Introduction
Seed cells and scaffold material are two main issues
that are addressed in cartilage tissue engineering. Bone marrow
mesenchymal stem cells (BMMSCs), being among the most commonly used
seed cells in tissue engineering, have the ability to differentiate
multi-directionally and can be generously amplified in
vitro. Therefore, BMMSCs may be induced to differentiate into
chondroblasts through various methods in order to construct
cartilage-engineered tissue and provide seed cells.
BMMSCs in vivo mostly exist in the bone
marrow at a static state, but when stimulated by physiological and
pathological factors, their proliferation ability is demonstrated
and BMMSCs differentiate into fat cells, bone cells and
chondroblasts (1). Studies have
shown that during the differentiation process from BMMSCs to
chondrocytes, TGF-β1, one of the most important growth factors
(2), can induce BMMSCs to
differentiate into chondrocytes and promote the secretion of type
II collagen and the synthesis and accumulation of extracellular
matrix (3,4). Therefore, the TGF-β1 gene can be
transferred into BMMSCs using gene transfer technology to ensure
stable expression of TGF-β1, and BMMSCs can be persistently
induced. This has become an ideal method in cartilage tissue
engineering. Transcriptional enhancer factor TAZ (transcriptional
coactivator with PDZ-binding motif) is one type of side line gene
of the Yes-associated protein (YAP) that can regulate the
transcription expression of Smad, BMP-2 and Runx, while the
induction of TGF-β1 in BMMSCs is closely related to Smad, BMP-2,
Runx and others (5,6). After transfection of TGF-β1, during
the differentiation of BMMSCs to phenotypic chondrocytes, the
method for effectively altering TAZ expression is still
questionable. The present study was designed for a preliminarily
investigation of this issue.
Most cell growth in vivo is wrapped in a
three-dimensional environment similar as a niche box. For example,
wrapped chondrocytes in vivo grow in cartilage matrix. At
present, the antilinear of prepared and applicated porous scaffolds
are usually much larger than that of cells; the cells are planted
in such materials and can only spread and grow in an adherent
manner, which is actually a two-dimensional (monolayer) culture
model. This two-dimensional (monolayer) culture is not an effective
manner to simulate cell growth in vivo in a
three-dimensional micro-environment. Studies have shown that a
three-dimensional environment is crucial for the maintenance of
cell morphology and biological activity. Gel material has
hydrophilicity which is suitable for cell embedding. It highly
simulates the in vivo environment of cell growth, and
provides a space similar to the natural substrate and chemical
structure and signal transduction environment for cell growth
(7). Alginate calcium is a type of
saccharan composed of a different number of gulonic and mannuronic
acids. Alginate calcium itself is biodegradable and biocompatible.
This study was designed to investigate the feasibility of
constructing tissue-engineered cartilage in a three-dimensional
culture in vitro after embedding hTGF-β1 gene-transferred
BMMSCs in alginate gel material.
Materials and methods
Experimental animals
Wistar rats, 120±15 g, male or female, were
purchased from the Experimental Animal Center of Wuhan University
(Wuhan, China).
Reagents
Fetal calf serum, L-DMEM medium containing 10% fetal
calf serum, tryptase, rat anti-human TGF-β1 and collagen II
polyclonal antibody were obtained. Goat anti-rat IgG secondary
antibody, an immunohistochemistry kit, TRIzol reagent and a western
blotting kit were obtained. The adenovirus with the EGFP gene
(Ad-EGFP) and adenovirus with human transforming growth factor
(Ad-hTGF)-β1 gene were constructed and preserved in our laboratory.
Sodium alginate, calcium chloride and PCR primers were synthesized
by Shanghai Biological Engineering Company.
Procurement and culture of BMMSCs
After ether anesthesia, the rats were sacrificed and
soaked in 75% ethanol degeneration for 10 min. The femur was
removed and the soft tissues were cleanly shaved. Both sides of the
bone were opened with a rongeur, and the two femurs were placed in
10 ml L-DMEM medium containing 10% fetal calf serum. The bone
marrow cavity was repeatedly flushed until turning white using
medium in a 5-ml sterile syringe. The obtained cell suspension was
repeatedly pipetted and mixed, and then the cell suspension was
seeded in 60-mm sterile Petri dishes, and placed in a 95%
humidified incubator at 37°C in 5% CO2 for incubation.
The medium was replaced every 3–4 days, when the cells covered
70–80% of the dish. The cells were subsequently digested and
subcultured with trypsin containing 0.25% EDTA. Third-generation
cells were selected for use in the experiment.
Cell transfection and experimental
groups
The third generation cells were seeded in 12-well
plates at 1×105/ml (400 μl/well). When the cells
reached 60% confluence, the cells were divided into three groups:
the Ad-hTGF-β1-transfected group [serum-free medium with Ad-hTGF-β1
(MOI value of 200)], the Ad-EGFP-transfected group [serum-free
medium with Ad-EGFP (MOI value of 200)]. The media in the two
groups were replaced by complete medium after >48 h. The control
group was cultured in complete medium with no treatment. The three
groups of cells were placed in a 95% humidified incubator at 37°C
in 5% CO2.
mRNA expression of TGF-β1 and TAZ in
BMMSCs in the three groups as detected by real-time PCR
The various cultured cells were obtained 7 days
after transfection, and total-RNA was extracted with TRIzol reagent
according to the manufacturer’s instructions. β-actin was used as
the housekeeping gene. Embedded dye SYBR-Green I was used for
real-time quantitative PCR amplification. The primers were: TGF-β1
upstream primer, CGCAACAACGCAATCTATG and downstream primer,
ACCAAGGTAACGCCAGGA, base length 204 bp. The PCR amplification
system (25 μl) contained 2.5 μl Plus solution, 12.5
μl SYBR-Green mix, 1 μl of each of the upstream and
downstream primer (5 pmol/μl), 2.5 μl cDNA and 5.5
μl deionized water. Amplification conditions were: 50°C for
2 min, 95°C for 2 min, then a 95°C denaturation for 15 sec, and
58°C annealing for 15 sec, a 72°C extension for 45 sec, for a total
of 40 cycles, then 72°C for 10 min.
Western blotting for the detection of
TGF-β1 protein expression in the transfected BMMSCs
The cultured cells were obtained 7 days after
transfection and were rinsed three times with cold PBS at 4°C. The
lysis buffer-containing cocktail (1 ml lysis buffer plus 20
μl cocktail, prepared 5 min prior to use) was added at 4°C
and schizolysis was performed for 30 min. Percussion was carried
out with a pipette 10 times every 5 min to collect the cell
lysates. After centrifugation at 12,000 rpm for 10 min, the
supernatant was collected at room temperature. Fifteen microliters
was added/lane in an SDS-polyacrylamide gel electrophoresis tank,
in Tris-glycine electrophoresis buffer for electrophoresis and was
transferred to acetate film. The film was blocked with non-fat dry
milk for 2 h in room temperature. Then blocking fluid and rat
anti-human TGF-β1 (dilution 1:200) polyclonal antibody was added
and oscillated for 2 h at 4°C and washed three times with PBS.
Tris-Cl solution was added and incubated for 10 min at room
temperature, then blocking solution and goat anti-rat IgG secondary
antibody were added and incubated at room temperature for 1 h. The
membrane was then transferred to Tris-Cl and incubated for 10 min.
Horseradish peroxidase was added to produce the color images.
Results were repeated three times, and GAPDH was used as a
housekeeping gene.
Preparation of the cell-calcium alginate
gel compound
Preparation of the sodium
polymannuronate suspension
The sodium alginate powder (Sigma) was dissolved in
double distilled water at 12 g/l at room temperature agitated with
a magnetic stirrer overnight. To fully dissolve the sodium alginate
solution, the pH was adjusted to 7.0–8.0, and eas then reserved
after high temperature and high pressure sterilization.
Preparation of compound cell-calcium
alginate gel
The successfully transfected BMMSCs were digested,
centrifugated and counted, and were separately and thoroughly mixed
with sterile sodium alginate suspension at a final concentration of
1.0×107 cells/ml. Excess calcium chloride was added at
102 mmol/l solution, and was kept standing for 20–30 min. Then the
cells were washed 3–4 times with D-Hank’s solution, and the
formative cell-calcium alginate gel compound was suspended in
culture medium at 37°C in a 95% humidified incubator for culture.
The culture medium was replaced every 3–4 days, and the cells were
determined after 10 days of culture.
Proliferation of transfected cells
determined by MTT assay and the number of BMMSCs in the calcium
alginate gel material
The transfected cells in each group were obtained
and digested with pancreatic enzyme containing 0.25% EDTA into a
single-cell suspension, and the cell concentration was adjusted to
1×105/ml and cells were seeded into a 96-well cell
culture plate (200 μl/well). At the same time point at 1, 2,
3, 4, 5, 6 days after transfection, the cells from three wells were
randomly selected from the cells in each group, the culture medium
was discarded, 20 μl MTT was added (5 g/l) and culturing was
carried out for 4 h. After removal of the supernatant, 150
μl DMSO was added and oscillated for approximately 10 min.
The extinction A value (OD) of each well was determined at 490 nm
wavelength in a microplate reader, and the cell growth curve was
drawn according to the measured values.
At the same time points 1, 2, 3, 4, 5, 6 days after
cells were seeded in the alginate gel material, the cells were
extracted and suspensed, and the cell concentration was adjusted to
1×105/ml. The cells from the three wells were randomly
selected from the cells in each group, the culture medium was
discarded, 20 μl MTT was added (5 g/l) and culturing was
carried out for 4 h. After removing the supernatant, 150 μl
DMSO was added and oscillated for approximately 10 min. The
extinction A value (OD) in each well was determined at 490 nm
wavelength in the microplate reader and the cell growth curve was
drawn according to the measured values.
Histological and histochemical
staining
Ten days after the culture of the cell-calcium
alginate gel compound in vitro, the compound was extracted
and washed with PBS. The compound was fixed in 4% paraformaldehyde
and embedded in paraffin according to the conventional method. It
was cut into 4-μm slices, and H&E, Masson’s and
toluidine blue staining were performed, respectively, according to
conventional methods, amd the results were examined under an
inverted optical microscope.
Collagen II protein determined by
immunohistochemistry
After the 10-day culture in vitro, the
cell-calcium alginate gel compound was extracted and washed with
PBS. The compound was fixed in 4% paraformaldehyde and embedded in
paraffin according to a conventional method. It was cut into
4-μm slices. SABC assay was used to detect collagen II
expression. The primary antibody of collagen II was rat anti-human
collagen II polyclonal antibody (1:100), and the secondary antibody
was goat anti-rat IgG (1:200). The protocol was according to the
manufacturer’s instructions, and the results were observed and
tested under an inverted optical microscope and photographed.
Data analysis
Data are expressed as the means ± SD, said the
group. The comparison of group differences was carried out using
the analysis of variance. SPSS13.0 statistical software was used
for statistical analysis, and P<0.05 indicated a significant
difference.
Results
Morphology of BMMSC in primary culture
and cells cultured in calcium alginate gel
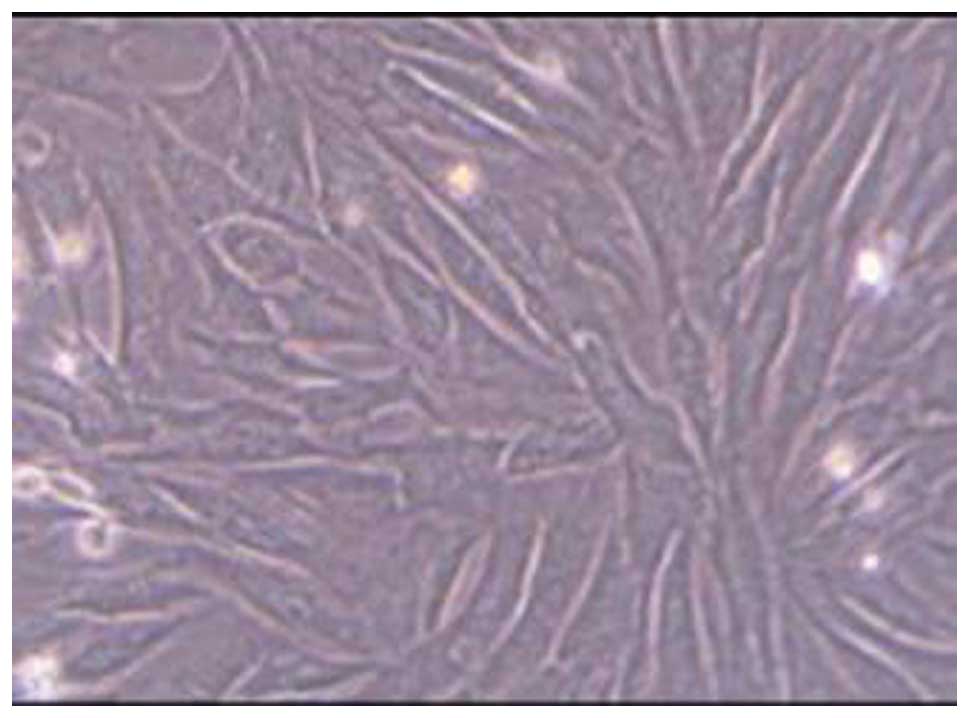
After 72 h of primary culture, BMMSCs were observed
to exhibit adherence and colony formation. The cells were
spindle-shaped and polygonal-based which continued for the 7- to
9-day culture. When the cells reached 80–90% confluence, the speed
of cell proliferation was significantly rapid after passage. The
cells were uniform and continued to grow. After 2–3 days, the cells
reached 80–90% confluence, and the cells exhibited a long
spindle-shape (Fig. 1). After
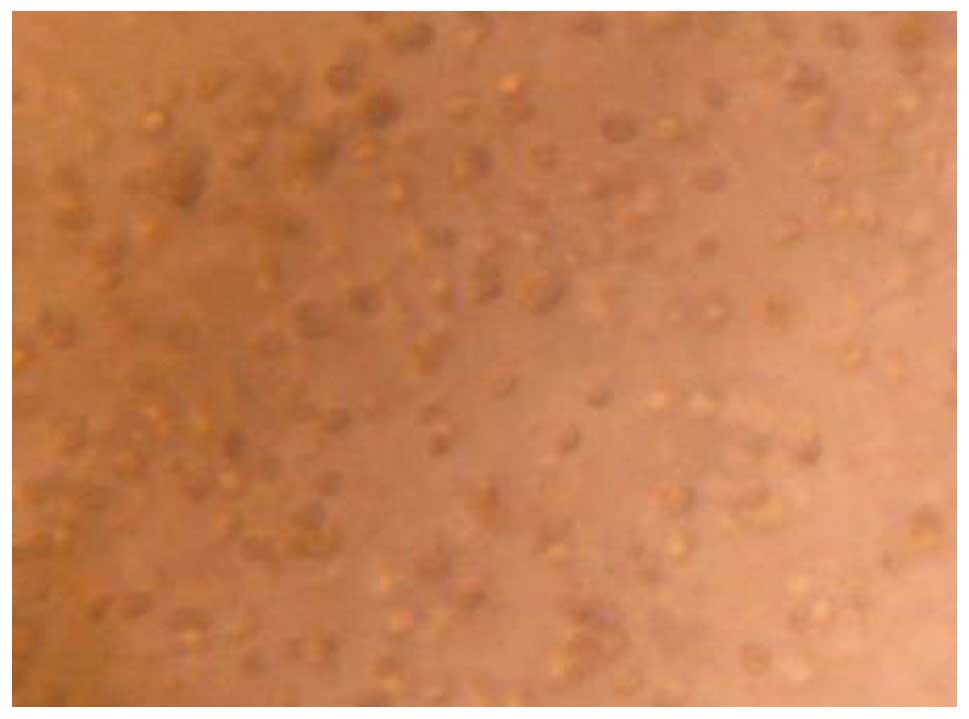
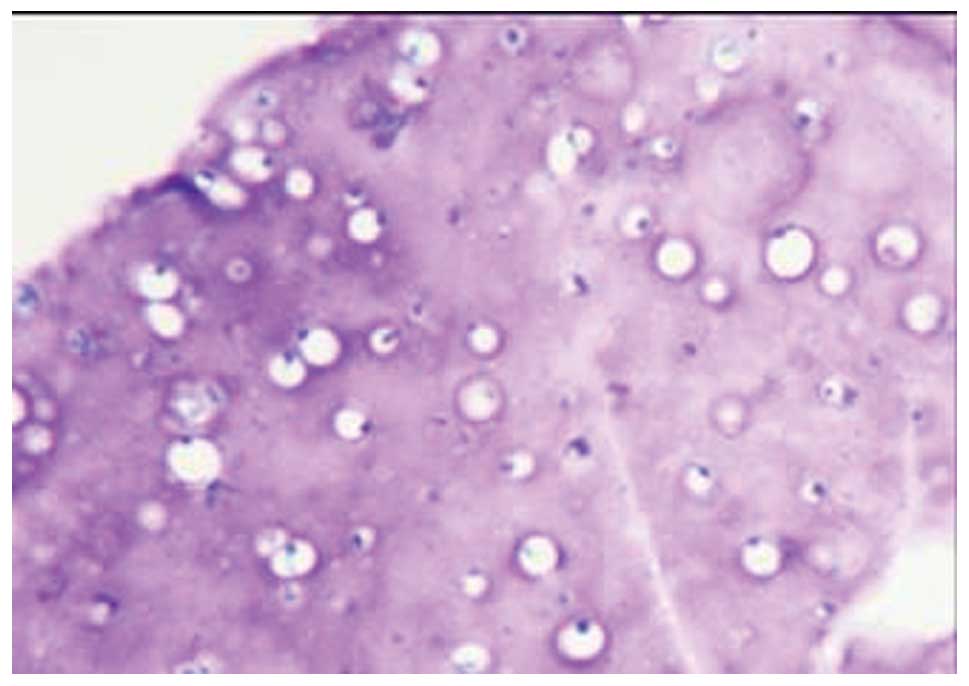
BMMSCs were grown in calcium alginate gel, they were evenly
distributed in the gel layer, and their shape was spherical
(Fig. 2).
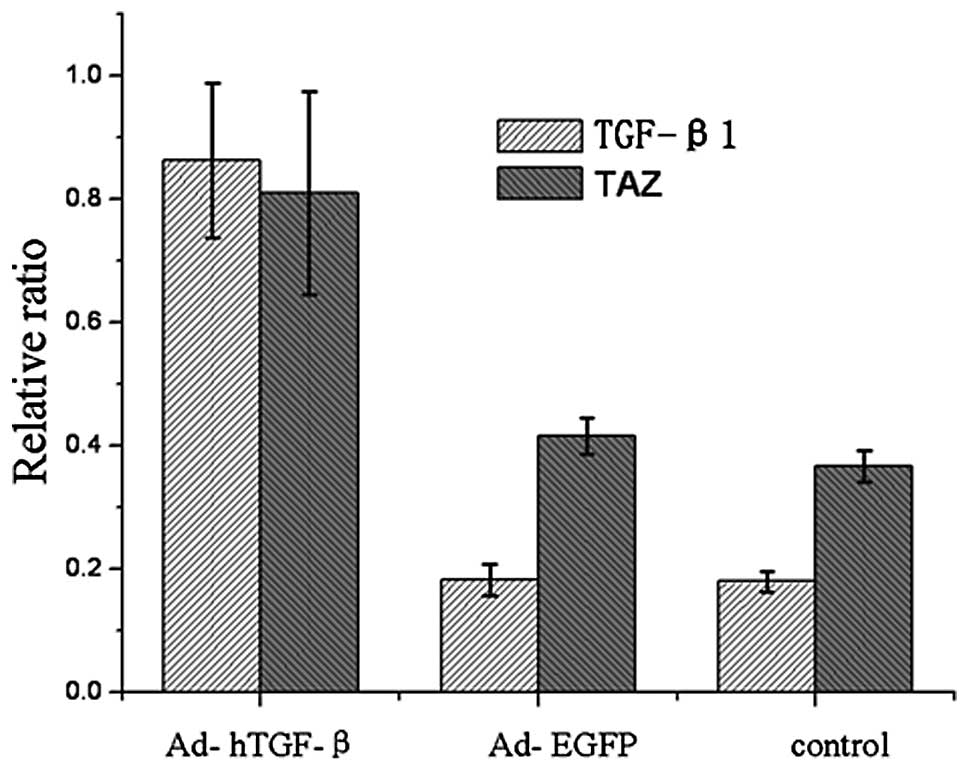
mRNA expression of TAZ and TGF-β1 in
the transfected cells as determined by real-time PCR
As shown in Fig. 3,
7 days after transfection, the
mRNA expression of TGF-β1 and TAZ in the Ad-hTGF-β1-transfected
group was significantly higher than levels in the
Ad-EGFP-transfected and the control groups.
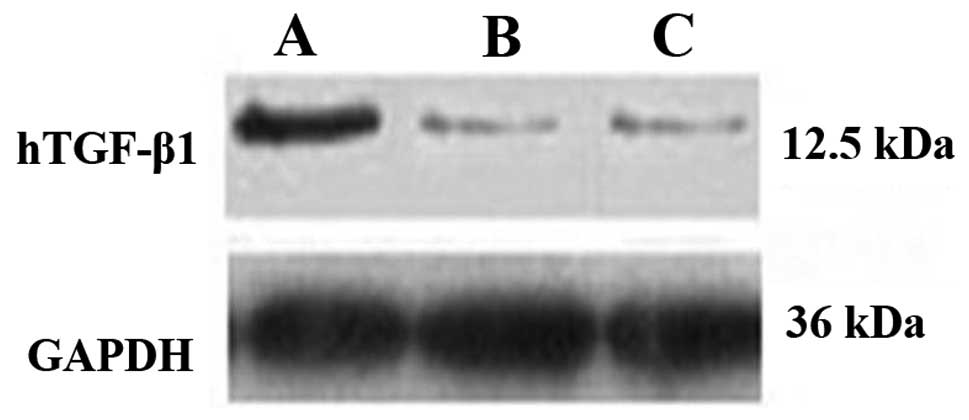
Protein expression of TGF-β1 in the
transfected BMMSCs as determined by western blotting
As shown in Fig. 4,
7 days after transfection, the
protein expression of TGF-β1 in the Ad-hTGF-β1-transfected group
was significantly higher than that of the Ad-EGFP-transfected and
control groups (Fig. 4).
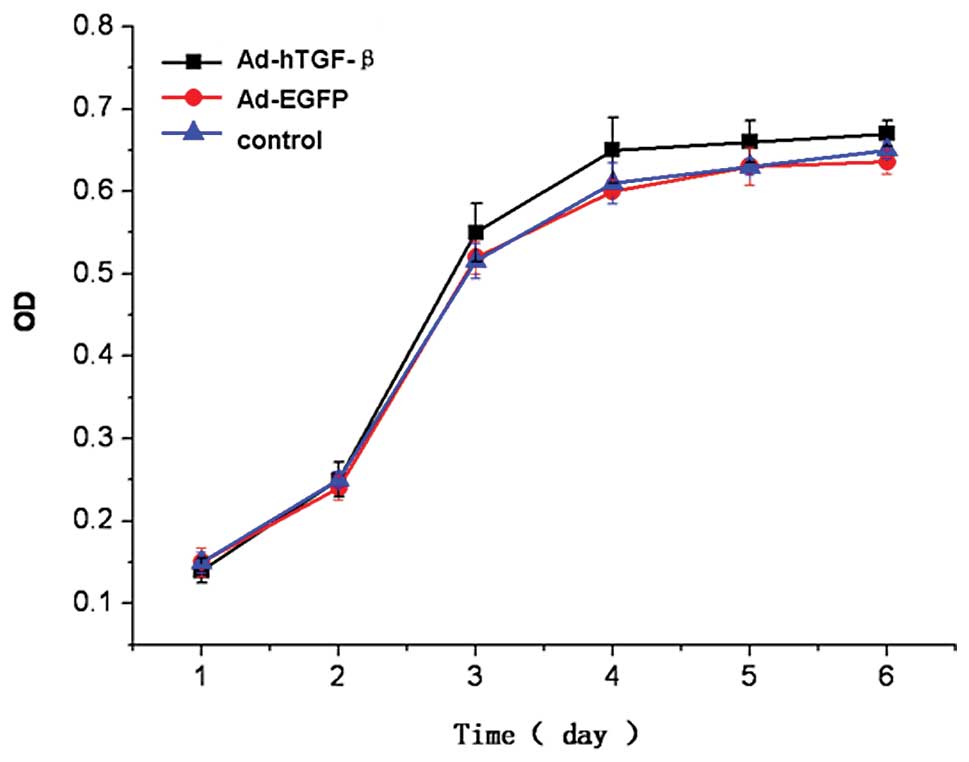
Cell proliferation ability in the
transfected BMMSCs as determined by MTT assay
The results from the MTT colorimetric assay showed
that after transfection, days 1 and 2 after cell seeding in the
three groups was considered to be a period of delitescence, day 3
was considered as the logarithmic growth phase, day 4 was
considered as the platform phase. The cell growth curves for the
three groups were similar, and the difference in absorbance (OD) at
each time point was not statistically significant (P=0.718, 0.670,
0.246, 0.192, 0.172, 0.094) (Fig.
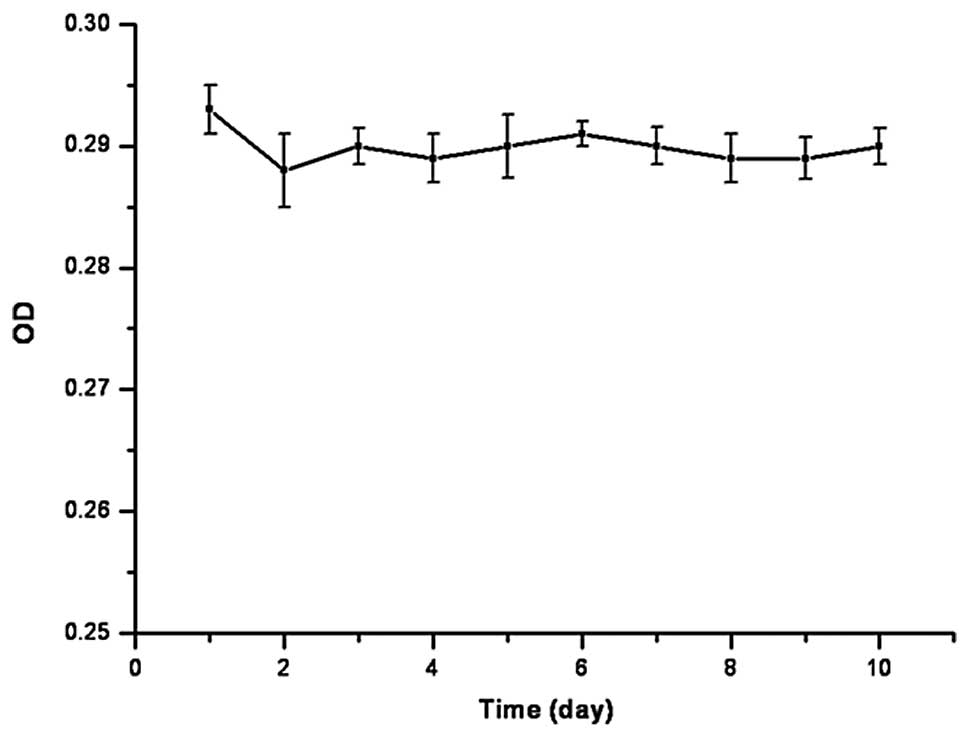
5). At the same time, after the cells were seeded on the
calcium alginate gel, the cell growth curve did not change
significantly, and difference in the absorbance (OD) at each time
point was not statistically significant (P=0.473) (Fig. 6)
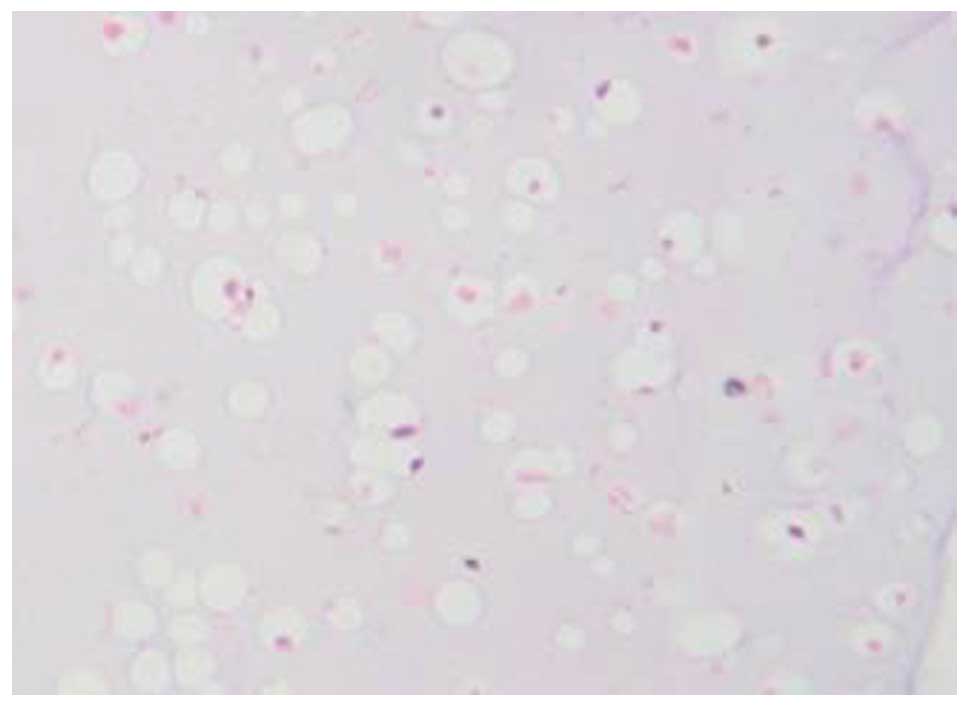
Histological and histochemical
observations
H&E staining showed that a large number of
cartilage lacunae were formed in the gel material, and the
nucleolus was clearly visible (Fig.
7). Masson staining showed the synthesis and secretion of type
II collagen in the gel material (Fig.
8). Toluidine blue staining confirmed the synthesis and
secretion of proteoglycan in the gel material (Fig. 9).
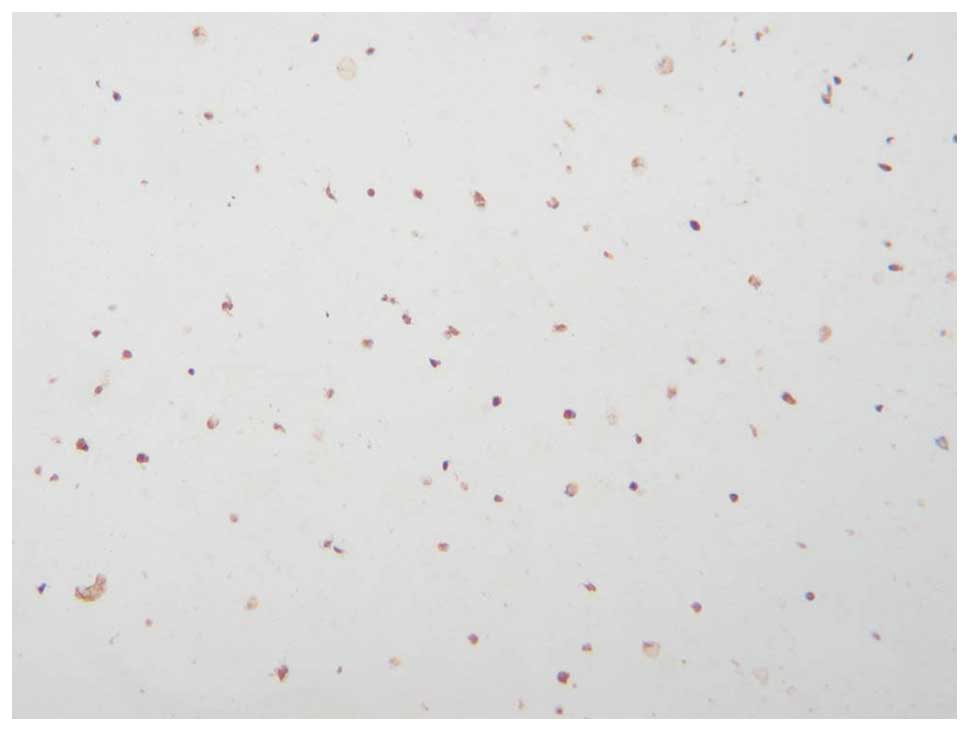
Type II collagen determined by
immunohistochemistry
The results from type II collagen determined by
immunohistochemistry showed that 10 days of culture in vitro
after the Ad-hTGF-β1-transfected cells were seeded in the gel
material, brown particles were present in the gel material. This
indicated that BMMSCs synthesized and secreted type II collagen
(Fig. 10).
Discussion
Seed cells for application in the tissue engineering
of cartilage include chondrocytes and mesenchymal stem cells, yet
autologous chondrocytes are not easily obtained, the source is
limited and their use easily leads to cartilage damage of draw
material parts. Previous studies have shown that only
1.8–4.5×105 chondrocytes can be extracted from a trauma
patient with knee injury with a single femoral condyle articular
cartilage (2), which is not enough
for chondrocyte transplantation (3). In contrast, mesenchymal stem cells
are easily obtained, can be amplified in vitro and have a
strong ability to differentiate into cartilage cells, particularly
mesenchymal stem cells. Thus, we chose bone marrow mesenchymal stem
cells as seed cells in this study. TGF-β1 is known to be a growth
factor in bone that can induce BMMSCs to differentiate into
chondrocytes, TGF-β1 is also one of the most important factors
causing BMMSCs to differentiate into cartilage (4). In view of these facts, gene transfer
technology was used to transfer the TGF-β1 gene into BMMSCs using a
standard method. TGF-β1 can steadily induce BMMSC differentiation
but does not affect the proliferation rate of BMMSCs. In this
study, after BMMSCs were transfected with Ad-hTGF-β1, the results
from real-time PCR and western blotting showed that TGF-β1
expression in the Ad-hTGF-β1-transfected cells was significantly
higher than levels in the Ad-EGFP-transfected and control group
cells; a fact indicating that we successfully constructed BMMSCs
with high expression of TGF-β1.
The role of TGF-β1 in the differentiation of BMMSCs
was found to be affected by Smad, BMP-2 and Runx genes (5). TAZ is one of the YAP sideline genes,
and regulates the expression of Smad, BMP-2 and Runx at the
transcription level, and it also affects the life of BMMSCs
(6). In this study, we designed
primer sequences to detect TAZ mRNA, and the expression differences
of TAZ mRNA were detected between the experimental and control
groups through real-time quantitative PCR. The results showed that
the level of TAZ mRNA in the Ad-hTGF-β1-transfected cell group was
significantly higher than levels in the Ad-EGFP-transfected and
control groups (P<0.05). This indicates that BMMSCs transfected
by Ad-hTGF-β1 induced BMMSC differentiation into chondrocytes due
to the increased expression of TGF-β1, while the mRNA level of TAZ
was also increased in this process. Therefore, we speculated that
TAZ may play a regulatory role in the process of TGF-β1-induced
BMMSC differentiation to chondrocyte phenotype. However, several
studies have also demonstrated that in the process of
TGF-β1-induced BMMSC differentiation into osteoblasts, the
expression level of TAZ is increased (7,8).
Therefore, it remains to be investigated how TAZ regulates the
process of TGF-β1-induced BMMSC differentiation to chondrocytes and
osteoblasts.
In a healthy organism, in addition to blood cells in
the circulatory system, other cells are grown in a relatively
stable three-dimensional space structure. In this structure, the
cells are packaged in the extracellular matrix, and soluble growth
factors distributed in the extracellular matrix are able to bind to
receptors on the cell surface and play a role in regulating the
activity of cell biology (9). At
the same time, interactions between cells also play a regulatory
role in the biological activity of cells. For mesenchymal stem
cells, the extracellular matrix and cell interactions also play an
important role in maintaining the morphology of mesenchymal stem
cells, a fact that directly affects the proliferation and
differentiation activity of mesenchymal stem cells themselves
(10,11). These features require us to take
into account the in vivo environment of the mesenchymal stem
cells in the study of tissue engineering, and thus we tried to
simulate the three-dimensional spatial structure for the growth of
cells in order to maintain the biological activity of mesenchymal
stem cells when constructing tissue-engineering scaffolds.
Research has revealed that a two-dimensional (single
layer) condition is not a good method for maintaining the
biological and biochemical properties of cells (1), particularly those of mesenchymal stem
cells. If the cells are placed in a single- or two-dimensional
culture environment, the ability of cells to differentiate into
cartilage is difficult to maintain. Therefore, material chemistry,
structure, processing technology and bioauxology can be used to
design a three-dimensional cell culture matrix for tissue
engineering with an adequate geometric and chemical composition
similar to the natural extracellular matrix signaling system. In
addition, this unique structure is conducive to the transmission of
soluble molecules, as well as the exchange of nutrients and cell
metabolism waste (12). The
traditional material for cartilage tissue engineering mainly
includes polyvinyl alcohol, polyglycolic acid, polylactic acid,
chitosan and a mixture according to different proportions. The
porous structure of the scaffolds is ensured by a freeze-drying
method, and the seed cells are then seeded in this scaffold.
Concerning the porous scaffold prepared using this method, the pore
size is generally between 200–400 μm (13), while the diameter of the cell is
only a few microns; much smaller than the pore size of the
scaffold. The cells seeded to grow in material with such pores
inevitably make the cells spread and attach to the surface of the
material in an adherent manner, which is actually a two-dimensional
(single) model. It is difficult to maintain the spherical shape of
cells, and the latter is an indispensable condition for mesenchymal
stem cells exerting their biological properties (14). In contrast, gel-like material is
prepared according to the physical and chemical properties of the
biological material itself rather than by a freeze-drying method.
The pore of this material is very small, while a large number of
charged groups are on the surface of gel, which makes the material
highly hydrophilic. This precisely simulates the body’s natural
growth environment of cartilage cells (15). This is extremely beneficial for the
differentiation of mesenchymal stem cells to cartilage cells.
Alginate (e.g. sodium alginate), which is a type of
anionic polysaccharide without a side chain derived from brown
algae, exists as a solid when it interacts with polyvalent cations
(e.g. calcium) (1,16). Alginate displays outstanding
biocompatibility with the host and seed cells in condition of
maintaining their own invariant characteristics (1). In animal experiments, gel complexes
with cartilage cells and alginate has been successfully used in
cartilage transplantation (17,18).
Alginate calcium arises from the crosslink of sodium alginate and
calcium chloride. It has superior biocompatible and absorptive
abilities, and has a porous structure that contributes to material
exchange required by the metabolism (19). In addition, the hydrophilic ability
of alginate makes cells difficult to attach to the surface of
alginate material, a fact that helps to maintain the spherical
structure and stabilize the differentiation of cells (2). A number of studies have shown that
alginate can be used as a three-dimensional material to culture
chondrocytes, since alginate material can simulate the environment
of articular cartilage and induce cartilage cells to secrete
proteoglycans and maintain the surrounding chondrocytes (20). However, it is still unclear whether
BMMSCs gene-modified using an alginate calcium-gel complex can be
used to successfully construct tissue engineered cartilage in
vitro. In the present study, after BMMSCs were successfully
transfected by Ad-hTGF-β1, they were generously amplified in
vitro. The cells at 1.0×107/ml were mixed in sodium
alginate (17) to become a cell
suspension, and calcium chloride was added and fully cross-linked
with the sodium alginate-cell suspension and finally made into a
hydrogel combined with calcium alginate-cells. BMMSCs were observed
in the gel material in a uniformly distributed manner under an
inverted microscope and grew in a spherical state (Fig. 2), which is required to maintain the
stability of the cell morphology, and simulate the grow morphous of
cells in the body to ensure the biological and biochemical
characteristics of cells. It effectively avoids the shortcoming of
adherent growth of cells cultured in a common two-dimensional
(single) manner. We used MTT assay in vitro, to detect the
cell number and found that the number of cells in the alginate gel
at different times was not significantly different (P=0.473). This
suggests that the cells embedded in the alginate gel can obtain the
required nutrients from the medium in vitro to ensure the
required energy of normal cells and maintain the cell activity, yet
at the same time, the cells do not undergo rapid proliferation in
the gel material as the gel material is unable to provide adequate
space for cell proliferation.
To confirm the differentiation of cartilage cells in
gel using BMMSCs modified by hTGF-β1 combined with alginate
calcium, after 10 days of culture in vitro, we adopted
H&E, toluidine blue and Masson’s staining for application to
the alginate-cells combined with gel materials. The results showed
that a large number of lacunae and a great amount of cartilage
proteoglycans and collagen was found in the gel material. The
results from immunohistochemistry showed that type II collagen can
be produced in the cultured cells from gel material. This indicates
that after the Ad-TGF-β1-transfected mesenchymal stem cells were
cultured in alginate calcium gel they were steadily differentiated
into cartilage.
Our preliminarily experimental results showed that
BMMSCs modified by the hTGF-β1 gene can be used as seed cells for
the tissue engineering of cartilage, while calcium alginate gel
material provides the required three-dimensional structure for
cartilage differentiation of seed cells. Alginate gel can be used
as a suitable carrier material for cartilage tissue engineering.
However, a key defect of gel material is that the mechanical
properties cannot reach the requirements of vito-dynamics. We
expect that in future studies, three-dimensional alginate gel
materials will be able to be combined with the superior mechanical
ability of a porous scaffold to develop more comprehensive tissue
engineering scaffolds. In addition, the detailed mechanism involved
in the three-dimensional culture environment affecting stem cell
proliferation and differentiation remains to be further
investigated.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (grants 30770574) as
well as by the Science and Technology Foundation of Hubei Health
Department (JX3B21).
References
|
1
|
Ghidoni I, Chlapanidas T, Bucco M, et al:
Alginate cell encapsulation: new advances in reproduction and
cartilage regenerative medicine. Cytotechnology. 58:49–56. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin YJ, Yen CN, Hu YC, et al: Chondrocytes
culture in three-dimensional porous alginate scaffolds enhanced
cell proliferation, matrix synthesis and gene expression. J Biomed
Mater Res A. 88:23–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kanda C, Barry WO and Marie-Paule I:
Effects of growth factors on cell proliferation and matrix
synthesis of low-density, primary bovine chondrocytes cultured in
collagen I gels. J Orthop Res. 20:1070–1078. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yao Xiawan, Wei Liu, Tianyi Liu, et al:
Application of bone marrow stromal cells in vitro tubular cartilage
tissue engineering experiments. Chin J Microsurg. 28:328–330.
2005.
|
|
5
|
Jian Zhao and Wei Xu: Bone marrow
mesenchymal stem cells and embryonic stem cells into cartilage
cells into possible molecular mechanisms [J]. Tissue Eng
Reconstructive Surg. 2:53–57. 2006.PubMed/NCBI
|
|
6
|
Hong JH, Hwang ES, McManus MT, et al: TAZ,
a transcriptional modulator of mesenchymal stem cell
differentiation. Science. 309:1074–1078. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao L, Jiang S and Hantash BM: TGF-beta1
induces osteogenic differentiation of murine bone marrow stromal
cells. Tissue Eng Part A. 16:725–733. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hong JH and Yaffe MB: TAZ: a
beta-catenin-like molecule that regulates mesenchymal stem cell
differentiation. Cell Cycle. 5:176–179. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lund AW, Yener B, Stegemann JP and Plopper
GE: The natural and engineered 3D microenvironment as a regulatory
cue during stem cell fate determination. Tissue Eng Part B Rev.
15:371–380. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Purpura KA, Aubin JE and Zandstra PW:
Sustained in vitro expansion of bone progenitors is cell density
dependent. Stem Cells. 22:39–50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McBeath R, Pirone DM, Nelson CM, et al:
Cell shape, cytoskeletal tension, and RhoA regulate stem cell
lineage commitment. Dev Cell. 6:483–495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu WF and Chen CS: Cellular and
multicellular form and function. Adv Drug Deliv Rev. 59:1319–1328.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burg KJ, Porter S and Kellam JF:
Biomaterial developments for bone tissue engineering. Biomaterials.
21:2347–2359. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benya PD and Shaffer JD: Dediferentiated
chondrocytes reexpress the differentiated collagen phenotype when
cultured in agarose gels. Cell. 30:215–224. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vinatier C, Guicheux J, Daculsi G, et al:
Cartilage and bone tissue engineering using hydrogels. Biomed Mater
Eng. 16:S107–S113. 2006.PubMed/NCBI
|
|
16
|
Dobratz EJ, Kim SW, Voglewede A and Park
SS: Injectable cartilage: using alginate and human chondrocytes.
Arch Facial Plast Surg. 11:40–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang SC, Rowley JA, Tobias G, et al:
Injection molding of chondrocyte / alginate constructs in the shape
of facial implants. J Biomed Mater Res. 55:503–511. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang SC, Tobias G, Roy AK, Vacanti CA and
Bonassar LJ: Tissue engineering of autologous cartilage for
craniofacial reconstruction by injection molding. Plast Reconstr
Surg. 112:793–799. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yi Guoquan and Shibi Lu: Alginate
properties and applications in cartilage tissue engineering. Chin J
Orthopaedic Surg. 14:216–218. 2006.
|
|
20
|
Kamishina H, Miyabayashi T, Clemmons RM,
et al: Three-dimensional culture of feline articular chondrocytes
in alginate microspheres. J Vet Med Sci. 68:1239–1242. 2006.
View Article : Google Scholar : PubMed/NCBI
|