications for white
matter degeneration. J Neurotrauma. 21:754–774. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu XZ, Xu XM, Hu R, et al: Neuronal and
glial apoptosis after traumatic spinal cord injury. J Neurosci.
17:5395–5406. 1997.PubMed/NCBI
|
|
8
|
Martin LJ: Neuronal cell death in nervous
system development, disease, and injury (Review). Int J Mol Med.
7:455–478. 2001.PubMed/NCBI
|
|
9
|
Brostrom MA and Brostrom CO: Calcium
dynamics and endoplasmic reticular function in the regulation of
protein synthesis: implications for cell growth and adaptability.
Cell Calcium. 34:345–363. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Almeida SF, Fleming JV, Azevedo JE,
Carmo-Fonseca M and de Sousa M: Stimulation of an unfolded protein
response impairs MHC class I expression. J Immunol. 178:3612–3619.
2007.PubMed/NCBI
|
|
11
|
Foufelle F and Ferré P: Unfolded protein
response: its role in physiology and physiopathology. Med Sci
(Paris). 23:291–296. 2007.(In French).
|
|
12
|
Kaneko M and Nomura Y: ER signaling in
unfolded protein response. Life Sci. 74:199–205. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang F, Song W, Brancati G and Segatori L:
Inhibition of endoplasmic reticulum-associated degradation rescues
native folding in loss of function protein misfolding diseases. J
Biol Chem. 286:43454–43464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cox DJ, Strudwick N, Ali AA, Paton AW,
Paton JC and Schröder M: Measuring signaling by the unfolded
protein response. Methods Enzymol. 491:261–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oida Y, Shimazawa M, Imaizumi K and Hara
H: Involvement of endoplasmic reticulum stress in the neuronal
death induced by transient forebrain ischemia in gerbil.
Neuroscience. 151:111–119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Silva RM, Ries V, Oo TF, et al:
CHOP/GADD153 is a mediator of apoptotic death in substantia nigra
dopamine neurons in an in vivo neurotoxin model of parkinsonism. J
Neurochem. 95:974–986. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Basso DM, Beattie MS, Bresnahan JC, et al:
MASCIS evaluation of open field locomotor scores: effects of
experience and teamwork on reliability. Multicenter Animal Spinal
Cord Injury Study. J Neurotrauma. 7:343–359. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dang AB, Tay BK, Kim HT, Nauth A,
Alfonso-Jaume MA and Lovett DH: Inhibition of MMP2/MMP9 after
spinal cord trauma reduces apoptosis. Spine (Phila Pa 1976).
33:E576–E579. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen KB, Uchida K, Nakajima H, et al:
Tumor necrosis factor-alpha antagonist reduces apoptosis of neurons
and oligodendroglia in rat spinal cord injury. Spine (Phila Pa
1976). 36:1350–1358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan J and Yankner BA: Apoptosis in the
nervous system. Nature. 407:802–809. 2000. View Article : Google Scholar
|
|
22
|
Eldadah BA and Faden AI: Caspase pathways,
neuronal apoptosis, and CNS injury. J Neurotrauma. 17:811–829.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferri KF and Kroemer G: Organelle-specific
initiation of cell death pathways. Nat Cell Biol. 3:E255–E263.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lehotský J, Kaplán P, Babusíková E,
Strapková A and Murín R: Molecular pathways of endoplasmic
reticulum dysfunctions: possible cause of cell death in the nervous
system. Physiol Res. 52:269–274. 2003.PubMed/NCBI
|
|
25
|
Sakurai M, Takahashi G, Abe K, Horinouchi
T, Itoyama Y and Tabayashi K: Endoplasmic reticulum stress induced
in motor neurons by transient spinal cord ischemia in rabbits. J
Thorac Cardiovasc Surg. 130:640–645. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ogawa S, Kitao Y and Hori O:
Ischemia-induced neuronal cell death and stress response. Antioxid
Redox Signal. 9:573–587. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nagata T, Ilieva H, Murakami T, et al:
Increased ER stress during motor neuron degeneration in a
transgenic mouse model of amyotrophic lateral sclerosis. Neurol
Res. 29:767–771. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
DeGracia DJ and Montie HL: Cerebral
ischemia and the unfolded protein response. J Neurochem. 91:1–8.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chiribau CB, Gaccioli F, Huang CC, Yuan CL
and Hatzoglou M: Molecular symbiosis of CHOP and C/EBP beta isoform
LIP contributes to endoplasmic reticulum stress-induced apoptosis.
Mol Cell Biol. 30:3722–3731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ling ZQ, Tian Q, Wang L, et al: Constant
illumination induces Alzheimer-like damages with endoplasmic
reticulum involvement and the protection of melatonin. J Alzheimers
Dis. 16:287–300. 2009.PubMed/NCBI
|