Introduction
Pancreatic carcinoma is one of the most frequently
occurring gastrointestinal malignancies and the incidence rate has
shown an upward trend worldwide (1). The prognosis for patients with
advanced pancreatic carcinoma remains poor with a 5-year survival
rate of <5% (1). Among the most
significant determinants of the poor prognosis associated with this
malignancy are the highly aggressive loco-regional invasion and
early metastasis that characterise this malignancy, such that the
majority of patients present with advanced, surgically unresectable
disease (2). Gemcitabine and
erlotinib are the only agents that are approved for the treatment
of pancreatic carcinoma. However, both drugs induce a poor response
in patients and their use can result in patients developing
multiple drug resistance (3,4).
Although in recent years, great progress has been observed with
regard to investigations on the molecular pathogenesis of
pancreatic carcinoma, the clinical treatment of pancreatic
carcinoma remains a challenge. Therefore, novel therapeutic
approaches to this malignancy are needed.
Gene-direct enzyme/prodrug therapy (GEPT), or
suicide gene therapy, aims to improve the therapeutic efficacy of
conventional cancer radio- and chemotherapy without side-effects
(5,6). This system has received a great deal
of attention for its clinical and therapeutic potential to treat
cancer. At present, a large number of enzyme/prodrug systems have
been developed for GEPT, two of which are the herpes simplex virus
thymidine kinase/ganciclovir (HSV-TK/GCV) and cytosine
deaminase/5-fluorocytosine (CD/5-FC) (7). Recently, some studies (8,9) have
tried to enhance the therapeutic effect of suicide gene therapy by
combining it with other gene therapies. However, the combination of
fusion suicide gene therapy with anti-angiogenesis gene therapy for
pancreatic carcinoma has yet to be reported.
Carcinoembryonic antigen-related cell adhesion
molecule (CEACAM)6, also known as CD66c or NCA-90, as well as
another 6 members of the CEACAM subgroup, belong to the human
carcinoembryonic antigen (CEA) family (10). CEACAM6 overexpression was found in
a wide variety of epithelial cancer types such as lung, breast,
colorectal, and hepatocellular carcinomas (11–14).
CEACAM6 has also become a target for pancreatic cancer therapy
(15,16). Overexpression of CEACAM6 was found
in >90% of invasive pancreatic adenocarcinomas (16). RNA interference (RNAi) is a process
involving sequence-specific and post-transcriptional gene
silencing. CEACAM6-specific RNAi decreases cancer cell
proliferation, metastasis and angiogenesis in pancreatic cancer
(15).
In the present study, we aimed to test the
feasibility of a novel therapeutic vector system involving a
combination of suicide gene therapy and antiangiogenesis gene
therapy. The in vitro experiments on pancreatic carcinoma
SW1990 cells were studied using a triple-gene vector expressing
CEACAM6-shRNA and the fusion suicide gene yCDglyTK.
Materials and methods
Cell lines and cell culture
The SW1990 human pancreatic carcinoma cell line (CEA
positive) was obtained from the Cancer Research Institution,
Central South University (Hunan, China). Cells were cultured in
RPMI-1640 medium (Invitrogen Inc., Carlsbad, CA, USA) with 10%
fetal bovine serum at 37°C in a humidified atmosphere of 5%
CO2 and 95% air.
Construction of the triple-gene plasmid
of pcDNA3.1(-) shCEACAM6-yCDglyTK
pcDNA3.1(-)CV-yCDglyTK was constructed in our
previous study (17). Expression
of the fusion suicide gene yCDglyTK was regulated by CMV- enhanced
CEA promoter and was expressed specifically in CEA-positive cells.
Oligonucleotides encoding the corresponding small hairpin RNA
(termed CEACAM6-shRNA) was generated by ligation of inserts
targeting the following sequences into a hU6 promoter-contained
pUC57-simple plasmid: 5′-CCG GACAGTTCCATGTATATTCAAGACGTATACATGGAAC
TGTCGTTTTTT-3′ (sense: CCGGACAGTTCCATGTATA; loop: TTCAAGACG;
antisense: TATACATGGAACTGT CCGG; termination code: TTTTTT).
shCEACAM6 and pcDNA3.1(-)yCDglyTK were then fused by NheI
and XbaI restriction endonuclease overnight at 37°C to form
a recombinant plasmid of shCEACAM6 and the fusion suicide gene
yCDglyTK .Thus, a novel triple-gene vector pcDNA3.1(-)
shCEACAM6-yCDglyTK was developed. Double enzyme cutting and
sequencing were performed to prove the accuracy of the new
plasmid.
Stable transfection in vitro
The novel triple-gene vector,
pcDNA3.1(-)shCEACAM6-yCDglyTK plasmid was mixed with Lipofectamine
2000 at the combination rate of 1/2 to 1/3. SW1990 human pancreatic
carcinoma cells were seeded in 6-well plates at a density of
2×105 cells per well. When the cell monolayer reached
70–80% confluence, pcDNA3.1(-) null, pcDNA3.1(-)yCDglyTK and
pcDNA3.1(-)shCEACAM6-yCDglyTK were added to different 6-well
plates. The following day, a 1:10 passage of the transfected SW1990
cells was performed, followed by G418 selection (400 μg/ml).
Approximately 3 weeks later, the resistant colonies were picked and
transferred to 96-well plates. These clones were maintained in
selective culture medium (with 200 μg/ml of G418). Surviving
colonies transfected with pcDNA3.1(-)null, pcDNA3.1(-) yCDglyTK, or
pcDNA3.1(-)shCEACAM6-yCDglyTK were designated as SW/null, SW/CDTK,
or SW/shCEACAM6-CDTK, respectively, and subjected to further
study.
Reverse transcription polymerase chain
reaction (RT-PCR) and western blot analysis
Total RNA from parental SW1990 cells and three
different transfected cells was extracted using TRIzol reagent
(Invitrogen Inc.). The quantity and quality of RNA was assessed by
absorbance at 260 and 280 nm, respectively. The RT reaction was
carried out using the ReverTra Ace® RT Kit (Toyobo Co.,
Ltd., Osaka, Japan) as per the manufacturer’s instructions.
Subsequently, we performed PCR on the cDNA product. For yCDglyTK, a
PCR product of 707 bp was produced by the forward primer
5′-GGGAGATT AGAGGGCAAAGTGT-3′ and reverse primer 5′-ACGGCGT
CGGTCACGGCATAA-3′. For CEACAM6, a PCR product of 356 bp was
produced by the forward primer 5′-CGTTCAAT GTCGCAGAGGG-3′ and
reverse primer 5′-CGCTGAGTA GAGTGAGGGT-3′. β-actin was used as an
internal control and a PCR product of 285 bp was produced by the
forward primer 5′-AGCGAGCATCCCCCAAAGTT-3′ and reverse primer
5′-GGGCACGAAGGCTCATCATT-3′.
For western blot analysis, cells were collected 72 h
after transfection and lysed in loading buffer (20 mmol/l Tris-HCl,
pH 7.5; 150 mmol/l NaCl, 1 mmol/l EDTA, 5 mmol/l DTT, 1% Triton
X-100). The lysates were centrifuged at 12,000 × g for 15 min at
4°C. The supernatant was collected and protein concentrations were
determined by the BCA protein assay. Protein (40 μg) was
separated via 15% SDS-PAGE and transferred to PVDF film (Amersham
Pharmacia Biotech, Piscataway, NJ, USA). The films were incubated
in blocking solution, consisting of 5% skimmed milk in TBS-T [10 mM
Tris-HCl (pH 8.0), 150 mM NaCl and 0.1% Tween-20], for 1 h at room
temperature, then probed with mouse anti-CEACAM6 antibody (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA), rabbit anti-TK
antibody (QED Bioscience, Inc., San Diego, CA, USA) or mouse
anti-β-actin antibody (Sigma-Aldrich, St. Louis, MO, USA). This was
followed by incubation with their respective peroxidase-conjugated
secondary antibodies. Hybridization was visualized using the ECL
chemiluminescence detection system (Kodak).
CDTK/5-FC, shCEACAM6-CDTK/5-FC
system-induced cytotoxicity
SW1990 cells (transfected and untransfected) were
seeded in 96-well plates at a density of 8,000 cells per well and
incubated at 37°C and 5% CO2 in humidified air for 24 h.
The following day, 5-fluorocytosine (5-FC) was added into the
culture medium at a final concentration of 200 μg/ml. MTT
assays were conducted at 24-, 48-, 72- and 96-h incubation time
points to analyze cell viability. Twenty microliters of MTT
solution (5 mg/ml, Sigma-Aldrich) were added to each well and
incubated for 4 h. Dimethylsulfoxide (DMSO, Promega Corporation,
Madison, WI, USA) was added to dissolve the blue crystal. The
optical density (OD) was then determined using a multi-well plate
reader (Awareness, model Stat-Fax-2100, USA) by measuring
absorbance at 570 nm (OD570), with the absorbance at 690 nm as
reference. The background absorbance of medium was also subtracted.
Samples were assayed in triplicate, and the mean for each
experiment was calculated. Cell growth curves were plotted, with
culture time on the horizontal axis and OD570 on the vertical
axis.
Invasion and migration assay
The invasion assay was performed using an 8
μm pore size transwell chamber in 24-well plates (Corning
Costar, Cambridge, MA, USA). SW1990 cells stably expressing
pcDNA3.1(-), pcDNA3.1(-)yCDglyTK, pcDNA3.1(-) shCEACAM6-yCDglyTK or
untransfected SW1900 cells in 500 μl of serum-free MEM
medium were loaded into the top chamber with fetal bovine serum
placed in the bottom chamber as a chemoattractant. After further
incubation at 37°C for 10 h, the cells on the top of the filters
were removed with cotton swabs. The cells on the lower surface of
the filters were fixed in 4% paraformaldehyde and stained with 0.1%
crystal violet. The crystal violet was removed and the cells were
washed three times with phosphate-buffered saline (PBS). The
remaining crystal violet staining of the migrated cells was eluted
with one wash with 33% acetic acid. The OD540 nm of the eluted
crystal violet was determined as a measure of migrated cells. Each
experiment was performed in triplicate. The invasion of different
groups was observed under a microscope. The cell migration assay
was performed in a similar mode, except that cells were seeded into
the uncoated filter and incubated for 24 h. Each measurement was
performed in at least three independent experiments.
Statistical analysis
Statistical analysis was performed by one-way
analysis of variance (ANOVA) test. P<0.05 was considered to
indicate a statistically significant difference. Numeric data were
presented as the mean values ± standard deviation (SD).
Results
Recombinant plasmid was successfully
constructed
An interfering plasmid targeting CEACAM6 was
initially constructed. The CEACAM6-shRNA expression cassette was
subcloned into pcDNA3.1(-)CV-yCDglyTK to construct the novel vector
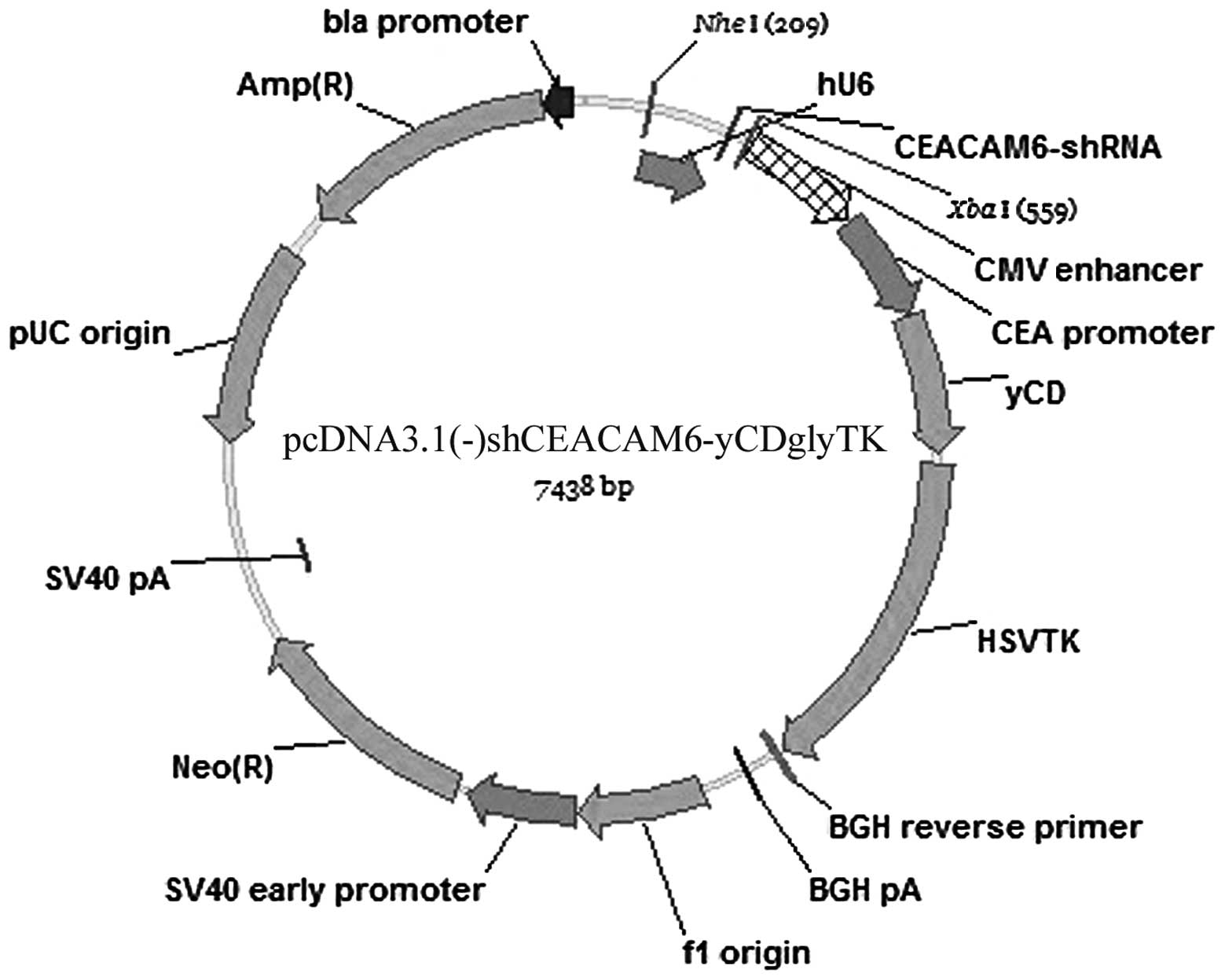
pcDNA3.1(-)shCEACAM6-yCDglyTK (Fig. 1). In this novel triple-expressing
plasmid, the CEACAM6-shRNA sequence was placed under control of the
U6 promoter, while the fusion suicide gene yCDglyTK was driven by a
CMV-enhanced CEA promoter. Newly constructed gene plasmid
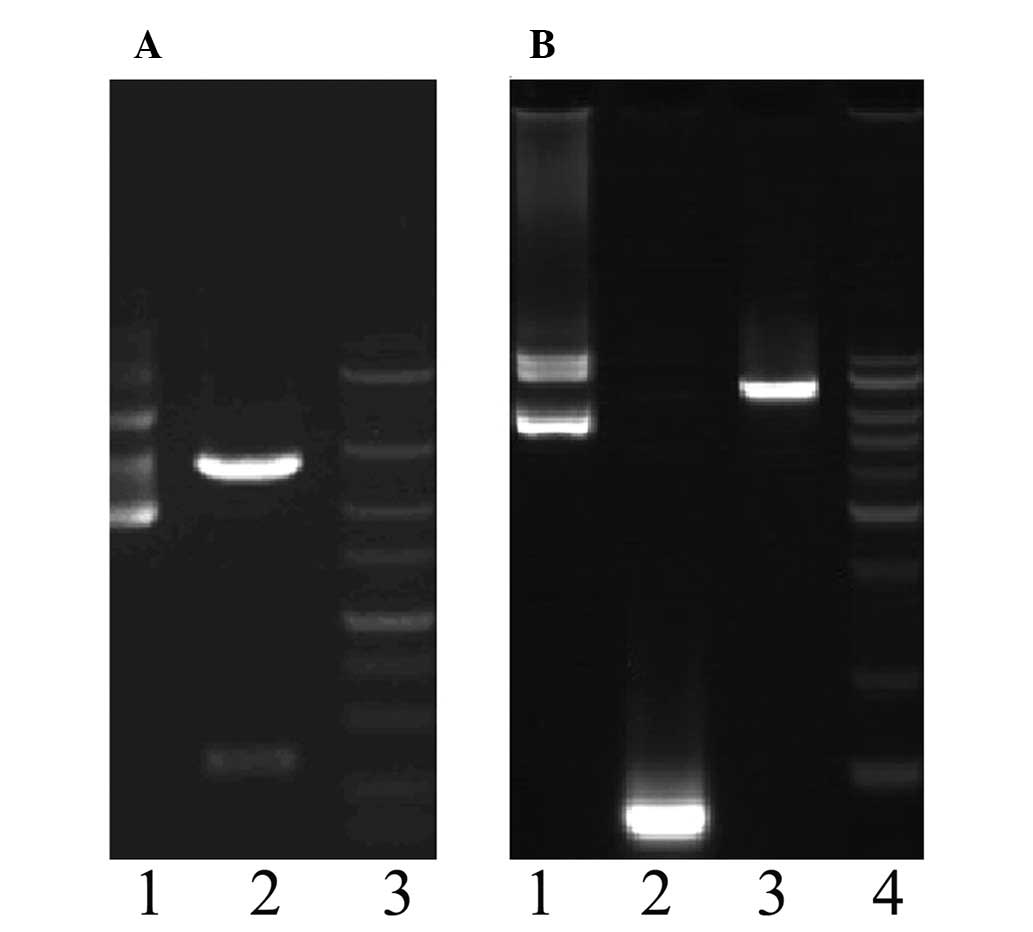
pcDNA3.1(-)shCEACAM6-yCDglyTK was identified by double-enzyme
cutting and sequencing. As expected, the size of the newly
constructed plasmid fragment by enzyme cutting was 7.5 kb (Fig. 2). Sequencing results showed that
the newly constructed target-combined double suicide gene plasmid
was in concordance with pcDNA3.1(-) shCEACAM6-yCDglyTK (data not
shown).
Recombinant plasmid was effectively
delivered into SW1990 cells in vitro
This novel vector was delivered into SW1990 cells
and stably transfected cell lines were obtained by G418 selection.
At the same time, SW1990 cells stably transfected with three other
plasmids, pcDNA3.1(-)null, pcDNA3.1(-) yCDglyTK and
pcDNA3.1(-)shCEACAM6-yCDglyTK were also established. The expression
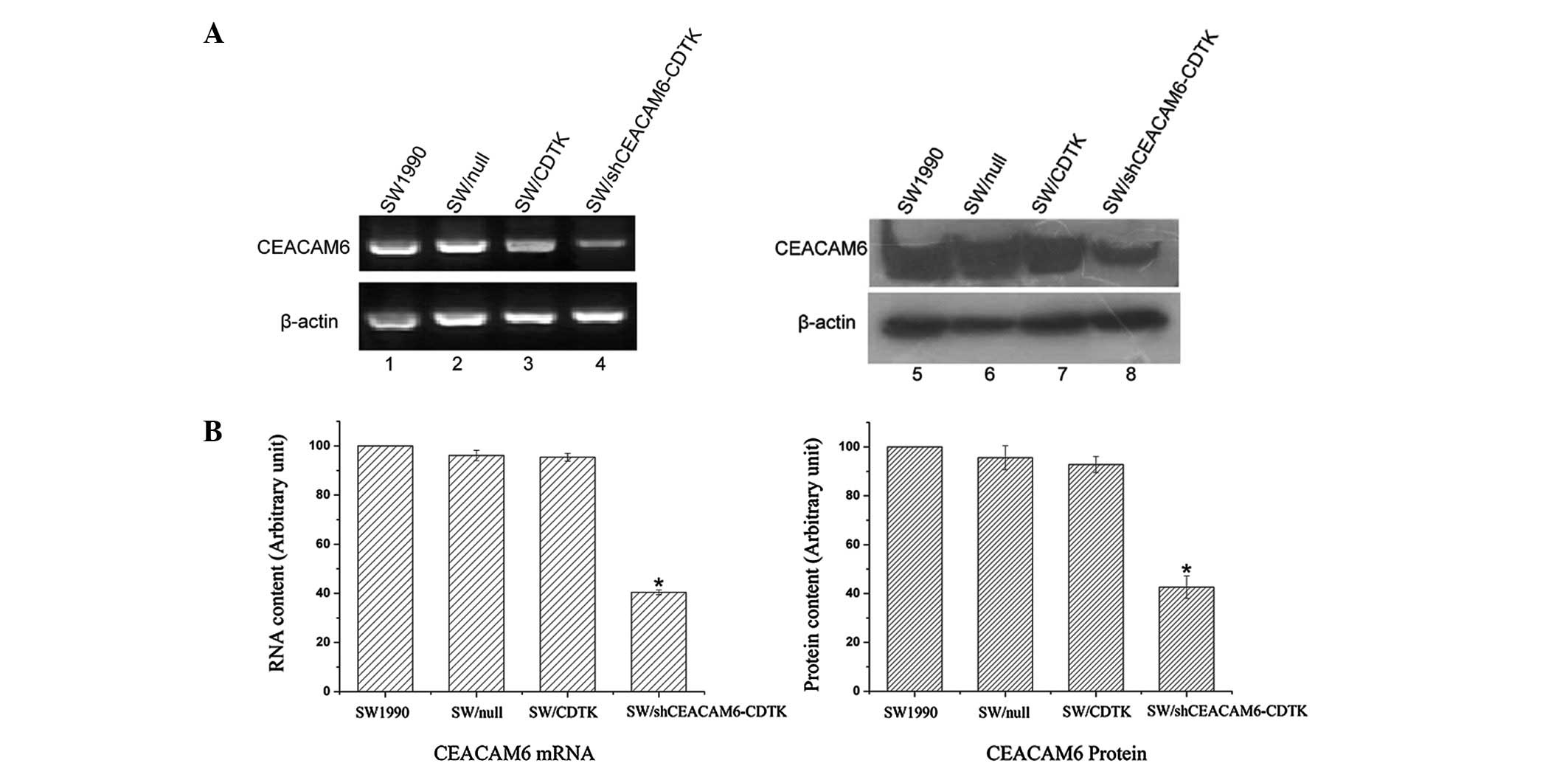
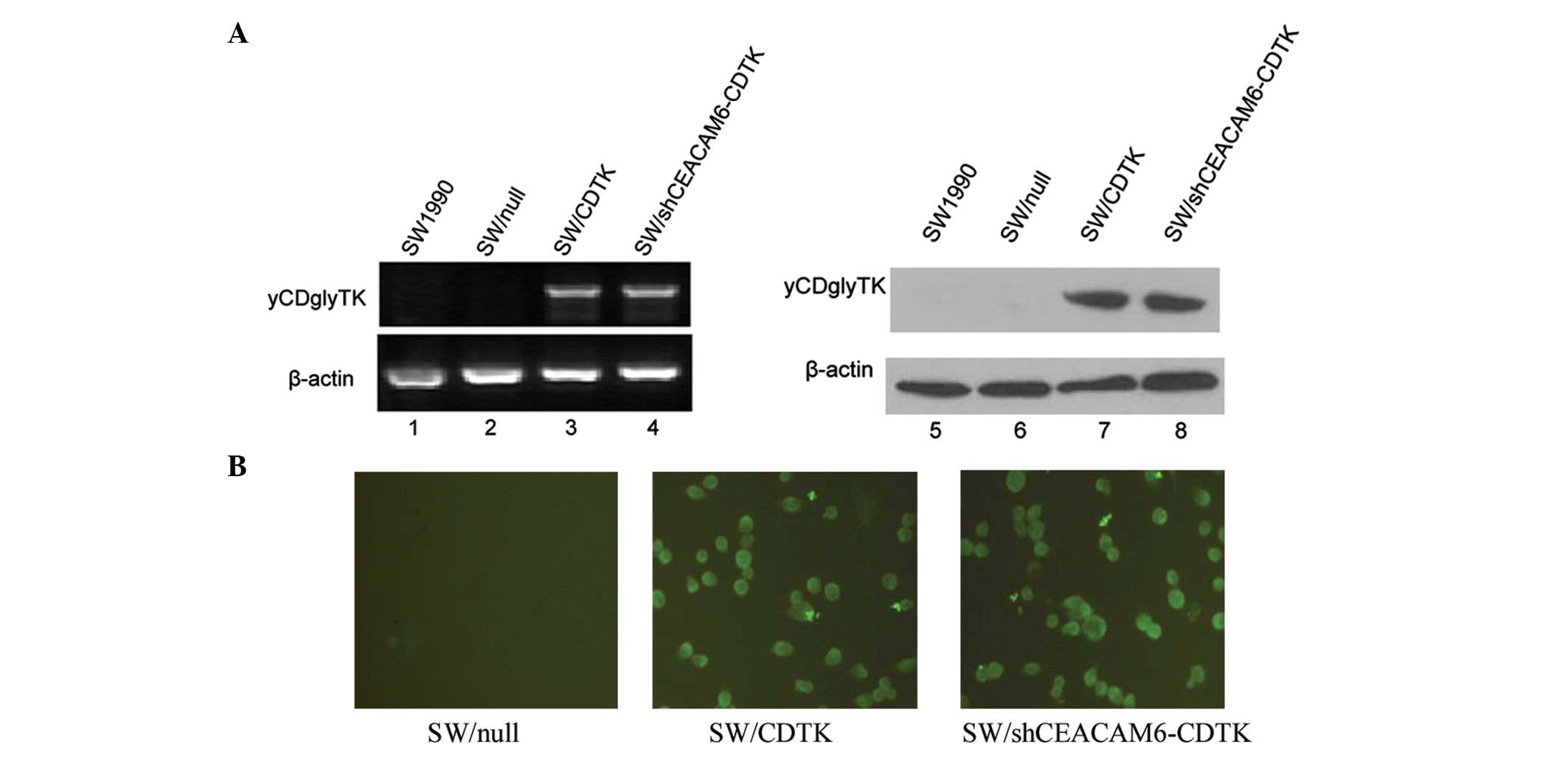
of CEACAM6 and yCDglyTK were determined via RT-PCR, western blot
analysis and immunofluorescence. Compared with parent SW1990 cells
and SW/null, mRNA and protein levels of CEACAM6 were significantly
decreased in SW/shCEACAM6-CDTK (Fig.
3). yCDglyTK was confirmed to be expressed in SW/CDTK and
SW/shCEACAM6-CDTK cells, but not in the parent SW1990 cells and
SW/null (Fig. 4).
Recombinant plasmid pcDNA3.1(-)shCEACA M6
- yCDglyTK/5-FC system resulted in cytotoxicity in SW1990
cells
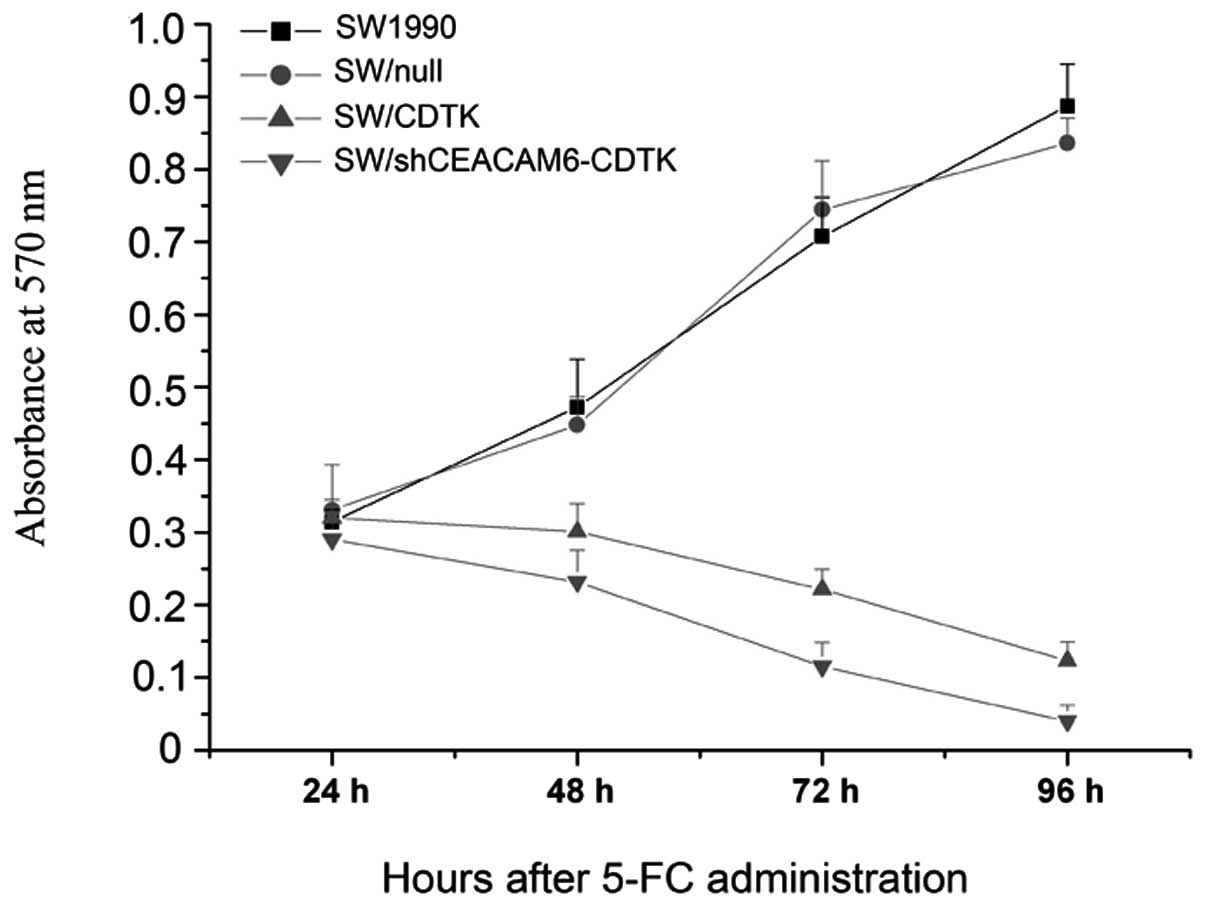
After a 24-h treatment with 5-FC, the OD570 of
SW/CDTK cells and SW/shCEACAM6-CDTK cells decreased significantly
compared to SW1990 or SW1990/null cells (P<0.01), as shown in
the cell growth curve in Fig. 5.
As time progressed, a high proliferation rate was maintained in
untransfected SW1990 and SW/null cells. After 48 h, the OD570 did
not increase in SW/CDTK or SW/shCEACAM6-CDTK cells. At 72- and 96-h
treatment with 5-FC, a decrease was observed for the OD570,
suggesting that most cells in the process of being killed. Low
shCEACAM6-CDTK cell viability was detected.
Recombinant plasmid
pcDNA3.1(-)shCEACAM6-yCDglyTK inhibited SW1990 cell invasion and
migration
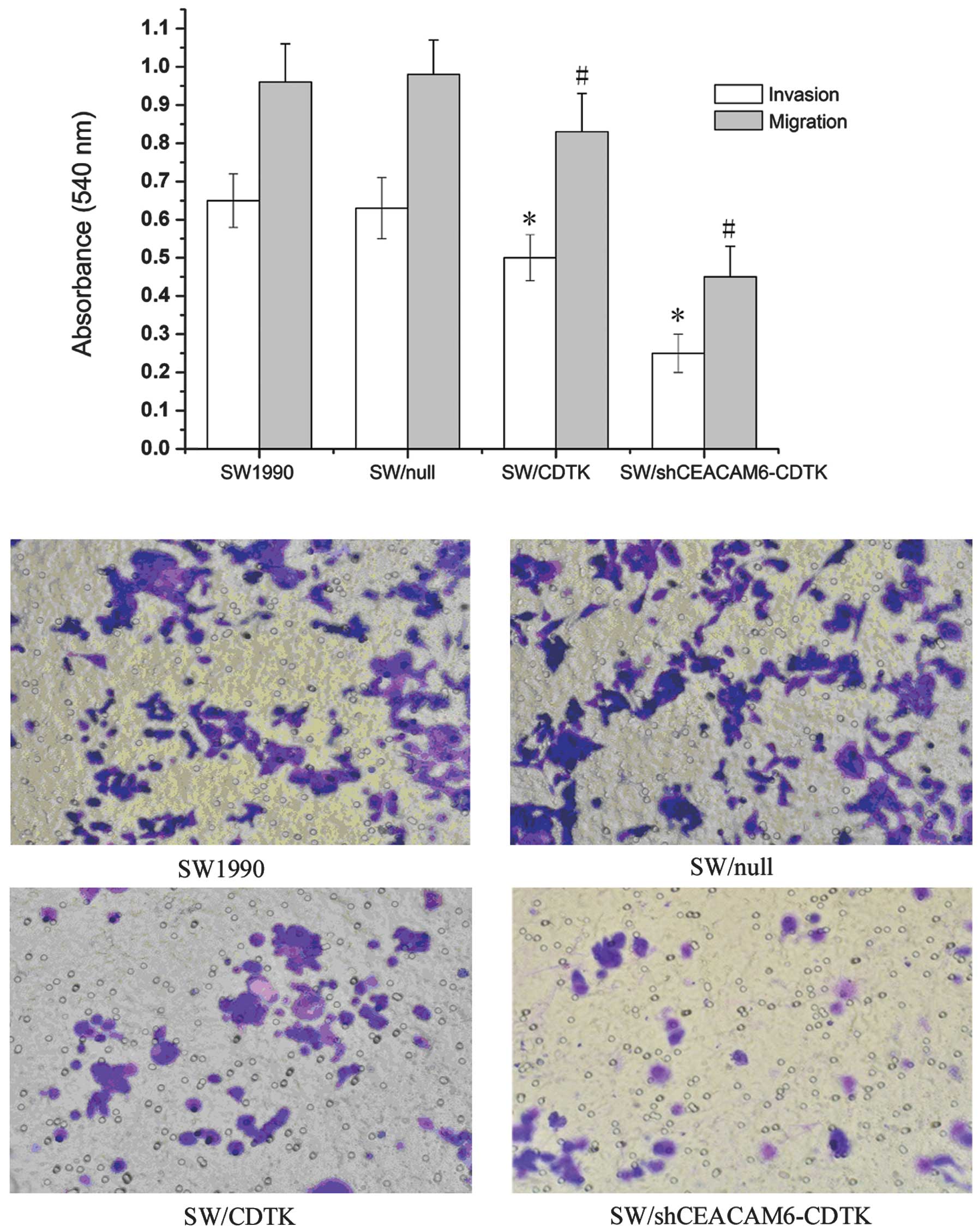
The motility of three different transfected cells
across transwell polycarbonate membranes was evaluated. As shown in
Fig. 6A, compared with SW1990 and
SW/null, the cell invasiveness and migration of SW/CDTK were
attenuated to 50.41 and 80.12%, respectively (P<0.01), while
those of SW/shCEACAM6-CDTK cells were reduced more significantly to
24.61 and 45.17%, respectively (P<0.01). By contrast, no
differences were observed between SW1990 and SW/null (P>0.05)
(Fig. 6B).
Discussion
Mounting evidence suggests that combination cancer
therapy has the potential to be effective in combating
malignancies. Combination gene therapy has the advantages of gene
therapy, elevates the therapeutic efficacy and overcomes the
shortcomings of single gene therapy (18). Although suicide gene therapy is a
potentially effective method for killing tumor cells in
vitro and in vivo, the results of clinical trials
indicate a need for greater efficacy (19). In previous studies, the co-transfer
of vectors carrying different genes to enhance anti-tumor effect
has been attempted (20).
Combination of TK/CD gene therapy gene therapy with lipiodol
embolism in the treatment of liver cancer may effectively inhibit
cancer growth and prolong the survival time (9). In this study, to test the feasibility
of a novel therapeutic vector system involving a combination of
suicide and RNAi-based gene therapy, we initially constructed the
novel vector pcDNA3.1(-)shCEACAM6-yCDglyTK, which was regulated by
a U6 promoter, while the fusion suicide gene yCDglyTK was driven by
a CMV-enhanced CEA promoter. Normal expression of each gene was
confirmed by RT-PCR and western blot analysis. Pancreatic carcinoma
cell lines stably expressing the CEACAM6 shRNA and yCDglyTK gene
were then established and anti-tumor efficacy of the recombinant
plasmid was evaluated in vitro.
Suicide genes are viral or bacterial enzymes capable
of converting non-toxic prodrugs into toxic metabolites that cause
tumor cell death when introduced into tumor sites. CD and HSV-TK
are typical suicide genes that convert non-toxic prodrugs, such as
5-FC and GCV, into cytotoxic metabolites, such as 5-fluorouracil
(5-FU) and GCV-TP, respectively (20–23).
Combination of HSV-TK/GCV and CD/5-FC might have synergistic
effects (24). Previous studies
showed that yCD, a yeast-derived CD, is efficient at deaminating
5-FC to yield 5-FU, whereas bacterial CD (bCD) has a poor
conversion efficiency (25).
Moriuchi et al(26)
reported that TK was able to mediate the phosphorylation of 5-FU
metabolites, and might reduce the cytotoxicity of CD/5-FC system.
GCV had the potential to interfere with 5-FC in the yCDglyTK
system, thus 5-FC was used as the only prodrug in our study.
Cytotoxicity of the yCDglyTK gene in the presence of prodrugs using
MTT assay was tested. The mean cell viability decreased in a
time-dependent manner in yCDglyTK- and
shCEACAM6-yCDglyTK-transfected SW1990 cells, although not in
untransfected SW1990 cells. Our results have shown that yCDglyTK
was confirmed to be expressed in SW/CDTK and SW/shCEACAM6-CDTK
cells, rendering the new system efficient in delivering suicide
gene into cancer cells and inducing cytotoxicity.
CEACAM6 is a single-chain GPI-anchored
immunoglobulin (Ig)-like glycoprotein and is a member of the human
CEA family (27). Jantscheff et
al(28) showed that CEACAM6
overexpression was associated with poor clinical outcome in
colorectal cancer. CEACAM6 overexpression independently predicted
poor overall survival and disease-free survival, whereas CEACAM1 or
CEACAM5 was not significantly associated with these outcomes.
CEACAM6 overexpression leads to morphology changes that are similar
to epithelium-messenchymal-transformation (29), increased invasiveness (29), increased chemoresistance (30) and resistance to anoikis (31–33),
whereas CEACAM6 appears to exert its pro-invasive effect in a
c-Src-dependent manner, at least in part through the upregulation
of MMP-9 activity (34). It has
also been proposed that low levels of E-cadherin-mediated
cell-to-cell interaction are important in tumor invasiveness and
metastasis (35,36). Suppressing CEACAM6 gene expression
or inhibiting CEACAM6 function can reverse these effects.
Inhibition of CEACAM6 function using an antibody fragment can
affect cell migration, invasion and adhesion in
vitro(12,34). RNAi offers a unique opportunity to
silence the expression of individual genes with a high degree of
specificity, allowing the roles of individual genes to be dissected
(37). In the present study, we
investigated the invasion and migration-inhibitory effects of the
yCDglyTK- and shCEACAM6-yCDglyTK-transfected SW1990 cells. The
invasion and migration were significantly suppressed (P<0.01) in
the two transfected groups. The inhibitory rates of
shCEACAM6-yCDglyTK-transfected SW1990 cells were more prominent
than those of yCDglyTK-transfected SW1990 cells. Suppression of the
CEACAM6 transcripts using siRNA of CEACAM6 leading to a reduction
in cancer cell invasiveness, may be associated with an increase in
E-cadherin promoter activity (35). CEACAM6-siRNA or yCDglyTK alone has
the potential to kill cancer cells and cause tumor growth delay, as
well as inhibit tumor invasion and migration. However, a
combination of the two genes has been shown to achieve a stronger
anti-tumor effect, demonstrating a synergistic effect between
CEACAM6-siRNA and yCDglyTK.
The novel recombinant plasmid was able to silence
functional genome CEACAM6, inhibit tumor invasion and metastasis.
However, there are potential defects with this new system. CEA
protein only overexpresses in a majority of pancreatic cancer
cells. In our triple-expressing vector pcDNA3.1(-)
shCEACAM6-yCDglyTK, we used a CEA promoter to drive the expression
of yCDglyTK, a treatment that specifically killed CEA-positive
cancer cells. The novel shCEACAM6-yCDglyTK system had little effect
on the CEA-negative pancreatic cancer cells. Moreover, a low level
of CEACAM6 protein expression has been noted in a variety of normal
human tissues, including granulocytes and epithelia from various
organs (38) and this expression
is also associated with infectious diseases (39,40).
The novel system may therefore not only target specific tumor
tissues. Strategies aiming to improve the safety of RNAi-based gene
therapy are therefore necessary.
In conclusion, the results from the present study
have demonstrated that CEACAM6-targeted RNAi with suicide gene
therapies had a synergistic effect. Additionally, the combination
gene therapy system may be a valid and viable strategy to inhibit
the proliferation, as well as attenuate the invasiveness and
metastasis of pancreatic carcinoma SW1990 cells in vitro.
The present study provides a novel gene therapy strategy that is
effective, not only for pancreatic cancer, but also for other
CEACAM6-expressing tumors.
Abbreviations:
|
CEACAM6
|
carcinoembryonic antigen-related cell
adhesion molecule
|
|
CEA
|
carcinoembryonic antigen
|
|
shRNA
|
short hairpin RNA
|
|
GEPT
|
gene-direct enzyme/prodrug therapy
|
|
RNAi
|
RNA interference
|
|
CD
|
cytosine deaminase
|
|
HSV-TK
|
herpes simplex virus thymidine
kinase
|
|
5-FC
|
5-fluorocytosine
|
|
GCV
|
ganciclovir
|
Acknowledgements
This study was supported partially by
the Project from Science and Technology Department, Hunan, China
(no. 2010FJ4087), a grant from Science and Technology Bureau,
Changsha, China (no. K1203051-31) and the Innovation Program of
Central South University, China (no. YB10071).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics. CA Cancer J Clin. 60:277–300. 2010.
|
|
2
|
Bond-Smith G, Banga N, Hammond TM and
Imber CJ: Pancreatic adenocarcinoma. BMJ. 344:e24762012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arlt A, Gehrz A, Muerkoster S, et al: Role
of NF-kappaB and Akt/PI3K in the resistance of pancreatic carcinoma
cell lines against gemcitabine-induced cell death. Oncogene.
22:3243–3251. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stathis A and Moore MJ: Advanced
pancreatic carcinoma: current treatment and future challenges. Nat
Rev Clin Oncol. 7:163–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hedley D, Ogilvie L and Springer C:
Carboxypeptidase-G2-based gene-directed enzyme-prodrug therapy: a
new weapon in the GDEPT armoury. Nat Rev Cancer. 7:870–879. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nawa A, Tanino T, Luo C, et al: Gene
directed enzyme prodrug therapy for ovarian cancer: could GDEPT
become a promising treatment against ovarian cancer. Anticancer
Agents Med Chem. 8:232–239. 2008. View Article : Google Scholar
|
|
7
|
Shah K: Mesenchymal stem cells engineered
for cancer therapy. Adv Drug Deliv Rev. 64:739–748. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahn YH, Yi H, Shin JY, et al: STAT3
silencing enhances the efficacy of the HSV.tk suicide gene in
gastrointestinal cancer therapy. Clin Exp Metastasis. 29:359–369.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niu HX, Du T, Xu ZF, Zhang XK and Wang RG:
Role of wild type p53 and double suicide genes in interventional
therapy of liver cancer in rabbits. Acta Cir Bras. 27:522–528.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barnett T, Goebel SJ, Nothdurft MA and
Elting JJ: Carcinoembryonic antigen family: characterization of
cDNAs coding for NCA and CEA and suggestion of nonrandom sequence
variation in their conserved loop-domains. Genomics. 3:59–66. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuroki M, Matsushita H, Matsumoto H,
Hirose Y, Senba T and Yamamoto T: Nonspecific cross-reacting
antigen-50/90 (NCA-50/90) as a new tumor marker. Anticancer Res.
19:5599–5606. 1999.PubMed/NCBI
|
|
12
|
Blumenthal RD, Hansen HJ and Goldenberg
DM: Inhibition of adhesion, invasion, and metastasis by antibodies
targeting CEACAM6 (NCA-90) and CEACAM5 (carcinoembryonic antigen).
Cancer Res. 65:8809–8817. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Poola I, Shokrani B, Bhatnagar R, DeWitty
RL, Yue Q and Bonney G: Expression of carcinoembryonic antigen cell
adhesion molecule 6 oncoprotein in atypical ductal hyperplastic
tissues is associated with the development of invasive breast
cancer. Clin Cancer Res. 12:4773–4783. 2006. View Article : Google Scholar
|
|
14
|
Blumenthal RD, Leon E, Hansen HJ and
Goldenberg DM: Expression patterns of CEACAM5 and CEACAM6 in
primary and metastatic cancers. BMC Cancer. 7:22007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duxbury MS, Matros E, Ito H, Zinner MJ,
Ashley SW and Whang EE: Systemic siRNA-mediated gene silencing: a
new approach to targeted therapy of cancer. Ann Surg. 240:667–676.
2004.PubMed/NCBI
|
|
16
|
Strickland LA, Ross J, Williams S, et al:
Preclinical evaluation of carcinoembryonic cell adhesion molecule
(CEACAM) 6 as potential therapy target for pancreatic
adenocarcinoma. J Pathol. 218:380–390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu T, Zhang G, Chen Y, et al: Tissue
specific expression of suicide genes delivered by nanoparticles
inhibits gastric carcinoma growth. Cancer Biol Ther. 5:1683–1690.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SW, Lee YL, Lee YJ, et al: Enhanced
antitumor effects by combination gene therapy using MDR1 gene shRNA
and HSV1-tk in a xenograft mouse model. Cancer Lett. 291:83–89.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rainov NG: A phase III clinical evaluation
of herpes simplex virus type 1 thymidine kinase and ganciclovir
gene therapy as an adjuvant to surgical resection and radiation in
adults with previously untreated glioblastoma multiforme. Hum Gene
Ther. 11:2389–2401. 2000. View Article : Google Scholar
|
|
20
|
Boucher PD, Im MM, Freytag SO and Shewach
DS: A novel mechanism of synergistic cytotoxicity with
5-fluorocytosine and ganciclovir in double suicide gene therapy.
Cancer Res. 66:3230–3237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rubsam LZ, Davidson BL and Shewach DS:
Superior cytotoxicity with ganciclovir compared with acyclovir and
1-beta-D-arabinofuranosylthymine in herpes simplex virus-thymidine
kinase-expressing cells: a novel paradigm for cell killing. Cancer
Res. 58:3873–3882. 1998.
|
|
22
|
Tomicic MT, Thust R and Kaina B:
Ganciclovir-induced apoptosis in HSV-1 thymidine kinase expressing
cells: critical role of DNA breaks, Bcl-2 decline and caspase-9
activation. Oncogene. 21:2141–2153. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang JF, Wei F, Wang HP, et al: Potent
anti-tumor activity of telomerase-dependent and HSV-TK armed
oncolytic adenovirus for non-small cell lung cancer in vitro and in
vivo. J Exp Clin Cancer Res. 29:522010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miyagi T, Koshida K, Hori O, et al: Gene
therapy for prostate cancer using the cytosine deaminase/uracil
phosphoribosyltransferase suicide system. J Gene Med. 5:30–37.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kievit E, Bershad E, Ng E, et al:
Superiority of yeast over bacterial cytosine deaminase for
enzyme/prodrug gene therapy in colon cancer xenografts. Cancer Res.
59:1417–1421. 1999.PubMed/NCBI
|
|
26
|
Moriuchi S, Wolfe D, Tamura M, et al:
Double suicide gene therapy using a replication defective herpes
simplex virus vector reveals reciprocal interference in a malignant
glioma model. Gene Ther. 9:584–591. 2002. View Article : Google Scholar
|
|
27
|
Hammarstrom S: The carcinoembryonic
antigen (CEA) family: structures, suggested functions and
expression in normal and malignant tissues. Semin Cancer Biol.
9:67–81. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jantscheff P, Terracciano L, Lowy A, et
al: Expression of CEACAM6 in resectable colorectal cancer: a factor
of independent prognostic significance. J Clin Oncol. 21:3638–3646.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lewis-Wambi JS, Cunliffe HE, Kim HR,
Willis AL and Jordan VC: Overexpression of CEACAM6 promotes
migration and invasion of oestrogen-deprived breast cancer cells.
Eur J Cancer. 44:1770–1779. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Duxbury MS, Ito H, Benoit E, Waseem T,
Ashley SW and Whang EE: A novel role for carcinoembryonic
antigen-related cell adhesion molecule 6 as a determinant of
gemcitabine chemoresistance in pancreatic adenocarcinoma cells.
Cancer Res. 64:3987–3993. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ordonez C, Screaton RA, Ilantzis C and
Stanners CP: Human carcinoembryonic antigen functions as a general
inhibitor of anoikis. Cancer Res. 60:3419–3424. 2000.PubMed/NCBI
|
|
32
|
Zhu Z, Sanchez-Sweatman O, Huang X, et al:
Anoikis and meta-static potential of cloudman S91 melanoma cells.
Cancer Res. 61:1707–1716. 2001.PubMed/NCBI
|
|
33
|
Duxbury MS, Ito H, Zinner MJ, Ashley SW
and Whang EE: CEACAM6 gene silencing impairs anoikis resistance and
in vivo metastatic ability of pancreatic adenocarcinoma cells.
Oncogene. 23:465–473. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Duxbury MS, Ito H, Benoit E, Ashley SW and
Whang EE: CEACAM6 is a determinant of pancreatic adenocarcinoma
cellular invasiveness. Br J Cancer. 91:1384–1390. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Takeichi M: Cadherins in cancer:
implications for invasion and metastasis. Curr Opin Cell Biol.
5:806–811. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Birchmeier W and Behrens J: Cadherin
expression in carcinomas: role in the formation of cell junctions
and the prevention of invasiveness. Biochim Biophys Acta.
1198:11–26. 1994.PubMed/NCBI
|
|
37
|
Shi Y: Mammalian RNAi for the masses.
Trends Genet. 19:9–12. 2003. View Article : Google Scholar
|
|
38
|
Kuroki M, Matsuo Y, Kinugasa T and
Matsuoka Y: Three different NCA species, CGM6/CD67, NCA-95, and
NCA-90, are comprised in the major 90 to 100-kDa band of
granulocyte NCA detectable upon SDS-polyacrylamide gel
electrophoresis. Biochem Biophys Res Commun. 182:501–506. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Barnich N, Carvalho FA, Glasser AL, et al:
CEACAM6 acts as a receptor for adherent-invasive E. coli,
supporting ileal mucosa colonization in Crohn disease. J Clin
Invest. 117:1566–1574. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Carvalho FA, Barnich N, Sivignon A, et al:
Crohn’s disease adherent-invasive Escherichia coli colonize
and induce strong gut inflammation in transgenic mice expressing
human CEACAM. J Exp Med. 206:2179–2189. 2009.
|