Introduction
Osteomyelitis is an inoculation of bacteria into
bone as a result of hematogenous seeding, surgical contamination,
spread of infection from an adjacent area, trauma coinciding with
contamination or injury to a limb without the protective soft
tissue envelope (1). The
implantation of foreign bodies increases the likelihood of
infection. In this disease, bone is colonized with microorganisms,
with associated inflammation and bone destruction (2). Chronic infection is difficult to
eradicate. Aggressive surgical debridement of the infected or
necrotic tissue and extensive bone and soft tissue reconstruction
are usually required to cure the disease (1). Foreign bodies need to be removed in
the majority of cases in order to eradicate infection. A small
proportion of seriously ill patients require amputation. Besides
surgical treatment, intravenous antibiotics for extended periods of
time is another form of treatment for osteomyelitis. However, due
to the locally compromised blood supply, a therapeutic level of
antibiotics is rarely achieved (1,3).
The gram-positive organism Staphylococcus
aureus (S. aureus) is the principle causative agent of
osteomyelitis, accounting for 80% of all human cases (4,5).
Adhesion molecules of S. aureus facilitate its binding to
the bone matrix. Toxin secretion such as interleukin (IL)-1, IL-6
and tumor necrosis factor (TNF)-α may produce S.
aureus-induced osteomyelitis and stimulate bone resorption
(6). The bacterial biofilm is
considered to be the main reason for refractoriness of
osteomyelitis. However, previous research demonstrated that S.
aureus not only colonized bone matrix, but also invaded
osteoblasts (2,5,7–9).
This phenomenon has been demonstrated in vitro and in
vivo(10). The capability of
S. aureus to invade and survive within osteoblasts may be an
important reason as to why chronic osteomyelitis is difficult to be
eradicate. S. aureus internalized in osteoblasts avoids
immune responses, including engulfment by phagocytes, as well as
the action of many forms of antibiotics. S. aureus survived
in the intracellular environment of osteoblasts and maintained the
vitality to invade other osteoblasts (1,11).
This may explain the recurrence and chronic course of this disease
(3).
Numerous types of antibiotics have been employed in
the treatment of osteomyelitis, including gentamicin,
cephalosporins, vancomycin, clindamycin and rifampin. However, the
majority of these antibiotics cannot penetrate eukaryotic cells.
Rifampin is a hydrophobic antibiotic and is able to penetrate cell
membranes and enter osteoblasts. A study in vitro
demonstrated its bactericidal effect in the intracellular
environment (3). In clinical
practice, rifampin also demonstrates satisfactory results in the
treatment of osteomyelitis, particularly in prosthesis infections
(12–15). Other antibiotics capable of
penetrating eukaryotic cells, including clindamycin, also have
antibacterial effects in the intracellular environment (3).
In order to kill bacteria internalized in the
osteoblasts, it is necessary to increase the intracellular
concentration of antibiotics. The hydrophobic properties of the
drugs and the permeability of cell membranes are two important
factors when determining the concentration of a drug in the
intracellular environment. The aim of the present study was to
improve the permeability of the osteoblast membrane in order to
increase the concentration of antibiotics to kill S. aureus
in the intracellular environment.
The application of ultrasound as an adjunct has
showed satisfactory results in the treatment of tumors, infection
and DNA transfection in in vitro and in vivo
research. It increases the permeability of eukaryotic and
prokaryotic cells to drugs, DNA and nanoparticles. Previous
research has documented that ultrasound enhances the activity of a
number of antibiotics against certain bacteria in plankton and in
biofilms (16,17). Additionally, further research
revealed that ultrasound allows antibiotics to transport through
the biofilm more easily and therefore increases the drug
concentration surrounding the bacteria (18). Ultrasound perturbs the bacteria
cell membrane, rendering it more permeable to antibiotics.
Ultrasound-enhanced antibiotic efficacy has also been demonstrated
in planktonic bacteria. However, no research has reported on the
ultrasonic adjuvant effect to bacteria in the intracellular
environment. The present study tests the hypothesis that low
frequency, low-power ultrasound enhances the antibiotic (rifampin)
effect of S. aureus internalized in human osteoblasts
(19).
Materials and methods
Bacterial strains, media and
antibiotics
Cultures of S. aureus (ATCC12598) were grown
on nutrient agar plates. Tryptic soy broth (TSB; Difco, Detroit,
MI, USA) was inoculated with one colony from the plate and a
culture was grown overnight at 37°C with agitation to the
plate.
Cell culture
Normal human osteoblasts (sv 40) were incubated at
37°C under a humidified atmosphere containing 5% CO2 in
Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Beijing,China)
supplemented with 10% (v/v) fetal bovine serum. Once the cells had
reached ∼80% confluence, they were removed from the flasks using
0.025% trypsin and 0.01% ethylenediaminetetraacetic acid (EDTA) in
phosphate-buffered saline (PBS). The growth medium was changed
every 48 h after seeding. The study was approved by the ethics
committee of the Department of Orthopaedics, The Sixth Affiliated
People’s Hospital, Shanghai Jiao Tong University School of
Medicine, (Shanghai, China).
Internalization assay
S. aureus was grown overnight (16 h) in 5 ml
TSB in a water bath at 37°C with agitation. The bacteria were
harvested by centrifugation for 5 min at 5,000 rpm at 4°C and
washed twice in 5 ml PBS. The pellet was then resuspended in 5 ml
osteoblast growing medium (OBGM) without antibiotics. Confluent
cell layers of osteoblasts were washed three times with 5 ml PBS to
remove growth media containing antibiotics. Osteoblasts were then
infected at a multiplicity of infection (MOI) of 200:1 with S.
aureus. After infection for 45 min at 37°C, cell cultures were
washed and then incubated with OBGM containing 100 μg/ml
gentamicin for 4 h to kill the remaining extracellular S.
aureus. Gentamicin cannot penetrate normal eukaryotic cells, so
at this time, only intracellular bacteria remain.
Ultrasound treatment combined with
antibiotic activity
Non-focused ultrasonic transducers were used in this
experiment to deliver the ultrasound. A function generator (XinZhi,
Biotechnology Co., Ningbo, China) created a non-continuous wave at
a frequency of 20 kHz. The osteoblasts were divided into 4 groups
and treated as follows: in group A, osteoblasts with OBGM
containing 30 μg/ml of rifampin were exposed to ultrasound
for 60 cycles of 5 sec ultrasound with 2 sec intervals (200 W). In
group B, osteoblasts were exposed to rifampin without ultrasound.
In group C, the osteoblasts were exposed to ultrasound with OBGM
not containing rifampin and rifampin was added to OBGM 30 min after
exposure to ultrasound. In group D, the osteoblasts were not
exposed to ultrasound.
Assay to determine rifampin antibiotic
activity combined with ultrasound
After rifampin addition and ultrasound intervention
(6 h later), osteoblast cultures were washed with PBS 3 times and
subsequently lysed by the addition of 1 ml 0.2% Triton X-100
(Fisher Biotech, Fair Lawn, NJ, USA), followed by standard serial
dilution, plating on tryptic soy agar (TSA), incubation at 37°C
overnight and enumeration of resulting colony forming units.
Osteoblast viability following ultrasound
exposure (CCK-8)
Human osteoblasts (sv40) were inoculated in a
96-well plate. Once the cells had reached ∼80% confluence, they
were exposed to ultrasound as previously described. The osteoblasts
were then cultured in standard environment for another 24 h. The
Cell Counting Kit-8 (CCK-8; Beyotime, Shanghai, China) was employed
to quantitatively evaluate osteoblast viability (20). Briefly, the culture medium was
removed and the cultures were washed with PBS twice. Serum-free
DMEM (∼100 μl) and CCK-8 (10 μl) solution were added
to each well. Following incubation at 37°C for 2 h, a microplate
reader (BioTek Instruments, Winooski, VT, USA) was employed to
determine the optical density (OD) at 450 nm and compared with the
control group. Three duplicate experiments were performed to assess
the cell viability. The following equation was used: cell viability
= (ODsample / ODcontrol) × 100.
Statistical analysis
Each experiment was performed three to six times and
the quantitative results were expressed as mean ± standard
deviation. Differences between the means were analyzed by using
independent t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Internalization of S. aureus
We quantified the invasion of S. aureus into
osteoblasts using TSA plates. Following an internalization assay,
the osteoblast cultures were washed three times and subsequently
lysed by the addition of 1.2 ml 0.1% Triton X-100. Suspension
dilutions of the lysates were plated in triplicate on TSA plates
followed by incubation at 37°C overnight. Results showed that the
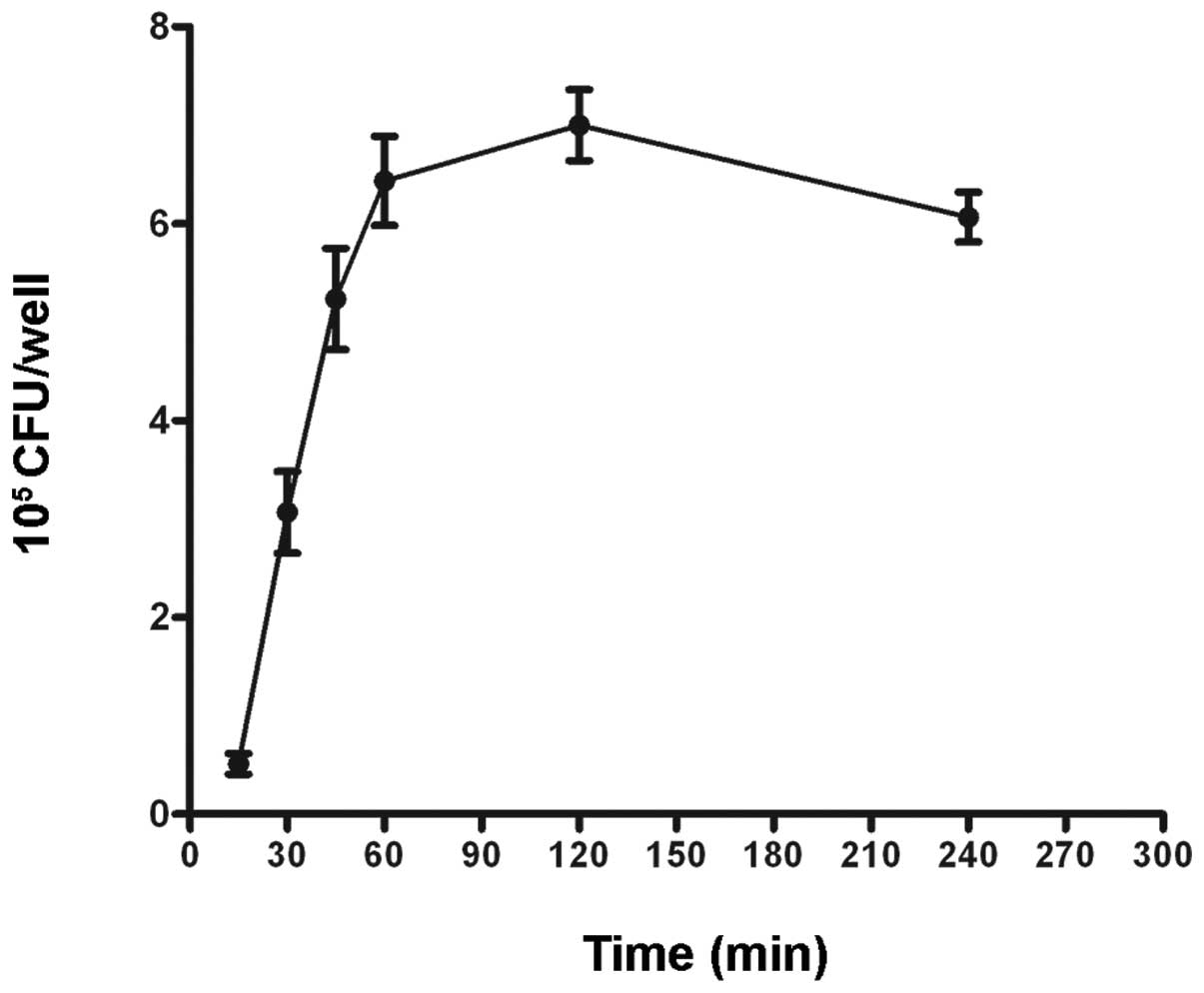
invasion of S. aureus increased in a time-dependent manner
following infection at an MOI of 200 (Fig. 1). Internalization of S.
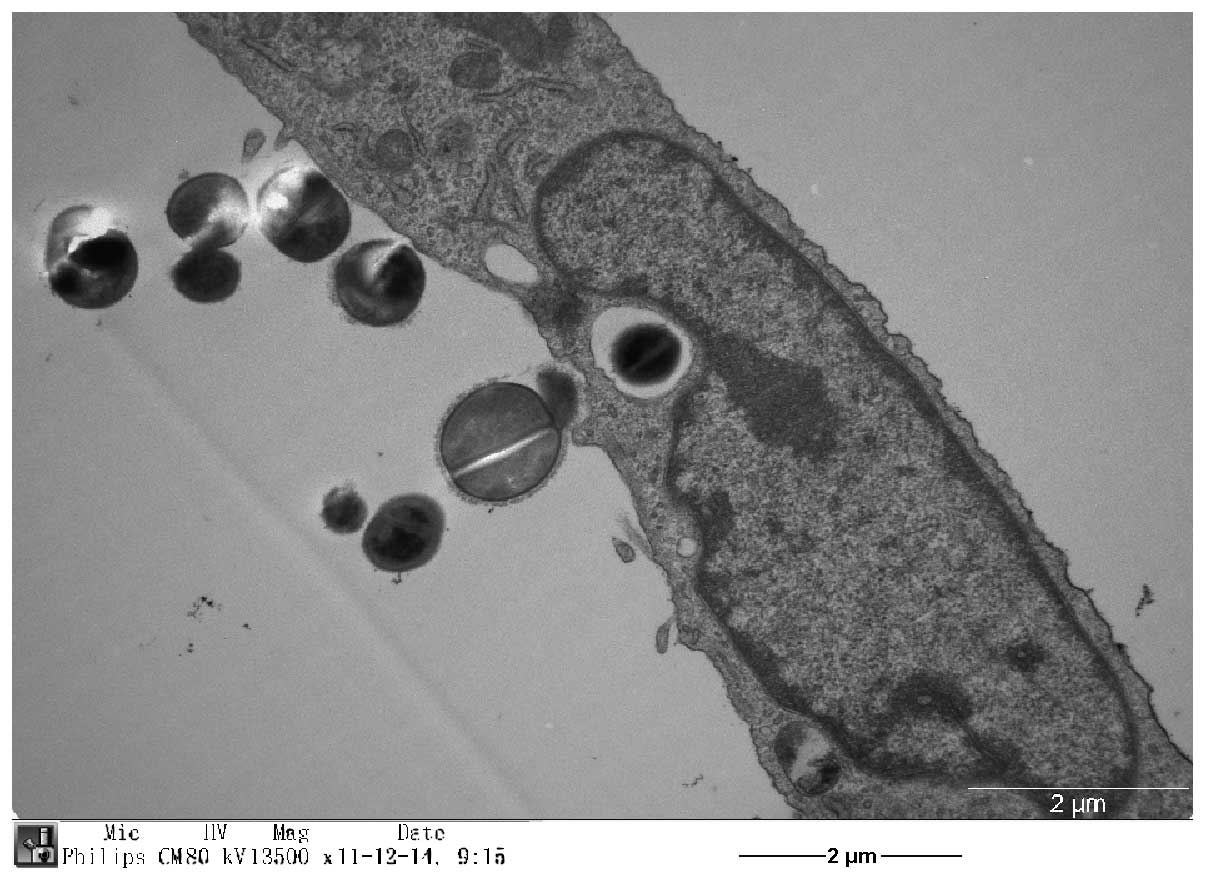
aureus by osteoblasts was confirmed by transmission electron
microscopy (Fig. 2).
Effect of ultrasound combined with
antibiotic inhibition of S. aureus internalized in osteoblasts
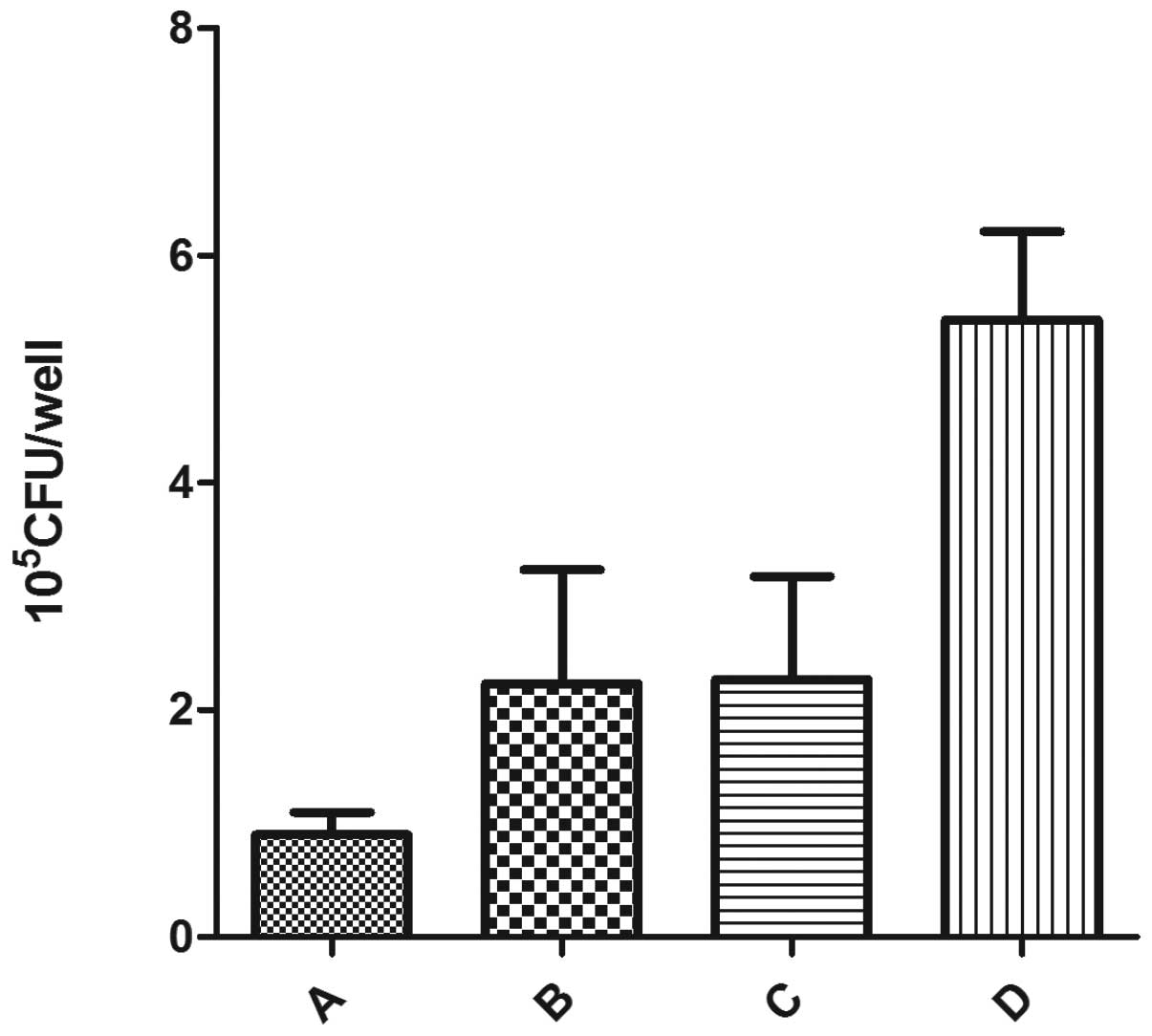
When S. aureus cells were exposed to
antibiotics combined with ultrasound, the number of viable
organisms in the intracellular environment of osteoblasts
decreased. Rifampin, a bactericidal nucleic acid synthesis
inhibitor, is readily able to penetrate eukaryotic cells. The
number of viable S. aureus cells within osteoblasts not
treated with ultrasound was smaller compared with the control
group, but larger compared with that of rifampin combined with
ultrasound. There was no significant difference between group B and
group C. These results suggest that rifampin successfully
penetrates osteoblasts and kills S. aureus internalized in
the osteoblasts, while ultrasound promotes this process (Fig. 3).
Osteoblast viability following ultrasound
exposure
The CCK-8 assay was employed to compare viability of
sv40 osteoblasts following exposure to ultrasound with normal
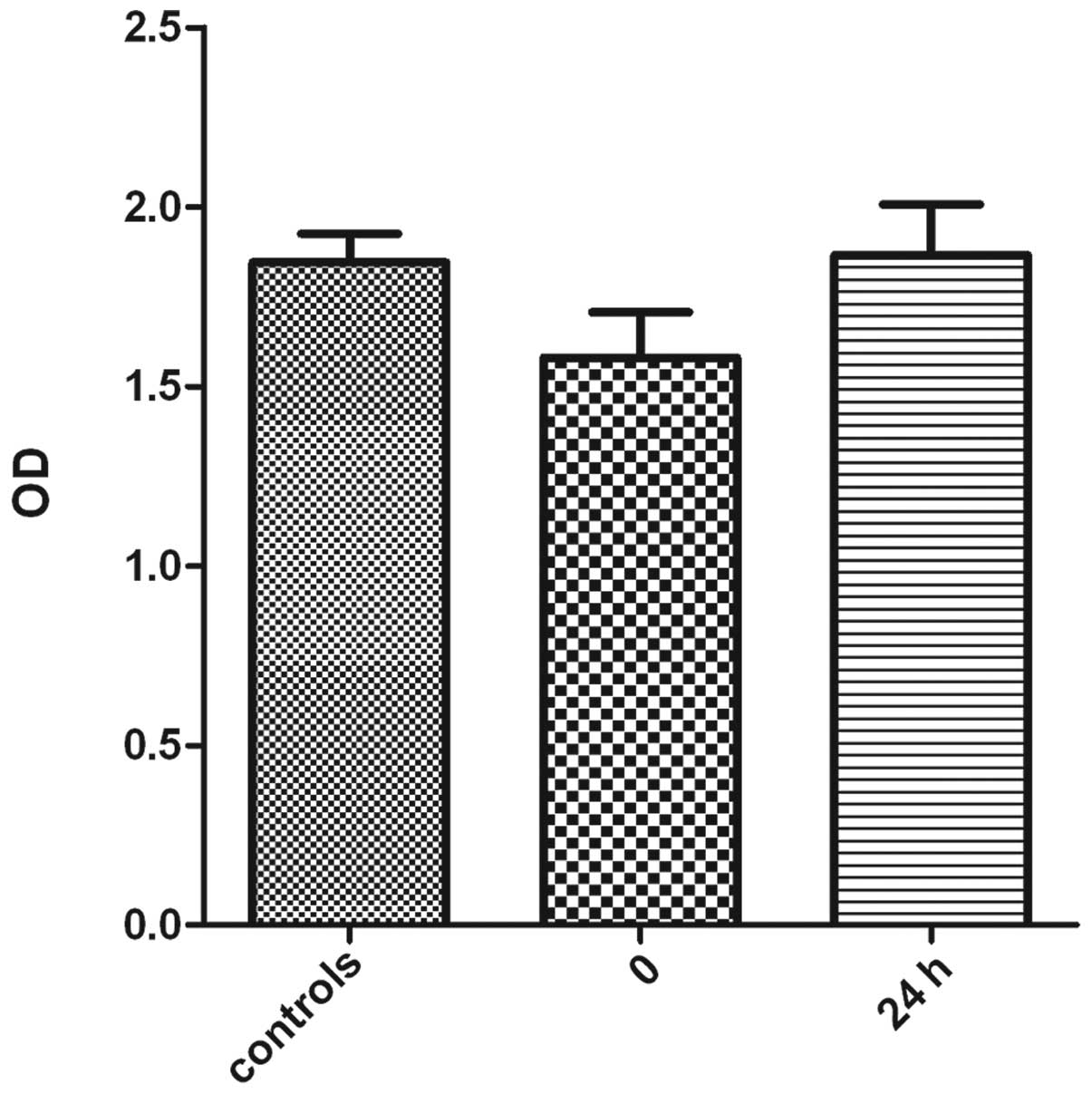
cells. The results revealed a decreased viability of the
osteoblasts immediately after exposure to ultrasound. However, the
viability of osteoblasts exposed to ultrasound increased after
being cultured in the standard atmosphere for 24 h. There was no
significant difference in viability of osteoblasts 24 h after
exposure to ultrasound and the control group (Fig. 4). These results suggest that
low-frequency ultrasound has a negative impact on cell viability of
osteoblast sv40; however, this damage is slight and reversible.
Discussion
Ultrasound has been used in a number of applications
in medicine and research (16,18,21,22).
Previous research has shown that low-frequency ultrasound, when
combined with antibiotics, significantly enhances the bactericidal
action of antibiotics (16,22,23).
Previous research has shown that ultrasound effectively enhances
the antibacterial effect of certain antibiotics against a culture
of bacteria in in vitro and in vivo biofilms
(17,23). Ultrasound increases the transport
of antibiotics through biofilms, which is a barrier for antibiotics
to penetrate in order to kill bacteria inside (18). Carmen et al demonstrated
that ultrasonication significantly increases the transport of
gentamicin through the biofilm. Insonation of biofilms for 45 min
more than doubles the gentamicin concentration compared to the
non-insonated counterparts. This enhanced transport may be
partially responsible for the increased killing of biofilm bacteria
exposed to combinations of antibiotics and ultrasound (18). Ultrasound-enhanced antibiotic
efficacy has also be conducted in in vivo research. Carmen
et al investigated the combination of low frequency
ultrasound and vancomycin in treating Staphylococcus
epidermidis infections in a rabbit model and the result showed
enhanced vancomycin activity against S. epidermidis
biofilms. At 48 h of insonation there were significantly fewer
viable bacteria in the insonated biofilm (17).
However, ultrasound-enhanced antibiotic activity in
planktonic cultures with no extensive exopolymer matrix to hinder
antibiotic transport, indicates that there must be other mechanisms
involved in the ultrasound-enhanced antibiotic effect on organisms.
Ultrasound is postulated to increase antibiotic concentration
inside cells by rendering the cell membrane more permeable to the
antibiotic (19). Perturbation
caused by ultrasound results in decreased stability of the
phospholipid bilayer. Rapoport et al observed that the
passage of stearic acid (lipophilic substances) into the outer
lipid bilayer was ultrasonically enhanced in Pseudomonas
aeruginosa(24,25).
A similar reversible destabilization may occur in
eukaryotic cell membranes. Studies have indicated that
low-frequency ultrasound destabilizes lipid layers in the skin,
thus enhancing the permeability of drugs (16,26).
It has also been demonstrated that plasmid DNA was effectively
delivered into Saccharomyces cerevisiae by using
low-frequency ultrasound (16,27).
Our current study indicates that ultrasound may create
perturbations in the outer membrane lipid bilayer of osteoblasts
sufficiently large for the passage of rifampin.
Recently, research into ultrasound-aided cancer
chemotherapy has been conducted to enhance the overall cytotoxic
effect of the therapeutic agent with no apparent hyperthermic event
(28). Sonoporation is an
ultrasound-induced means of increasing the permeability of cell
membranes to promote the passage of chemotherapy agents across the
cell membrane, thereby facilitating entry of the relevant drug into
the target cell (29–31). Our results are consistent with the
previous result. Rifampin is a lipophilic drug and the
ultrasonically-enhanced bactericidal capacity in the intracellular
environment may be due to the increased drug concentration in the
intracellular environment (19).
Sonoporation has shown promising prospects (32). Although the mechanism by which
ultrasound enhances antibiotic action is not fully known, it may be
due to perturbation of the cell membrane or stress responses by the
bacteria (22,33). Sonoporation enhances cell membrane
permeability allowing transfer of macromolecules.
Ultrasound-induced biological effects are commonly considered to be
caused by acoustic cavitation, which may collapse and lead to the
induction of transient holes in the cell membrane. It has been
hypothesized that antibiotics are transferred into cells across the
cell membrane via ultrasound-induced pores. This is a transient
phenomenon and our results also give indirect evidence of this, as
the cell viability decreased immediately after the application of
ultrasound, but recovered 24 h later (19). Ultrasound-induced pores have also
been confirmed by scanning election microscopy (SEM) observations,
although the size distribution could not be defined (32).
Ultrasonic waves pass directly through cells with
little absorption or scattering. The pressure oscillations of
ultrasound produces gas bubbles ranging in size from approximately
1 to 100 μm in diameter in the liquid. The oscillations of
bubbles, also called cavitation, are generally divided into
‘stable’ and ‘collapse’ types. Both types of cavitation are
reported to increase membrane permeability in eukaryotic cells
(19). Stable cavitation is the
low intensity oscillation of the bubbles without complete collapse
and collapse cavitation occurs at higher intensity levels.
There is an intensity threshold for the production
of collapse cavitation and collapse cavitation is absent below this
threshold; however, stable cavitation occurs readily (19,34).
The lower shear forces caused by stable cavitation may also be
sufficiently stressful to perturb the outer membrane (35). Ultrasound has been shown to enhance
fluorescent probe uptake in corneal endothelium and various cancer
cells, as well as allow penetration of Ara-C into HL-60 cells and
allow transfection of eukaryotes with plasmid DNA (19,28,32,36,37).
When these bubbles collapse, they violently accelerate the fluid
around them, producing a high temperature and free radicals as well
as generating high liquid shear force. Cell membranes may become
stressed or damaged by shear force, heat or free radicals (38). Stress and high velocity jet of
liquid toward the cell membrane during bubble expansion may
contribute to the cell membranes damage (19,39).
Although ultrasound is used as a means to lyse
bacteria, the low-frequency and lower intensities of ultrasound did
not directly lyse the bacterial cells or permanently damage the
outer bacterial membrane (19).
Indeed, ultrasound at the intensities used in our research did not
directly reduce viability of bacteria. In the present study, group
C, with ultrasound in the absence of antibiotics, demonstrated no
significant decrease in the number of bacteria (Fig. 3). In fact, in certain situations,
low-frequency ultrasound increased bacteria cell growth (38). Pitt et al used ultrasound to
irradiate bacterial cells (S. epidermidis, P.
aeruginosa and Escherichia coli cells) attached to
polyethylene surfaces. They found that low frequency ultrasound (70
kHz) of low acoustic intensity increased the growth rate of the
cells compared to growth without ultrasound. Ultrasound has also
demonstrated the ability to enhance planktonic growth of S.
epidermidis and other planktonic bacteria. These research
results may be due to an increased rate of transport of oxygen and
nutrients to the cells and waste products away from the cells by
ultrasound (38).
Ultrasound increases the permeability of cells
without killing them, to allow drugs to enter the cells (34). A major challenge to the application
of sonoporation in drug delivery is to optimize the ultrasound
parameters to increase the permeability of cell membranes and to
maintain good cell viability (40). Acoustic cavitation induced by
ultrasound increases cell permeability and facilitates drug
internalization in the cells. However, it may also damage or kill
the cells if the pores induced by cavitation are too large such
that the cell membrane cannot reseal quickly. For large molecules,
this is a more serious problem since formation of larger pores is
needed on the cell surface, risking disruption to the cell
membrane, loss of vital cytoplasmic compounds and resulting in cell
death. The results presented in this study show that a reasonable
result can be achieved under the correct ultrasound conditions. The
cell viability is as high as 80% compared with the control group
immediately after sonoporation. However, this is a transient
process, as shown in Fig. 4, since
cell viability returns 24 h after ultrasound exposure.
The use of ultrasound to transfect eukaryotes with
plasmid DNA indicates that the enhanced plasma membrane
permeability is transient and the cell membrane can seal or heal to
preserve viability (19,32). Mitragotri et al used
low-frequency ultrasound to destabilize lipid layers and enhance
permeability of drugs in the skin. Following ultrasonic treatment,
the original permeability was eventually restored (26). Freeze-fracture electron microscopy
has shown that, a few seconds after the electric pulse, the pores
began to reseal; 5 sec post-electroporation, the majority of the
pores became shallow and smaller, while at 10 sec the pore-like
structures on the cell membrane had almost disappeared (32,41).
Guzman et al reported similar results when cells were
exposed to low frequency ultrasound (24 kHz) (37,43,44).
Uptake of bovine serum albumin and calcein was completely abolished
when the compounds were added 1 and 2 min after ultrasound
exposure, respectively (18). The
short duration of membrane pore opening implies that, if the drug
is to be effectively internalized, it should be close to the
membrane when poration occurs (32,42–44).
Therefore, application of drugs and the interaction between drugs
and cells should be performed prior to sonication.
Chronic orthopedic infection is a serious concern in
clinical practice. Ultrasound-enhanced antibiotic efficiency to the
bacteria internalized in the osteoblast may contribute to the
control of chronic infection and reduce the recurrence. Further
research is required to allow for better understanding of these
fundamental issues and to fully exploit the potential use of
sonoporation in treatment of infections.
Acknowledgements
This study was financially supported
by the Characteristic Construction Foundation of the Sixth People’s
Hospital affiliated to Shanghai Jiaotong University.
References
|
1
|
Ellington JK, Harris M, Webb L, et al:
Intracellular Staphylococcus aureus. A mechanism for the
indolence of osteomyelitis. J Bone Joint Surg Br. 85:918–921.
2003.
|
|
2
|
Wright JA and Nair SP: Interaction of
staphylococci with bone. Int J Med Microbiol. 300:193–204. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ellington JK, Harris M, Hudson MC, Vishin
S, Webb LX and Sherertz R: Intracellular Staphylococcus
aureus and antibiotic resistance: implications for treatment of
staphylococcal osteomyelitis. J Orthop Res. 24:87–93.
2006.PubMed/NCBI
|
|
4
|
Ning R, Zhang X, Guo X and Li Q:
Attachment of Staphylococcus aureus is required for
activation of nuclear factor kappa B in human osteoblasts. Acta
Biochim Biophys Sin. 42:883–892. 2010.
|
|
5
|
Chihara S and Segreti J: Osteomyelitis.
Disease-a-Month. 56:6–31. 2010. View Article : Google Scholar
|
|
6
|
Ishida I, Kohda C, Yanagawa Y, Miyaoka H
and Shimamura T: Epigallocatechin gallate suppresses expression of
receptor activator of NF-kappaB ligand (RANKL) in Staphylococcus
aureus infection in osteoblast-like NRG cells. J Med Microbiol.
56:1042–1046. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin C and Flavell RA: Molecular mechanism
of NLRP3 inflammasome activation. J Clin Immunol. 30:628–631. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chauhan VS and Marriott I: Differential
roles for NOD2 in osteoblast inflammatory immune responses to
bacterial pathogens of bone tissue. J Med Microbiol. 59:755–762.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Webb LX, Wagner W, Carroll D, Tyler H,
Coldren F and Martin E: Osteomyelitis and intraosteoblastic
Staphylococcus aureus. J Surg Orthop Adv. 16:73–78.
2007.
|
|
10
|
Reilly SS: In vivo internalization of
Staphylococcus aureus by embryonic chick osteoblasts. Bone.
26:63–70. 2000.
|
|
11
|
Shi S and Zhang X: Interaction of
Staphylococcus aureus with osteoblasts (Review). Exp Ther
Med. 3:367–370. 2012.
|
|
12
|
Lefebvre M, Jacqueline C, Amador G, et al:
Efficacy of daptomycin combined with rifampicin for the treatment
of experimental meticillin-resistant Staphylococcus aureus
(MRSA) acute osteomyelitis. Int J Antimicrob Agents. 36:542–544.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khanlari B, Elzi L, Estermann L, et al: A
rifampicin-containing antibiotic treatment improves outcome of
staphylococcal deep sternal wound infections. J Antimicrob
Chemother. 65:1799–1806. 2010. View Article : Google Scholar
|
|
14
|
Garrigos C, Murillo O, Euba G, et al:
Efficacy of usual and high doses of daptomycin in combination with
rifampin versus alternative therapies in experimental foreign-body
infection by methicillin-resistant Staphylococcus aureus.
Antimicrob Agents Chemother. 54:5251–5256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
El Helou OC, Berbari EF, Lahr BD, et al:
Efficacy and safety of rifampin containing regimen for
staphylococcal prosthetic joint infections treated with debridement
and retention. Eur J Clin Microbiol Infect Dis. 29:961–967.
2010.PubMed/NCBI
|
|
16
|
Rediske AM, Hymas WC, Wilkinson R and Pitt
WG: Ultrasonic enhancement of antibiotic action on several species
of bacteria. J Gen Appl Microbiol. 44:283–288. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carmen JC, Roeder BL, Nelson JL, et al:
Ultrasonically enhanced vancomycin activity against
Staphylococcus epidermidis biofilms in vivo. J Biomater
Appl. 18:237–245. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carmen JC, Nelson JL, Beckstead BL, et al:
Ultrasonic-enhanced gentamicin transport through colony biofilms of
Pseudomonas aeruginosa and Escherichia coli. J Infect
Chemother. 10:193–199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Runyan CM, Carmen JC, Beckstead BL, Nelson
JL, Robison RA and Pitt WG: Low-frequency ultrasound increases
outer membrane permeability of Pseudomonas aeruginosa. J Gen
Appl Microbiol. 25:295–301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang L, Wang ZH, Shen CY, You ML, Xiao JF
and Chen GQ: Differentiation of human bone marrow mesenchymal stem
cells grown in terpolyesters of 3-hydroxyalkanoates scaffolds into
nerve cells. Biomaterials. 31:1691–1698. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Williams RG and Pitt WG: In vitro response
of Escherichia coli to antibiotics and ultrasound at various
insonation intensities. J Biomater Appl. 12:20–30. 1997.
|
|
22
|
Rediske AM, Roeder BL, Brown MK, et al:
Ultrasonic enhancement of antibiotic action on Escherichia
coli biofilms: an in vivo model. Antimicrob Agents Chemother.
43:1211–1214. 1999.
|
|
23
|
Carmen JC, Roeder BL, Nelson JL, et al:
Treatment of biofilm infections on implants with low-frequency
ultrasound and antibiotics. Am J Infect Control. 33:78–82. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rapoport N, Smirnov AI, Timoshin A, Pratt
AM and Pitt WG: Factors affecting the permeability of
Pseudomonas aeruginosa cell walls toward lipophilic
compounds: effects of ultrasound and cell age. Arch Biochem
Biophys. 344:114–124. 1997.PubMed/NCBI
|
|
25
|
Rediske AM, Rapoport N and Pitt WG:
Reducing bacterial resistance to antibiotics with ultrasound. Lett
Appl Microbiol. 28:81–84. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mitragotri S, Blankschtein D and Langer R:
Transdermal drug delivery using low-frequency sonophoresis. Pharm
Res. 13:411–420. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wyber JA, Andrews J and D’Emanuele A: The
use of sonication for the efficient delivery of plasmid DNA into
cells. Pharm Res. 14:750–756. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tachibana K, Uchida T, Tamura K, Eguchi H,
Yamashita N and Ogawa K: Enhanced cytotoxic effect of Ara-C by low
intensity ultrasound to HL-60 cells. Cancer Lett. 149:189–194.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li YS, Reid CN and McHale AP: Enhancing
ultrasound-mediated cell membrane permeabilisation (sonoporation)
using a high frequency pulse regime and implications for
ultrasound-aided cancer chemotherapy. Cancer Lett. 266:156–162.
2008. View Article : Google Scholar
|
|
30
|
Iwanaga K, Tominaga K, Yamamoto K, et al:
Local delivery system of cytotoxic agents to tumors by focused
sonoporation. Cancer Gene Ther. 14:354–363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kinoshita M and Hynynen K: Key factors
that affect sonoporation efficiency in in vitro settings: the
importance of standing wave in sonoporation. Biochem Biophys Res
Commun. 359:860–865. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mehier-Humbert S, Bettinger T, Yan F and
Guy RH: Plasma membrane poration induced by ultrasound exposure:
implication for drug delivery. J Control Release. 104:213–222.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qian Z, Sagers RD and Pitt WG:
Investigation of the mechanism of the bioacoustic effect. J Biomed
Mater Res. 44:198–205. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu J, Lewis TN and Prausnitz MR:
Non-invasive assessment and control of ultrasound-mediated membrane
permeabilization. Pharm Res. 15:918–924. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ananta E, Voigt D, Zenker M, Heinz V and
Knorr D: Cellular injuries upon exposure of Escherichia coli
and Lactobacillus rhamnosus to high-intensity ultrasound. J
Appl Microbiol. 99:271–278. 2005.PubMed/NCBI
|
|
36
|
Saito K, Miyake K, McNeil PL, Kato K, Yago
K and Sugai N: Plasma membrane disruption underlies injury of the
corneal endothelium by ultrasound. Exp Eye Res. 68:431–437. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guzman HR, McNamara AJ, Nguyen DX and
Prausnitz MR: Bioeffects caused by changes in acoustic cavitation
bubble density and cell concentration: a unified explanation based
on cell-to-bubble ratio and blast radius. Ultrasound Med Biol.
29:1211–1222. 2003. View Article : Google Scholar
|
|
38
|
Pitt WG and Ross SA: Ultrasound increases
the rate of bacterial cell growth. Biotechnol Prog. 19:1038–1044.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gracewski SM, Miao H and Dalecki D:
Ultrasonic excitation of a bubble near a rigid or deformable
sphere: implications for ultrasonically induced hemolysis. J Acoust
Soc Am. 117:1440–1447. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huber PE and Pfisterer P: In vitro and in
vivo transfection of plasmid DNA in the Dunning prostate tumor
R3327-AT1 is enhanced by focused ultrasound. Gene Ther.
7:1516–1525. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chang DC: Structure and dynamics of
electric field-induced membrane pores as revealed by rapid-freezing
electron microscopy. Guide to Electroporation and Electrofusion.
Academic Press, Inc; San Diego: pp. 9–27. 1992
|
|
42
|
Lee EK, Gallagher RJ, Campbell AM and
Prausnitz MR: Prediction of ultrasound-mediated disruption of cell
membranes using machine learning techniques and statistical
analysis of acoustic spectra. IEEE Trans Biomed Eng. 51:82–89.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guzman HR, Nguyen DX, Khan S and Prausnitz
MR: Ultrasound-mediated disruption of cell membranes. II.
Heterogeneous effects on cells. J Acoust Soc Am. 110:597–606. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Guzman HR, Nguyen DX, Khan S and Prausnitz
MR: Ultrasound-mediated disruption of cell membranes. I.
Quantification of molecular uptake and cell viability. J Acoust Soc
Am. 110:588–596. 2001. View Article : Google Scholar : PubMed/NCBI
|