Introduction
Acute lung injury (ALI) and acute respiratory
distress syndrome (ARDS) are common, costly and potentially lethal
diseases with mortality rates of ∼40%. They represent a spectrum of
acute respiratory failure with diffuse, bilateral lung injury and
severe hypoxemia caused by non-cardiogenic pulmonary edema. There
are several clinical disorders associated with the development of
ARDS, including sepsis, pneumonia, aspiration of gastric contents
and major trauma (1). These
pulmonary or extra-pulmonary insults may increase alveolar
epithelial-endothelial permeability, lead to alveoli flooding,
reduce lung compliance and deprive the lungs of adequate quantities
of surfactant. However, no specific pharmacological therapy has
proven effective for ARDS and therapy is mainly supportive with the
use of lung-protective mechanical ventilation, which may limitedly
improve survival but with high mortality (2). The effectiveness of pharmacological
treatments (including corticosteroids, acetylcysteine, alprostadil,
sivelestat sodium and pentoxifylline) is also not satisfactory and
remains controversial (3,4).
Pulmonary surfactant is a lipoprotein complex
consisting of phospholipids (90%) and surfactant-specific proteins
(10%) produced by type II alveolar cells. Surfactant reduces
alveolar surface tension, prevents alveolar collapse and enables
gas exchange and alveolar ventilation at low transpulmonary
pressures (5). One of the
characteristics of ARDS is reduced lung compliance, implicating
dysfunction or deficiency of the endogenous surfactant system.
Bronchoalveolar lavage fluid from patients with ARDS has low
concentrations of phosphatidylcholine, phosphatidylglycerol and
surfactant-specific proteins (6).
Therefore, treatment with an exogenous surfactant that may aid the
restoration or replenishment of the depleted endogenous surfactant
pool may improve ARDS outcome.
Intratracheal administration of exogenous surfactant
is an effective therapy for premature neonates and children with
acute respiratory failure (7).
Exogenous surfactant may improve oxygenation, but it has not been
shown to reduce mortality in adults with ALI/ARDS (8). One previous randomized multicenter
trial failed to demonstrate any improvement in mortality and
oxygenation following the bolus administration of exogenous natural
porcine surfactant to patients with ALI/ARDS (9). In addition, another trial showed that
recombinant surfactant protein C (rSP-C)-based surfactant was of no
clinical benefit to patients with severe direct lung injury
(10). However, a multicenter
study showed that early administration of Surfactant-BL (bovine
lung extract surfactant) led to reduced mortality in cardiac
patients who developed ARDS postoperatively (11).
Since the effectiveness of exogenous surfactant
administration in adults with ARDS remains unclear, in the present
study we performed a meta-analysis to analyze the effects of
exogenous surfactant treatment on 28–30-day mortality to address
this issue.
Materials and methods
Trial identification and search
strategy
We used systematic methods to identify randomized
controlled trials that compared administration of exogenous
pulmonary surfactant with an appropriate control group (standard
therapy or placebo) for adults diagnosed with ARDS. Trials that
reported mortality and/or pulmonary physiological parameters and
that used objective diagnostic criteria of ARDS were included. We
excluded studies reporting only physiological endpoints, studies in
children, abstracts, case reports, editorials, nonhuman studies and
reports not in English.
To identify all the relevant trials, we searched
Medline (1950-July 2011), Embase (1989-July 2011), the Cochrane
Database of Systematic Reviews and the Cochrane Central Register of
Controlled Trials (1994–2011), with a search strategy combining
medical subject headings and key words: <‘adult respiratory
distress syndrome’, ‘acute respiratory distress syndrome’, or
‘ARDS’>; <‘pulmonary surfactant’ or ‘lung surfactant’>;
and <‘adult’>.
Trial selection and quality
assessment
Two reviewers (L.N.Z. and J.P.S.) independently
assessed the eligibility of each study and resolved disagreements
by consensus. Candidate studies for inclusion were obtained and
reviewed in detail as indicated (Fig.
1).
Quality assessment of these studies was performed by
two investigators using a 10-point scoring system modified from a
previous meta-analysis (12). For
each trial, we evaluated the following aspects: methods of
randomization, allocation concealment, blinding, inclusion and
exclusion criteria defined, similar baseline at study entry,
treatment protocol clearly described, co-intervention that may
affect outcome, outcome definition, extent of follow-up described
clearly and intention-to-treat (ITT) analysis.
Data abstract and outcome measures
Two reviewers (L.N.Z. and J.P.S.) independently
abstracted data of relevant outcome measures with a standardized
spreadsheet. Disagreements were resolved by consensus among
authors.
The primary outcome measure was mortality 28–30 days
after randomization. Secondary outcome measures included the
oxygenation index (PaO2:FiO2 ratio), the
number of ventilation-free days, and the mean duration of
ventilation. Finally, we assessed the adverse events, including
hypoxia and hypotension.
Statistical analysis
We conducted a meta-analysis with a fixed-effects
model using Review Manager (RevMan) 5.0 software, unless there was
significant heterogeneity, and considered P≤0.05 (two-sided) to
indicate a statistically significant result. We reported binary
outcomes as risk ratios (RRs) and continuous outcomes as weighted
mean differences. A Z-test was performed to statistically evaluate
the treatment effects in different groups (13). Moreover, we assessed heterogeneity
between studies for each outcome using the I2 measure,
and considered an I2 value >50% to indicate
substantial heterogeneity (14). A
statistical test for funnel plot asymmetry was used to investigate
the publication bias.
Results
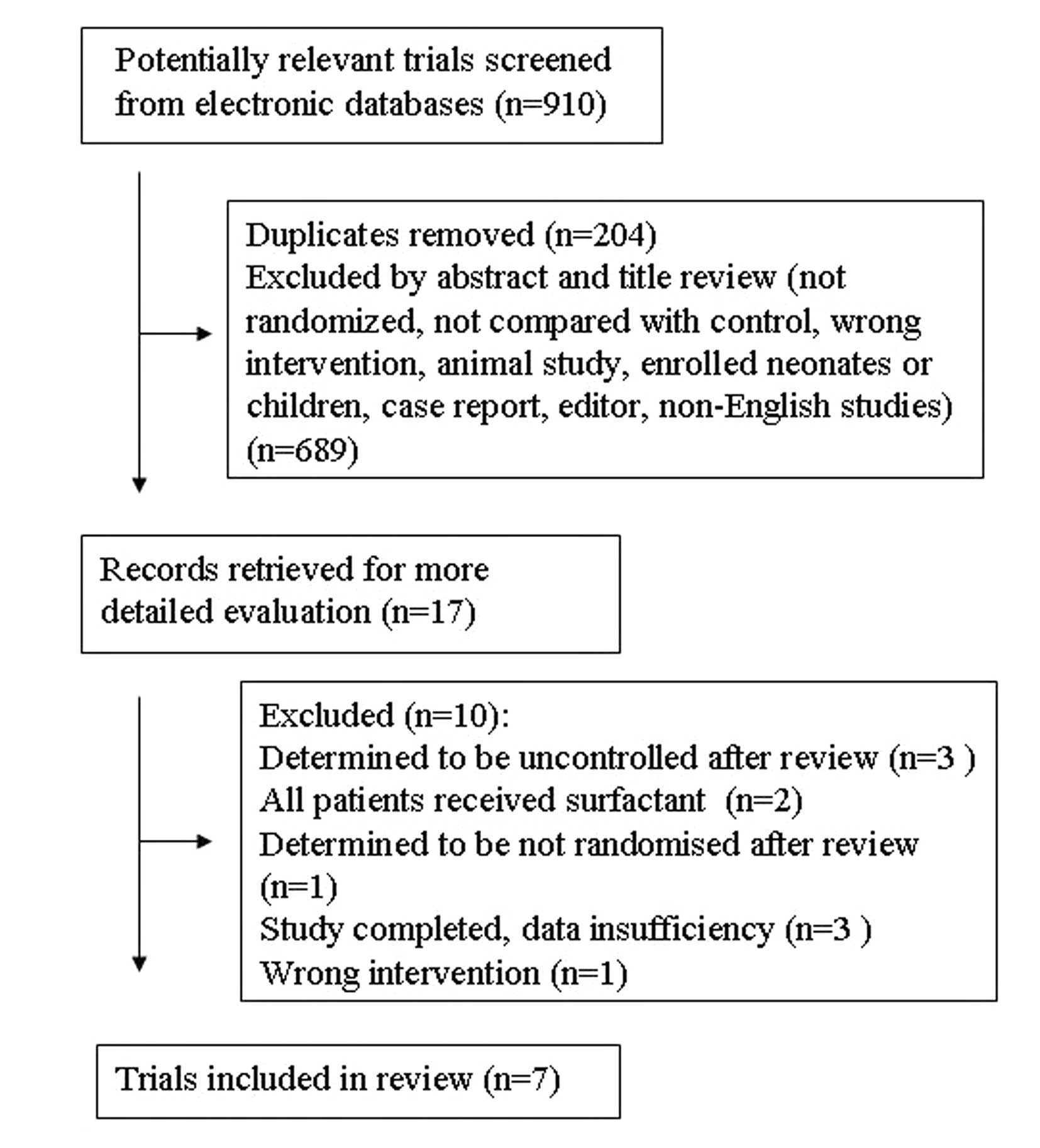
Trial flow
By searching electronic bibliographic databases, we
identified 910 citations, of which 204 were duplicate reports.
Next, we excluded 689 citations with irrelevant titles or abstracts
to obtain 17 studies, of which 7 met the criteria for review after
detailed evaluation (9,10,15–19)
(Fig. 1). Reviewers agreed on all
studies for inclusion.
Study characteristics
The seven included studies published between 1994
and 2011 were multicenter, randomized and controlled trials, in
which 2,144 patients were enrolled. Different types and doses of
surfactant were used in these trials. Two trials used synthetic
surfactant at different doses but without recombinant surfactant
proteins (15,16). One trial used modified natural
bovine surfactant containing surfactant proteins B and C (17). One trial employed modified natural
porcine surfactant containing surfactant proteins B and C (9). Three trials used synthetic surfactant
containing rSP-C at the same dose (10,18,19).
A variety of interventions were used in the control groups,
including standard therapy without placebo, or a placebo of 0.6 or
0.45% saline. Two studies included patients only with
sepsis-related ARDS (15,16). The other studies included patients
with direct lung injury (aspiration of gastric contents and
pneumonia) and indirect lung injury (trauma or surgery, multiple
blood transfusions, burn injury, pancreatitis and toxic injury). A
total of 418 patients with ALI/ARDS were included in the study by
Kesecioglu et al(9), but
only 327 patients (78.2%) had ARDS at baseline. Due to similar
issues in the study by Spragg et al(10), 440 patients without ARDS at
baseline were excluded from these studies. Baseline characteristics
of the patients are presented in Table
I. All trials had high methodological quality and low risk of
bias (Table II).
 | Table IBaseline characteristics of the
eligible trials. |
Table I
Baseline characteristics of the
eligible trials.
| First author, year
(ref.) | No. of patients | Age (years) | Gender (male %) | Type of
surfactant | Surfactant
dosing | Delivery method | Treatment
duration | Initial
PaO2/FiO2 | Initial APACHE II
score | Predisposing
event |
|---|
| Weg 1994 (15) | 51 | C, 51±19 | C, 29 | Exosurf (synthetic no
surfactant protein) | G 1, 21.9 mg
DPPC/kg/day | Aerosolized | Max. | C, 146.5±20.4 | C, 14.2±6.4 | Sepsis |
| C, 17 | G 1, 51±20 | G 1, 41 | G 2, 43.5 mg
DPPC/kg/day | | 120 h | G 1, 124.2±11.8 | G 1, 16.5±6.7 |
| S, 34 | G 2, 51±17 | G 2, 82 | | | | G 2, 161.5±16.2 | G 2, 15.7±6.6 |
| Anzueto 1996
(16) | 725 | C, 53±18 | C, 58 | Exosurf (synthetic no
surfactant protein) | 112 mg
DPPC/kg/day | Aerosolized | Max. | C, 140±64 | Not available | Sepsis |
| C, 361 | S, 50±17 | S, 59 | | | 5 days | S, 145±82 | |
| S, 364 | | | | | | | |
| Gregory 1997
(17) | 59 | C, 40±18.1 | C, 62.5 | Bovine lung extract
(containing SP-B.C) | G 1, 50 mg/kga | Intratracheal | Max. | C, 128 (71–286) | Not available | Trauma, aspiration,
transfusions, sepsis |
| C, 16 | G 1, 39.1±13.2 | G 1, 50 | G 2, 100
mg/kgb | | 96 h | G 1, 98 (84–402) | |
| S, 43 | G 2, 42.7±11.4 | G 2, 75 | G 3, 100
mg/kga | | | G 2, 124
(40–234) | |
| G 3, 42.8±15.4 | G 3, 68.4 | | | | G3, 133 (77–401) | |
| Spragg 2003 (18) | 40 | C, 51±5 | C, 38.4 | Venticute
(rSP-C-based surfactant) | G 1, 1 ml/kgb | Intratracheal | 24 h | C, 120.9±6.5 | C, 10.9±1.1 | Burn, aspiration,
sepsis, pneumonia, trauma, pancreatitis |
| C, 13 | G 1, 59±5 | G 1, 53.3 | G 2, 0.5
ml/kgb | | | G 1, 133.6±8.9 | G 1, 10.2±1.2 |
| S, 27 | G 2, 52±5 | G 2, 33.3 | | | | G 2, 113.9±8.3 | G 2, 10.1±1.7 |
| Spragg 2004
(19)d | 221 | C, 53.1±17.6 | C, 64 | rSP-C-based
surfactant | 1 ml/kgb | Intratracheal | 24 h | C, 130±39 | C, 17.9±6.6 | Trauma, aspiration,
transfusions, sepsis, burn, toxic injury |
| C, 115 | S, 56.5±17.8 | S, 61 | | | | S, 132±40 | S, 18.6±6.1 |
| S, 106 | | | | | | | |
| Spragg 2004
(19)e | 227 | C, 53.0±18.0 | C, 72 | rSP-C-based
surfactant | 1 ml/kgb | Intratracheal | 24 h | C, 136±39 | C, 16.6±5.8 | Trauma, aspiration,
transfusions, sepsis, burn, toxic injury |
| C, 109 | S, 50.6±17.5 | S, 68 | | | | S, 137±40 | S, 17.4±7.5 |
| S, 118 | | | | | | | |
| Kesecioglu 2009
(9) | 327 | C, 57.4±15.7 | C, 65.7 | Natural (porcine)
(containing SP-B.C) | 600 mg/kgc | Intratracheal | 36 h | C, 161.4±55.2 | C, 25.2±7.3 | Sepsis, trauma,
aspiration, shock, pneumonia |
| C, 163 | S, 57.2±15.9 | S, 63 | | | | S, 156.7±54.8 | S, 25.7±8.2 |
| S, 164 | | | | | | | |
| Spragg 2011
(10) | 494 | C, 56.5±0.83 | C, 67.2 | rSP-C based
surfactant | 1 ml/kga | Intratracheal | 96 h | C, 124.1±1.32 | C, 17.8±0.32 | Aspiration,
pneumonia |
| C, 249 | S, 57.5±0.8 | S, 66.1 | | | | S, 123.8±1.3 | S, 18±0.33 |
| S, 245 | | | | | | | |
 | Table IIMethodological quality scores. |
Table II
Methodological quality scores.
| First author, year
(ref.) | Randomization | Allocation
concealment | Blinding | Inclusion and
exclusion criteria defined | Similar baseline at
study entry | Treatment protocol
clearly described | Cointervention that
may affect outcome | Outcome
definition | Extent of follow-up
described clearly | ITT analysis | Final score |
|---|
| Weg 1994 (15) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Anzueto 1996
(16) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Gregory 1997
(17) | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Spragg 2003
(18) | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Spragg 2004
(19) | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Kesecioglu 2009
(9) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Spragg 2011
(10) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
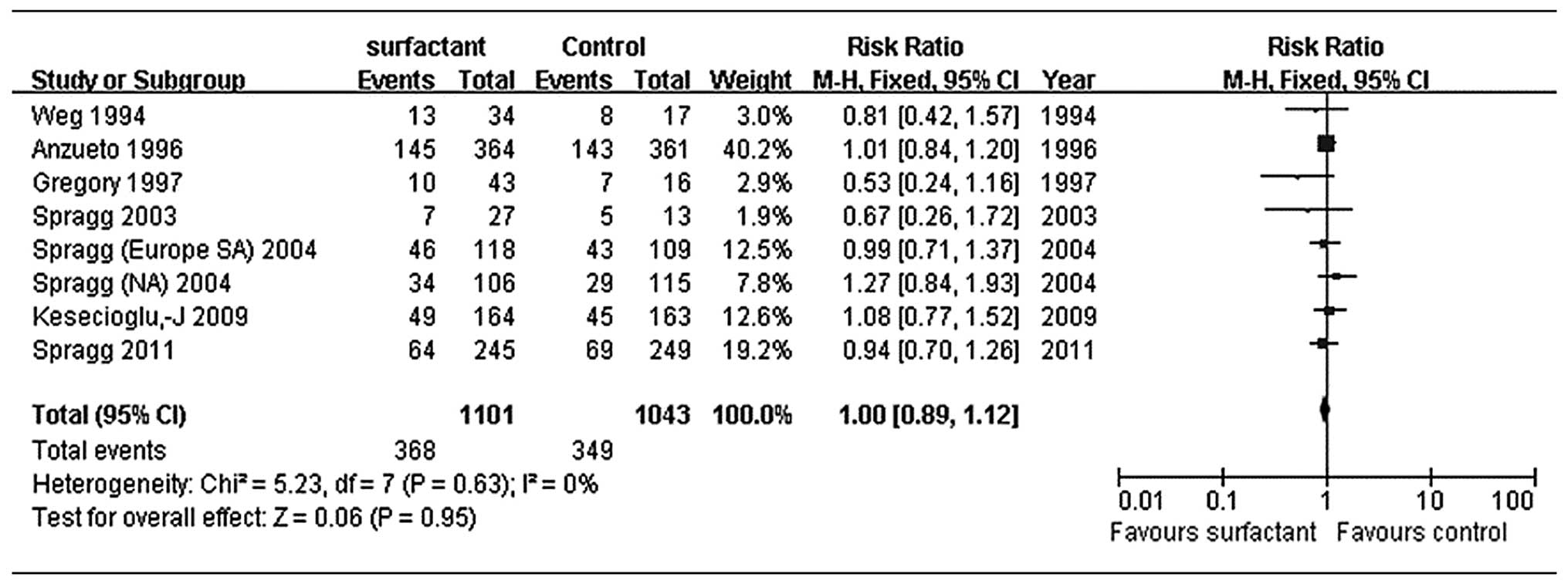
Primary outcome: mortality (28 to 30
days)
According to all the seven trials, the difference in
28–30-day mortality between the surfactant and control groups was
not statistically significant (P=0.95). Treatment with pulmonary
surfactant was not associated with reduced mortality compared with
controls [RR, 1.0; 95% confidence interval (CI), 0.89–1.12;
Fig. 2]. As there was no evidence
of heterogeneity (I2=0%), we assessed the data using a
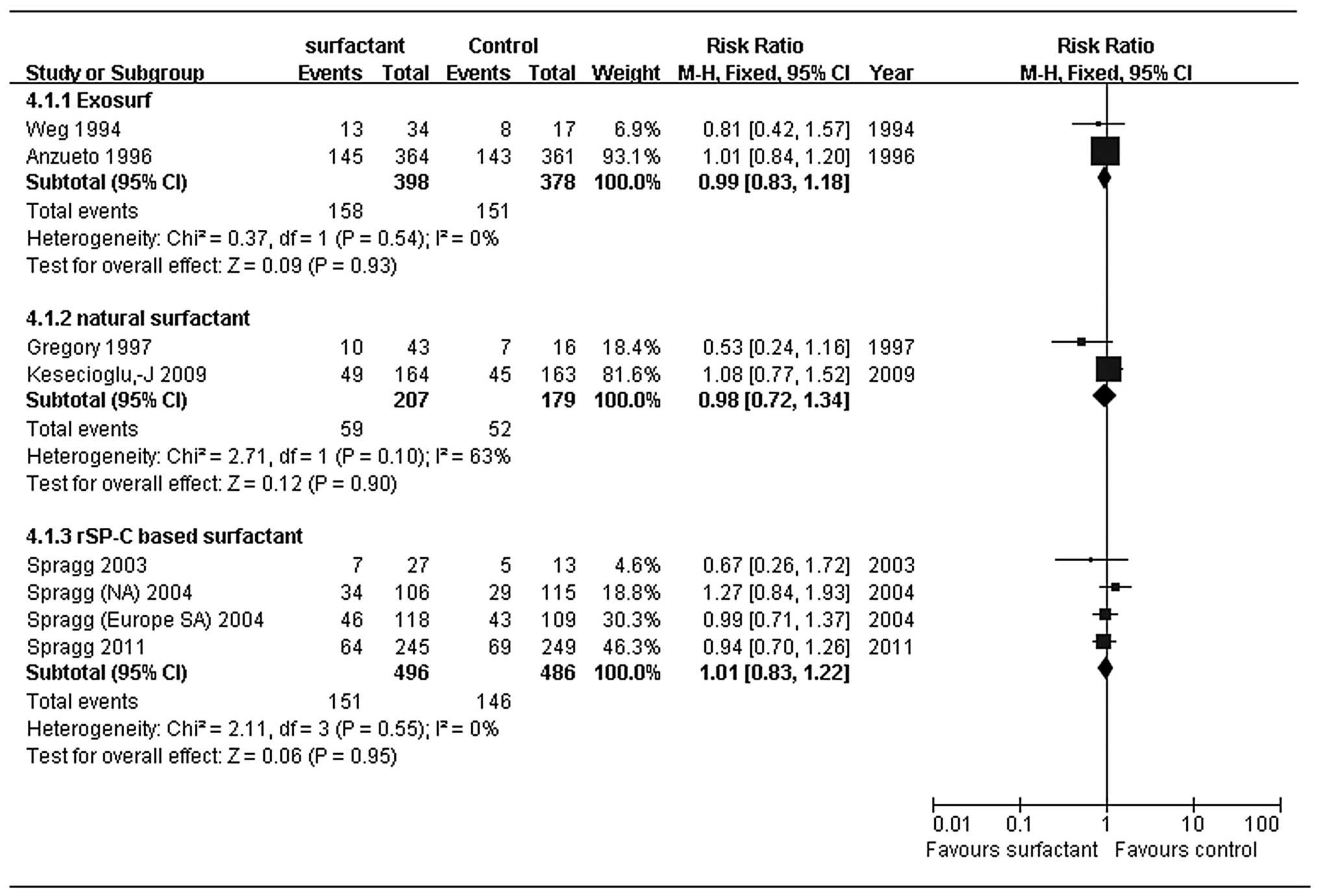
fixed-effects model. In the subgroup analysis, we found no
difference among various types of surfactant: synthetic surfactant
without surfactant protein (exosurf; RR, 0.99; 95% CI, 0.83–1.18),
modified natural surfactant (RR, 0.98; 95% CI, 0.72–1.34) and rSP-C
based surfactant (RR, 1.01; 95% CI, 0.83–1.22) were statistically
indistinguishable (Fig. 3). For
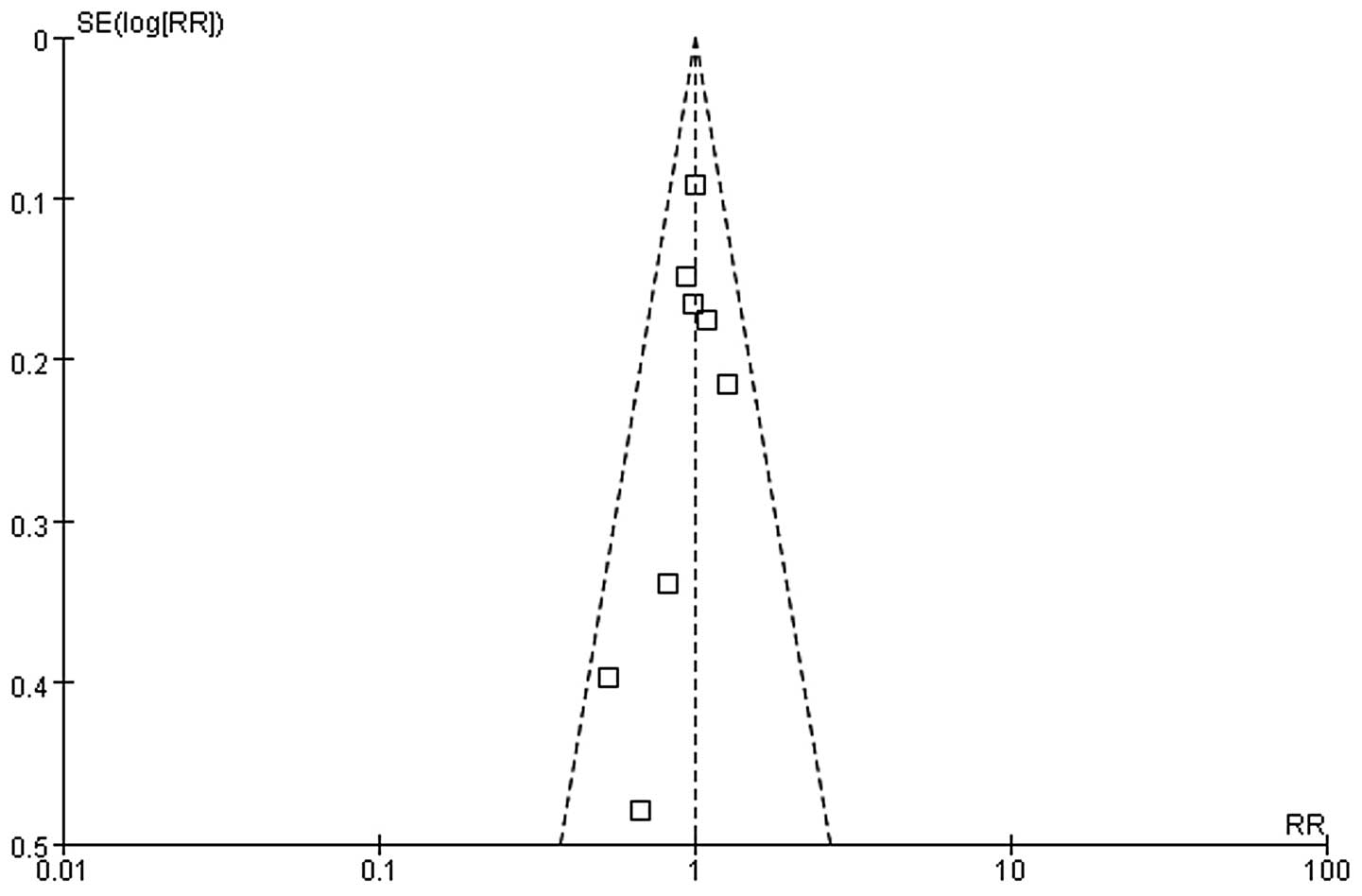
our primary outcome, we found no significant funnel plot asymmetry
based on visual inspection, suggesting no evidence of publication
bias (Fig. 4).
Secondary outcomes
The included trials all showed changes in
PaO2/FiO2 ratio following treatment with
surfactant, although the data forms and monitoring time points were
different. Some data was incomplete and thus difficult to analyze
with combined statistics. Only half of the patients in one trial
showed significant improvements in PaO2/FiO2
ratio at 24 h after surfactant treatment (19), while the other trials showed no
improvement in PaO2/FiO2 ratio. The outcomes
of ventilation-free days, mean duration of ventilation and APACHE
II scores could not be pooled and analyzed due to insufficient
data. Moreover, no trial showed improvement of these indicators in
the surfactant group.
Adverse events
Most trials reported that surfactant therapy was
well tolerated, and no patient was withdrawn from any trial due to
adverse events. Hypotension and hypoxia were the most commonly
reported adverse events in the included studies. Six trials
reported hypoxemia (9,10,16–19)
and four reported hypotension (9,16,17,19).
The following adverse events were reported in only one trial each:
increased secretions (16),
acidemia, air leak, bronchospasm, decreased consciousness, oxygen
desaturation, premature ventricular contractions, body rash, renal
failure, shock (17),
supraventricular tachycardia (18), bradycardia (19) and airway obstruction (10).
Discussion
Although a multitude of causes may lead to ARDS, the
reduction in the amount and function of endogenous surfactant is a
shared characteristic (20).
Exogenous surfactant replacement therapy may help restore or
replenish insufficient endogenous surfactant activity, thereby
improving the ARDS outcome. Our meta-analysis suggested that
administration of exogenous surfactant did not reduce 28–30-day
mortality in adults with ARDS (RR 1.0; 95% CI, 0.89–1.12).
Furthermore, subgroup analysis showed that all preparations of
surfactant similarly failed to reduce mortality. There were
insufficient data available for analysis of changes in oxygenation,
APACHE II scores and ventilation characteristics.
Heterogeneity of the primary outcome of 28–30-day
mortality in our study was low (I2=0%). The surfactants
used in the included trials consisted of synthetic surfactant
without surfactant protein (Exosurf), modified natural surfactants
and rSP-C-based surfactant. These surfactants may have had
different effects on outcomes due to differences in the composition
of phospholipids and surfactant proteins, so we performed subgroup
analysis. Exosurf and rSP-C-based surfactant subgroups exhibited
low heterogeneity (I2=0%), while the modified natural
surfactant subgroup had higher heterogeneity (I2=63%).
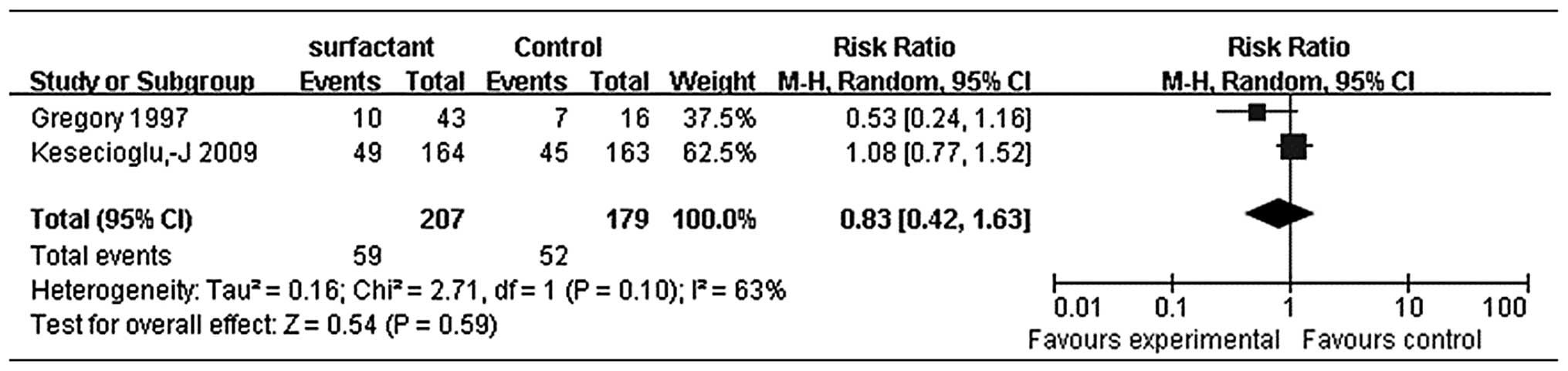
Gregory et al demonstrated that bovine lung extract
surfactant at 100 mg/kg LBW (maximum 4 or 8 doses) improved
survival compared with the control group (17), whereas Kesecioglu et al
found no benefit in outcome with the administration of exogenous
natural porcine surfactant (9).
The differences between these two trials may be caused by the use
of different natural surfactant types. Thus, we applied a
random-effects model (Fig. 5) and
these results did not show significantly reduced mortality (RR,
0.83; 95% CI, 0.42–1.63) either.
Our methods minimized bias by including a
comprehensive search strategy, abstracting data in duplicate, using
a predefined protocol outlining our hypotheses and including
methodological assessment of primary studies and planned
statistical analyses. All included trials had a high methodological
quality with scores between 8 and 10. Funnel plots showed low risk
of bias.
Davidson et al reported that exogenous
surfactant may improve oxygenation, but they detected no
improvement in mortality (8).
Oxygenation outcomes were only included in two trials, both of
which used rSP-C-based surfactant, and did not achieve statistical
significance. We included two additional trials to compare with
their meta-analysis, and generated additional subgroup analyses to
estimate the treatment effects more precisely. Our results were
consistent with theirs and indicated that exogenous pulmonary
surfactant has no significant effect on 28–30-day mortality.
Although the oxygenation outcome could not undergo pooled analysis
due to insufficient data, no improvement in oxygenation was
observed in the newly included trials.
We found that the natural surfactant subgroup had
higher heterogeneity. The trial that used bovine lung extract
surfactant showed significantly decreased risk of 28–30-day
mortality (17). One meta-analysis
demonstrated that bovine lung extract surfactant may significantly
decrease mortality in children with acute respiratory failure
(7). A multicenter study that used
a historical control group for comparison showed that early
administration of Surfactant-BL (bovine lung extract surfactant)
leads to reduced mortality and marked improvement in oxygenation at
24 h in cardiac patients who develop postoperative ARDS (11). However, trials in adults indicated
that surfactant is not effective in decreasing mortality. This is
probably due to differences in the etiologies of lung injury in
adults and children, design features of different trials, the mode
and timing of surfactant administration or the type and dose of
surfactant used (7). Therefore,
further randomized controlled studies of bovine lung extract
surfactant in treatment for adults with ARDS are required.
Our meta-analysis suggests that exogenous surfactant
does not reduce 28–30-day mortality in the treatment of adults with
ARDS. Nevertheless, we are unable to accurately define the effects
of exogenous surfactant on oxygenation from the included studies.
Therefore, clinicians who employ exogenous surfactant to treat
adult patients with ARDS should use it cautiously.
References
|
1
|
Ware LB and Matthay MA: The acute
respiratory distress syndrome. N Engl J Med. 342:1334–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Villar J, Kacmarek RM, Perez-Mendez L and
Aquierre-Jaime A: A high positive end-expiratory pressure, low
tidal volume ventilatory strategy improves outcome in persistent
acute respiratory distress syndrome: a randomized, controlled
trial. Crit Care Med. 34:1311–1318. 2000.
|
|
3
|
Adhikari N, Burns KE and Meade MO:
Pharmacologic treatments for acute respiratory distress syndrome
and acute lung injury: systematic review and meta-analysis. Treat
Respir Med. 3:307–328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iwata K, Doi A, Ohji G, et al: Effect of
neutrophil elastase inhibitor (sivelestat sodium) in the treatment
of acute lung injury (ALI) and acute respiratory distress syndrome
(ARDS): a systematic review and meta-analysis. Intern Med.
49:2423–2432. 2010. View Article : Google Scholar
|
|
5
|
Frerking I, Gunther A, Seeger W and Pison
U: Pulmonary surfactant: functions, abnormalities and therapeutic
options. Intensive Care Med. 27:1699–1717. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haitsma JJ, Papadakos PJ and Lachmann B:
Surfactant therapy for acute lung injury/acute respiratory distress
syndrome. Curr Opin Crit Care. 10:18–22. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duffett M, Choong K, Ng V, et al:
Surfactant therapy for acute respiratory failure in children: a
systematic review and meta-analysis. Crit Care. 11:R662007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davidson WJ, Dorscheid D, Spragg R, et al:
Exogenous pulmonary surfactant for the treatment of adult patients
with acute respiratory distress syndrome: results of a
meta-analysis. Crit Care. 10:R412006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kesecioglu J, Beale R, Stewart TE, et al:
Exogenous natural surfactant for treatment of acute lung injury and
the acute respiratory distress syndrome. Am J Respir Crit Care Med.
180:989–994. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spragg RG, Taut FJ, Lewis JF, et al:
Recombinant surfactant protein C-based surfactant for patients with
severe direct lung injury. Am J Respir Crit Care Med.
183:1055–1061. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bautin A, Khubulava G, Kozlov I, et al:
Surfactant therapy for patients with ARDS after cardiac surgery. J
Liposome Res. 16:265–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peter JV, John P, Graham PL, et al:
Corticosteroids in the prevention and treatment of acute
respiratory distress syndrome (ARDS) in adults: meta-analysis. BMJ.
336:1006–1009. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fleiss JL: The statistical basis of
meta-analysis. Stat Methods Med Res. 2:121–145. 1993. View Article : Google Scholar
|
|
14
|
Higgins JPT and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weg JG, Balk RA, Tharratt RS, et al:
Safety and potential efficacy of an aerosolized surfactant in human
sepsis-induced adult respiratory distress syndrome. JAMA.
272:1433–1438. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Anzueto A, Baughman RP, Guntupalli KK, et
al: Aerosolized surfactant in adults with sepsis-induced acute
respiratory distress syndrome. Exosurf Acute Respiratory Distress
Syndrome Sepsis Study Group. N Engl J Med. 334:1417–1421. 1996.
View Article : Google Scholar
|
|
17
|
Gregory TJ, Steinberg KP, Spragg R, et al:
Bovine surfactant therapy for patients with acute respiratory
distress syndrome. Am J Respir Crit Care Med. 155:1309–1315. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spragg RG, Lewis JF, Wurst W, et al:
Treatment of acute respiratory distress syndrome with recombinant
surfactant protein C surfactant. Am J Respir Crit Care Med.
167:1562–1566. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Spragg RG, Lewis JF, Walmrath HD, et al:
Effect of recombinant surfactant protein C-based surfactant on the
acute respiratory distress syndrome. N Engl J Med. 351:884–892.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsangaris I, Lekka ME, Kitsiouli E, et al:
Bronchoalveolar lavage alterations during prolonged ventilation of
patients without acute lung injury. Eur Respir J. 21:495–501. 2003.
View Article : Google Scholar : PubMed/NCBI
|