Introduction
Esophageal carcinoma is one of the most common
malignant tumors occurring in patients throughout the world, and
esophageal squamous cell carcinoma (ESCC) is the most common type
occurring in China. The 5-year overall survival (OS) rate of
esophageal carcinoma patients is approximately 20%, even when the
tumor is resected early on in the course of the disease (1). Therefore, early assessment of patient
prognosis and the development of individualized therapy are
essential for improving the survival of esophageal cancer patients.
Previous studies have suggested that tumor staging, length, and
grade of differentiation are powerful prognostic factors for
predicting patient survival (2,3).
18F-fluorodeoxyglucose positron emission
tomography-computed tomography (18F-FDG PET-CT), a
noninvasive molecular imaging tool, has been utilized extensively
in the diagnosis of disease for determining stage, predicting
outcome, and assessing the prognosis of cancer patients (4–6).
Studies have demonstrated that 18F-FDG uptake on PET
might be useful for assessing biological aggressiveness of tumor
in vivo(7,8), and the standardized uptake value
(SUV) as a semi-quantitative parameter of FDG PET may accurately
represent the intensity of metabolic activity of the primary tumor
(9). An SUV cutoff of 2.5 on FDG
PET was confirmed to accurately measure the length of gross tumor
volume (10). In several studies,
the maximum SUV (SUVmax) was considered to be associated with tumor
differentiation and clinical stage as well as predict survival for
most esophageal adenocarcinomas (9,11,12).
However, other studies did not find any significant correlation
between SUVmax and tumor differentiation and tumor-node-metastasis
(TNM) stage for non-small-cell lung cancer or ESCC (7,13).
To the best of our knowledge, the relationship between SUVmax and
the clinicopathological characteristics of tumors is still
controversial and information on esophageal carcinoma is scarce,
especially for ESCC.
Therefore, in the present study, we evaluated the
relationship between 18F-FDG uptake in primary lesions
and clinicopathological characteristics, including tumor length,
grade of differentiation and stage. We also included patient age
and gender in the evaluation in order to guide clinical
management.
Materials and methods
The institutional review board of Shandong Cancer
Hospital (Jinan, China) granted approval for this study. The
patients enrolled in the study provided written, informed
consent.
Patients
We reviewed the esophageal carcinoma patients who
received 18F-FDG PET-CT scanning at our institution from
June 2006 to July 2011. Only patients meeting the following
criteria were included in this retrospective study: i) detailed and
complete documentation for basic patient characteristics, such as
gender, age, histological type, no presence of another primary
tumor; ii) histological confirmation of ESCC and the grade of
differentiation; iii) whole body 18F-FDG PET-CT image
acquisition prior to treatment; iv) no signs of infection, no
administration of medications for increasing the leukocyte count
within one week of imaging, and no diagnosis of type I diabetes at
the time of PET-CT scan; and v) contrast-enhanced CT, magnetic
resonance imaging (MRI), bone scan, or clinical follow-up to
confirm the findings of PET-CT.
PET-CT scanning
The patients were asked to fast for at least 6 h
before the scan, and to rest for 15 min prior to consuming 500 ml
of water, which was followed by administration of 7–11 mCi of the
radioactive tracer (18F-FDG). Serum glucose levels were
measured to confirm levels <6.6 mmol/l. Patients rested in a
quiet room for at least 45 min after receiving the
18F-FDG injection. The patients were then assessed on a
whole-body PET-CT scanner. The emission scans were acquired from
the level of the calvaria to the thigh for 4 min/table position.
Each patient received a scan lasting 24–28 min in total that
covered 14.5 cm at an axial sampling thickness of 4.25 mm/slice.
The non-contrast spiral CT component was performed with a slice
thickness of 4.25 mm and a rotation speed of 0.8 sec/rotation. The
PET covered the identical axial field of view immediately after CT
scanning. PET images were reconstructed with CT-derived attenuation
correction using ordered-subset expectation maximization (OSEM)
algorithm. The attenuation-corrected PET images, CT images, and
fused PET-CT images displayed as coronal, sagittal, and transaxial
slices were viewed on a Xeleris workstation (GE Healthcare,
Waukesha, WI, USA). For semi-quantitative analysis of the FDG
uptake, the SUVmax of the tumor site was determined by a
region-of-interest technique with analysis software of the PET
scanner (14).
Length and staging
The primary lesion was diagnosed with a SUVmax over
2.5 on PET images and the lymph nodes with a maximal diameter
greater than or equal to 10 mm on CT scans or the SUVmax over 2.5
on PET scans. The PET length of ESCC was calculated by multiplying
the slice number by the slice thickness. The clinical staging of
patients was mainly determined by hybrid FDG PET-CT imaging
according to the American Joint Committee on Cancer (AJCC) staging
system (15). Any suspicious site
that included nodal or distant metastatic disease was further
verified by other anatomical imaging methods, such as contrast CT,
MRI or bone scan. The PET-CT scans were read by two experienced
nuclear medicine physicians independently, both with over five
years of experience in PET-CT imaging.
Statistical analysis
The descriptive analysis was expressed in terms of
frequency, mean and standard deviation. Comparisons of different
continuous parameters, including SUVmax and length, between
different groups were performed with independent sample t-tests or
the analysis of variance (ANOVA) method. The Pearson’s correlation
was used to determine an association between tumor length and
SUVmax. The Spearman’s rank correlation was used to analyze
associations between SUVmax and other parameters, including
differentiation, stage, age and gender. A partial correlation was
used to control the length factor for the relationship between T
stage and SUVmax. The statistical analyses were performed using the
SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference. The
tests were two-sided.
Results
One hundred and twelve patients with a median age of
59 years (range, 39–79) were enrolled in this study, and presented
with 26 well-differentiated, 53 moderately differentiated, and 33
poorly differentiated tumors. Of these patients, 52 were stage
I–II, 48 were stage III and 12 were stage IV. Among them, 24 were
T1–T2, 52 were T3 and 36 were T4. The mean values of SUVmax and
tumor length were 12.02±5.81 and 5.88±2.97 cm, respectively.
Patient characteristics and the differences in the
SUVmax from the different groups are summarized in Table I. Our results demonstrated a
significant difference in SUVmax among the different lengths and T
stages of the primary tumors (P=0.000 and 0.017, respectively).
However, no significant difference was found in the SUVmax and
grade of tumor differentiation (P= 0.383), clinical stage (P=
0.583), N staging (P= 0.387), M staging (P=0.886), age (P=0.752) or
gender (P=0.233).
 | Table IPatient characteristics and
differences in SUVmax of the different groups. |
Table I
Patient characteristics and
differences in SUVmax of the different groups.
| Characteristics | No. of cases (%) | SUV (mean ± SD) | F-value | P-value |
|---|
| Gender | | | 1.440 | 0.233 |
| Female | 14 (12.5) | 13.76±7.98 | | |
| Male | 98 (87.5) | 11.77±5.43 | | |
| Age (years) | | | 0.100 | 0.752 |
| <60 | 48 (42.9) | 12.22±4.89 | | |
| ≥60 | 64 (57.1) | 11.87±6.44 | | |
| Length of tumor
(cm) | | | 16.111 | 0.000 |
| <6 | 56 (50.0) | 9.96±5.93 | | |
| ≥6 | 56 (50.0) | 14.09±4.91 | | |
| Differentiation | | | 1.918 | 0.153 |
| Well | 26 (23.2) | 12.03±4.38 | | |
| Moderate | 53 (47.3) | 12.51±5.76 | | |
| Poor | 33 (29.5) | 13.95±5.47 | | |
| Clinical stage | | | 0.542 | 0.583 |
| I–II | 52 (46.4) | 11.42±6.56 | | |
| III | 48 (42.9) | 12.62±5.06 | | |
| IV | 12 (10.7) | 12.25±5.14 | | |
| T stage | | | 4.216 | 0.017 |
| T1–T2 | 24 (21.4) | 9.42±7.21 | | |
| T3 | 52 (46.4) | 2.03±4.91 | | |
| T4 | 36 (32.2) | 13.75±5.46 | | |
| N stage | | | 0.753 | 0.387 |
| N0 | 40 (35.7) | 11.38±5.99 | | |
| N1 | 72 (64.3) | 12.38±5.71 | | |
| M stage | | | 0.021 | 0.886 |
| M0 | 100 (89.3) | 11.99±5.90 | | |
| M1 | 2 (10.7) | 12.25±5.14 | | |
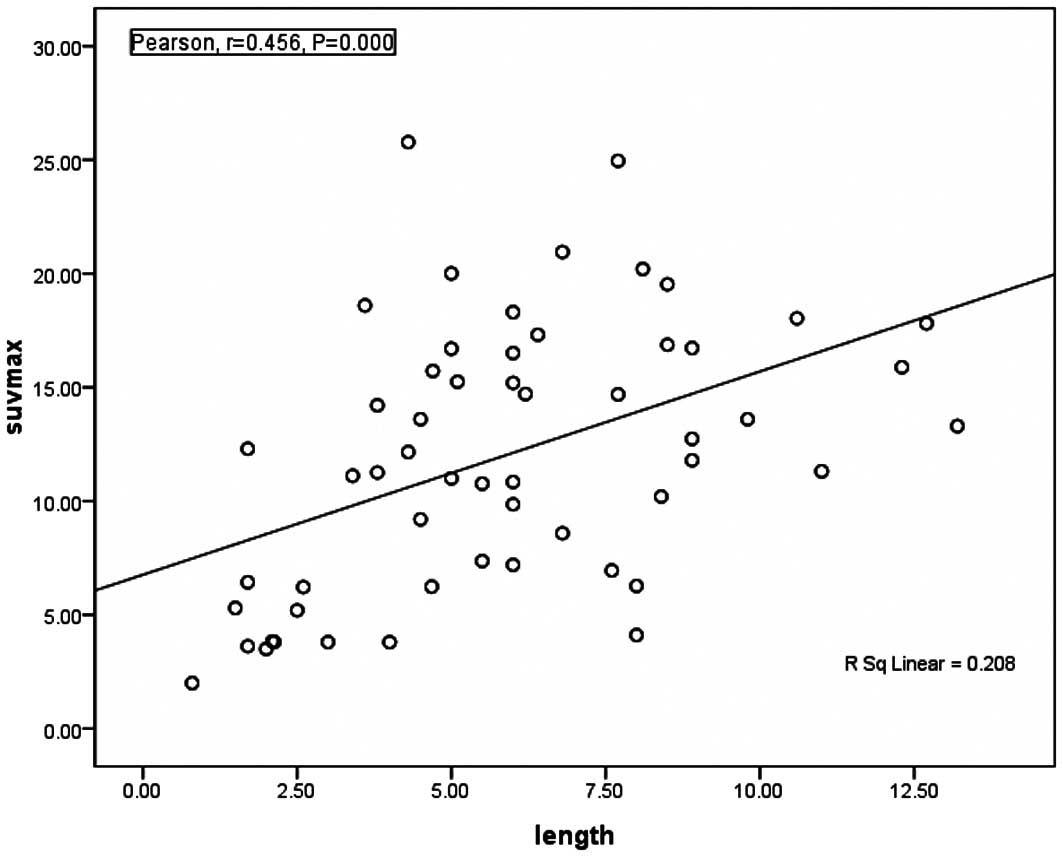
The correlations between the SUVmax and parameters
are listed in Table II. There was
a significant positive correlation between the SUVmax and the
length of the primary tumor (r= 0.456, P= 0.000; Fig. 1) and the depth of invasion of the
primary tumor (r= 0.257, P= 0.006; Table II). No significant relationship was
found between the SUVmax and other parameters. Moreover, the
partial correlation analysis using a controlled tumor length factor
did not find any statistically significant correlation between
SUVmax and T stage (r= 0.074, P=0.537). As a result, we concluded
that the SUVmax increased as the primary tumor length increased
(Tables I and II).
 | Table IICorrelations between SUVmax and ESCC
patient and tumor parameters. |
Table II
Correlations between SUVmax and ESCC
patient and tumor parameters.
|
Characteristics | r | P-value |
|---|
| Length of
tumor | 0.456 | 0.000 |
| T stage | 0.257 | 0.006 |
|
Differentiation | −0.191 | 0.061 |
| Clinical stage | 0.084 | 0.379 |
| N stage | 0.092 | 0.333 |
| M stage | 0.018 | 0.852 |
| Gender | 0.085 | 0.372 |
| Patient age | 0.018 | 0.847 |
Discussion
The 18F-FDG PET-CT procedure is
characterized by FDG uptake in a tumor and has been shown to
provide higher sensitivity of diagnosing a primary tumor, better
accuracy in detecting lymph node and distant metastasis, and better
prediction of prognosis of esophageal carcinoma than anatomical
structural imaging techniques based on morphological changes
(2,14,16).
The accumulation of SUV may be caused by inflammation and
represents an indicator of the potential malignancy. Buchmann et
al(17) concluded the maximum
FDG uptake by a tumor was weakly associated with tumor
proliferation. In addition, Mu et al(13) demonstrated a significant
correlation between SUV and glucose transporter-1 (Glut-1) protein
expression in esophageal cancer tissue and suggested that SUV
provides an indirect assessment of the proliferative capacity of
esophageal carcinoma tumors. Another study showed that the maximum
SUV of a tumor reflects the aggressive characteristics of a tumor
(7). Moreover, the SUVmax may
independently predict the extent of disease and survival of
esophageal carcinoma patients (9,14,18),
and therefore SUVmax may be a valuable marker that signifies the
biological behavior of a tumor. The prognosis of esophageal
carcinoma is largely dependent on tumor invasion, local and distant
metastases, tumor length and the grade of tumor differentiation.
However, a resection or biopsy is required to confirm the
diagnosis, guide therapy choices, and determine prognosis, which is
accompanied with certain risks. Therefore, the use of a noninvasive
PET-CT SUV has become an important focus in this setting.
The SUV has been shown to correlate with tumor
differentiation in lung carcinoma, head and neck cancers, and
esophageal cancer (8,19). However, previous studies on
esophageal carcinoma have been limited and the results were
inconsistent (12,13). Therefore, this study investigated
the relationship between the SUVmax and grade of tumor
differentiation of ESCC as well as other clinical parameters, such
as tumor stage or length. Cerfolio et al(14) showed that poorly differentiated
tumors tend to have a high SUVmax, and Feng et al(12) found that differentiation of ESCC
primary lesions positively correlate with the SUVmax. However, Mu
et al(13) found that there
was no significant difference between the SUVmax and
differentiation for a heterogeneous group of esophageal carcinoma
tumors. In this study, we analyzed a group of ESCC patients and did
not find any statistically significant difference between the
SUVmax and tumor differentiation. However, the SUVmax tended to
increase as tumor differentiation decreased. This discrepancy may
be due to the selected pool of heterogeneous patients, as a
previous study demonstrated that squamous cell carcinoma may have
greater FDG uptake than adenocarcinoma (14).
Tumor stage is used to describe the extent of
disease and tumor aggressiveness, and is an important parameter for
guiding treatment decisions and evaluating prognosis. A previous
study showed that PET-CT was a valuable tool for primary staging of
esophageal carcinoma (6) and that
SUVmax could identify the extent of tumor infiltration and nodal
involvement (20). Therefore,
based on these previous findings, the clinical TNM stage of the
tumors in our study was determined mainly by PET-CT scans in
combination with contrast CT and other imaging methods. Several
studies have investigated the relationship between the SUVmax and
tumor stage, and one study found that the SUVmax was significantly
correlated with clinical stage for adenocarcinoma lung cancer but
not squamous cell carcinoma (21).
In addition, Li et al(19)
indicated that the SUV was not related to the clinical staging of
nasopharyngeal carcinoma. However, Kato et al(18) found a significant association
between SUVmax and the stage of esophageal cancer, and increased
SUV uptake correlated with an advanced tumor stage (6,11,14,22,23).
However, our present study found no significant difference between
the SUVmax and clinical stage of ESCC, and did not demonstrate any
associated trends, which may most likely be due to the selection
bias of patients. However, a significant difference was found
between the T stage and the SUVmax, and the SUV increased with an
increasing depth of infiltration. After controlling for length, no
statistically significant correlation was found between the T stage
and FDG uptake values for ESCC. Therefore, we concluded that the
relationship between the T stage and SUVmax may be caused by the
length of the primary tumor. We did not find any correlation
between N or M staging and SUVmax, which is inconsistent with
previous studies (23). The
patients enrolled in our study were diagnosed with squamous cell
carcinoma, whereas other studies analyzed a heterogeneous group of
esophageal carcinoma patients that had been diagnosed with
adenocarcinoma, squamous cell carcinoma or other subtypes (6,11,14,18,23).
Primary tumor length has been shown to be a
prognostic factor of OS in ESCC patients (24,25).
Moreover, tumor length, as measured by PET-CT or PET, has been
shown to be associated with the stage and OS of esophageal cancer
(26). The metabolic response in
the reduction of SUV correlates with the tumor regression after
treatment (6). Feng et
al(12) demonstrated that the
SUVmax of primary tumors was positively correlated with tumor
length. Our study indicated that the length of ESCC tumors was
significantly correlated with SUVmax, and tumor lengths greater
than 6 cm had a higher SUVmax, which was similar to the findings of
a previous study showing that a higher SUV was associated with
longer tumors (P=0.0001) (11).
The SUVmax of primary esophageal carcinoma can predict tumor
length, which consequently provides preliminary information on
prognosis.
There were some limitations in our study. Firstly,
this study was retrospective in design and had a limited number of
patients. Secondly, most of the enrolled patients were nonsurgical
patients, and therefore accurate pathological TNM staging was not
available. However, we did acquire reliable clinical staging using
a combination of multi-imaging modalities. Thirdly, the FDG uptake
was positive correlated with the length of the primary tumor, but a
partial volume correction was not conducted. Fourthly, no survival
data were available to confirm our findings. Further research
should be conducted to determine the prognostic role and mechanism
of FDG uptake of ESCC.
In conclusion, tumor differentiation and clinical
stage were not significantly correlated with SUVmax, and no
significant differences in patient gender, age, N staging, and M
staging were found for SUVmax in this present study. The tumor
length influenced FDG uptake in ESCC tumors, and the T stage of the
primary tumor did not significantly correlate with the SUVmax after
controlling for length. Therefore, these data provide important
information for the management and evaluation of prognosis of ESCC
using an 18F-FDG PET-CT scan.
Acknowledgements
We thank all the PET-CT staff and the
technologists at our institute for their excellent support.
References
|
1
|
Lee JL, Park SI, Kim SB, et al: A single
institutional phase III trial of preoperative chemotherapy with
hyperfractionation radiotherapy plus surgery versus surgery alone
for resectable esophageal squamous cell carcinoma. Ann Oncol.
15:947–954. 2004. View Article : Google Scholar
|
|
2
|
Choi JY, Jang HJ, Shim YM, et al:
18F-FDG PET in patients with esophageal squamous cell
carcinoma undergoing curative surgery: prognostic implications. J
Nucl Med. 45:1843–1850. 2004.
|
|
3
|
Yuequan J, Shifeng C and Bing Z:
Prognostic factors and family history for survival of esophageal
squamous cell carcinoma patients after surgery. Ann Thorac Surg.
90:908–913. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Flamen P, Lerut A, Van Cutsem E, et al:
Utility of positron emission tomography for the staging of patients
with potentially operable esophageal carcinoma. J Clin Oncol.
18:3202–3210. 2000.PubMed/NCBI
|
|
5
|
Kato H, Miyazaki T, Nakajima M, et al: The
incremental effect of positron emission tomography on diagnostic
accuracy in the initial staging of esophageal carcinoma. Cancer.
103:148–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thurau K, Palmes D, Franzius C, Minin E,
Senninger N, Juergens KU and Bruewer M: Impact of PET-CT on primary
staging and response control on multimodal treatment of esophageal
cancer. World J Surg. 35:608–616. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang ZJ, Chen JH, Meng L, Du JJ, Zhang L,
Liu Y and Dai HH: 18F-FDG uptake as a biologic factor
predicting outcome in patients with resected non-small-cell lung
cancer. Chin Med J (Engl). 120:125–131. 2007.
|
|
8
|
Vesselle H, Schmidt RA, Pugsley JM, Li M,
Kohlmyer SG, Vallires E and Wood DE: Lung cancer proliferation
correlates with [F-18]fluorodeoxyglucose uptake by positron
emission tomography. Clin Cancer Res. 6:3837–3844. 2000.
|
|
9
|
Sepesi B, Raymond DP, Polomsky M, et al:
Does the value of PET-CT extend beyond pretreatment staging? An
analysis of survival in surgical patients with esophageal cancer. J
Gastrointest Surg. 13:2121–2127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhong X, Yu J, Zhang B, et al: Using
18F-fluorodeoxyglucose positron emission tomography to
estimate the length of gross tumor in patients with squamous cell
carcinoma of the esophagus. Int J Radiat Oncol Biol Phys.
73:136–141. 2009.
|
|
11
|
Suzuki A, Xiao L, Hayashi Y, et al:
Prognostic significance of baseline positron emission tomography
and importance of clinical complete response in patients with
esophageal or gastroesophageal junction cancer treated with
definitive chemoradiotherapy. Cancer. 117:4823–4833. 2011.
View Article : Google Scholar
|
|
12
|
Feng R, Li MH, Kong L, Shi F, Yang GR and
Yu JM: Correlation between PET-CT 18FDG uptake in
primary lesions and clinicopathological parameters in esophageal
carcinoma patients. Zhonghua Zhong Liu Za Zhi. 31:452–454. 2009.(In
Chinese).
|
|
13
|
Mu DB, Wang SP, Yang WF, Fu Z, Chen XX,
Sun XR and Yu JM: Correlation between FDG PET/CT and the expression
of glutl and ki-67 antigen in esophageal cancer. Zhonghua Zhong Liu
Za Zhi. 29:30–33. 2007.(In Chinese).
|
|
14
|
Cerfolio RJ and Bryant AS: Maximum
standardized uptake values on positron emission tomography of
esophageal cancer predicts stage, tumor biology, and survival. Ann
Thorac Surg. 82:391–394. 2006. View Article : Google Scholar
|
|
15
|
Greene FL, Page DL, Fleming ID, et al:
AJCC Cancer Staging Manual. 6th edition. Springer; New York, NY:
2002, View Article : Google Scholar
|
|
16
|
Kato H, Kuwano H, Nakajima M, et al:
Comparison between positron emission tomography and computed
tomography in the use of the assessment of esophageal carcinoma.
Cancer. 94:921–928. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Buchmann I, Haberkorn U, Schmidtmann I,
Brochhausen C, Buchholz HG, Bartenstein P and Hansen T: Influence
of cell proportions and proliferation rates on FDG uptake in
squamous-cell esophageal carcinoma: a PET study. Cancer Biother
Radiopharm. 23:172–180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kato H, Nakajima M, Sohda M, et al: The
clinical application of (18)F-fluorodeoxyglucose positron emission
tomography to predict survival in patients with operable esophageal
cancer. Cancer. 15:3196–3203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li J, Pan YD, Yin JL and Li XD: Analysis
of standard uptake values of 18F-FDG PET/CT in relation
to pathological classification and clinical staging of
nasopharyngeal carcinoma. Nan Fang Yi Ke Da Xue Xue Bao.
28:1923–1924. 2008.(In Chinese).
|
|
20
|
Hsu WH, Hsu PK, Wang SJ, Lin KH, Huang CS,
Hsieh CC and Wu YC: Positron emission tomography-computed
tomography in predicting locoregional invasion in esophageal
squamous cell carcinoma. Ann Thorac Surg. 87:1564–1568. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li M, Sun Y, Liu Y, et al: Relationship
between primary lesion FDG uptake and clinical stage at PET-CT for
non-small cell lung cancer patients: an observation. Lung Cancer.
68:394–397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rizk N, Downey RJ, Akhurst T, Gonen M,
Bains MS, Larson S and Rusch V: Preoperative
18[F]-fluorodeoxyglucose positron emission tomography
standardized uptake values predict survival after esophageal
adenocarcinoma resection. Ann Thorac Surg. 81:1076–1081. 2006.
|
|
23
|
van Westreenen HL, Plukker JT, Cobben DC,
Verhoogt CJ, Groen H and Jager PL: Prognostic value of the
standardized uptake value in esophageal cancer. AJR Am J
Roentgenol. 185:436–40. 2005.PubMed/NCBI
|
|
24
|
Liu H, Lu L, Zhu Q, et al: Cervical nodal
metastases of unresectable thoracic esophageal squamous cell
carcinoma: characteristics of long-term survivors after concurrent
chemoradiotherapy. Radiother Oncol. 99:181–186. 2011. View Article : Google Scholar
|
|
25
|
Eloubeidi MA, Desmond R, Arguedas MR, Reed
CE and Wilcox CM: Prognostic factors for the survival of patients
with esophageal carcinoma in the U.S.: the importance of tumor
length and lymph node status. Cancer. 95:1434–1443. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roedl JB, Sahani DV, Colen RR, Fischman
AJ, Mueller PR and Blake MA: Tumour length measured on PET-CT
predicts the most appropriate stage-dependent therapeutic approach
in oesophageal cancer. Eur Radiol. 18:2833–2840. 2008. View Article : Google Scholar : PubMed/NCBI
|