Introduction
Hyperfibrinolysis is a condition in which the
natural ability to dissolve blood clots is pathologically enhanced.
Hyperfibrinolysis occurs more frequently following primary
diseases, including severe trauma, liver surgery, organ
transplantation and obstetrical accidents, may cause hemorrhagic
manifestations of varying severity. It is present in 2–8% of trauma
patients and associated with shock and increased mortality
(1). No previous domestic or
foreign literature has reported hyperfibrinolysis following common
urological surgery. Our hospital recently reported the following
case.
Case report
A 25-year-old female was admitted to Shanghai
Songjiang Hospital in June 2011 with severe hydronephrosis in the
left kidney. Her past medical history was unremarkable. Physical
examination revealed soft percussion pain in the area of the left
kidney. The patient’s bilateral ureter and bladder region
demonstrated no sign of tenderness, and her body temperature was
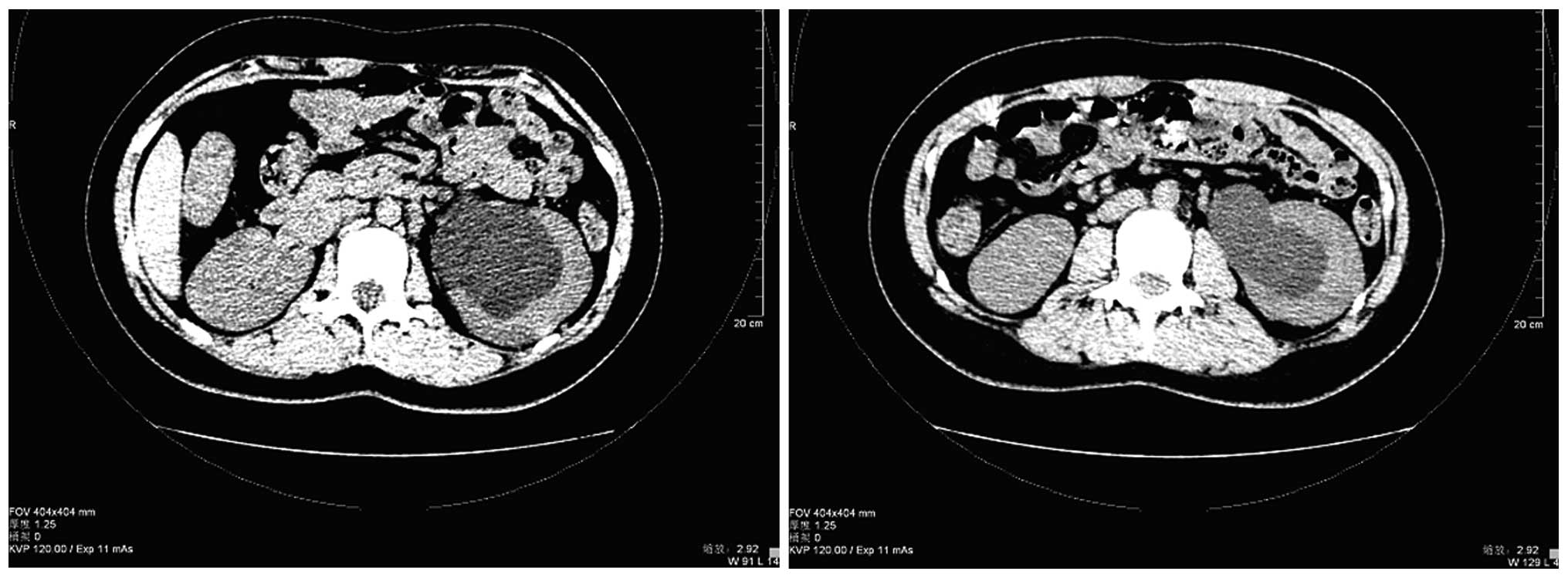
normal. All blood counts and biochemical data were normal. Computed
tomography (CT) of the abdomen revealed left hydronephrosis and a
parapelvic cyst, with no visible stones in the middle or distal
segments of both ureters and little fluid accumulated in the pelvic
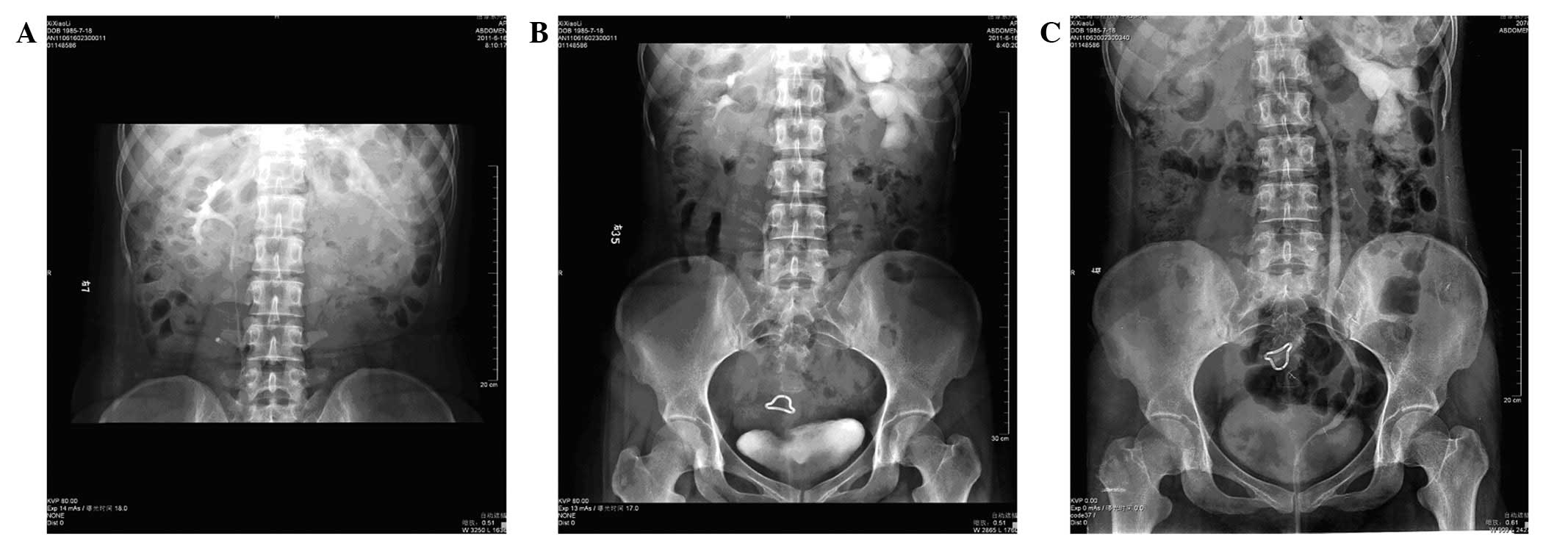
cavity (Fig. 1). An upper urinary
tract retrograde pyelography plus intravenous pyelogram (IVP) also
revealed left hydronephrosis and a parapelvic cyst (Fig. 1), with markedly dilated left renal
calyces (Fig. 2). A diagnosis of a
left parapelvic cyst was then made. On July 5th 2011, an unroofing
surgery of the left parapelvic cyst was successfully performed
under general anesthesia in 1.5 h. The patient was safely escorted
to the common ward.
On the night of July 7th (48 h post-surgery), the
patient developed a progressive heart rate, was pale and sweating,
and her blood pressure dropped to 85/50 mmHg. The immediate
peripheral blood cell counts revealed a hemoglobin (Hb) level of 62
g/l, a white cell count of 11,400/μl and a platelet (PLT) count of
135,000/μl (Fig. 3). The patient
was administered an immediate transfusion of 400 ml erythrocyte
suspension and was infused with appropriate fluids. The next day
the patient’s blood pressure and heart rate became stable again.
Further laboratory tests showed that the prothrombin time (PT) was
13.6 sec, the thrombin time (TT) was 15 sec, the activated partial
thromboplastin time (APTT) was 24.4 sec, the D-dimer assay recorded
0.3 mg/l and the fibrin degradation product (FDP) level was 8 mg/l.
The patient received further erythrocyte suspension transfusions of
400 ml on July 9th and 11th. The Hb level did not improve (range,
53–69 g/l), but the PLT count returned to normal
(159,000–258,000/μl) afterwards.
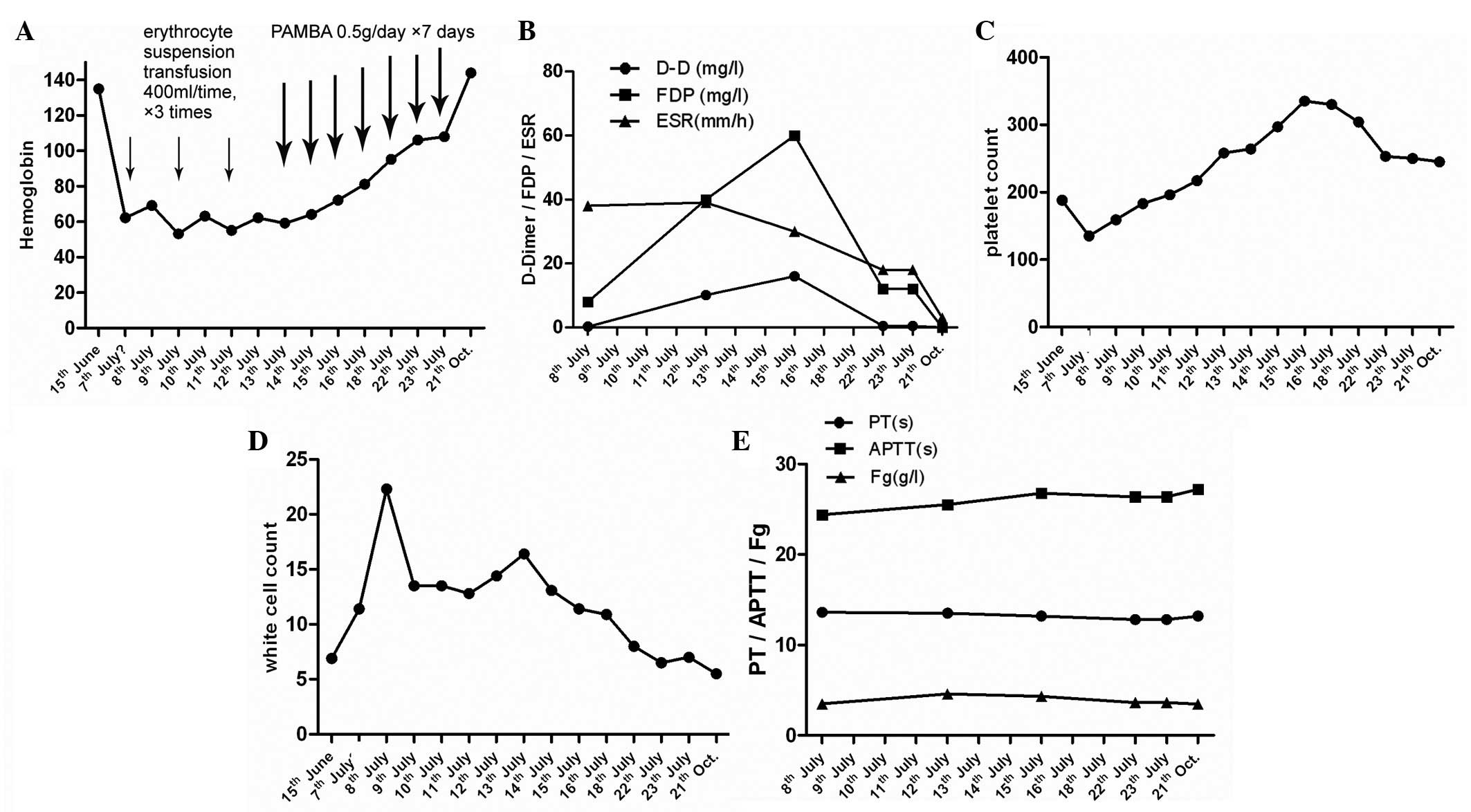 | Figure 3Blood coagulation and fibrinolytic
data statistics of clinical course. (A) The change of hemoglobin.
Hemoglobin levels increased gradually after PAMBA therapy. (B) The
changes of D-dimer and FDP, which increased sharply after
hemorrhage and decreased synchronously when hemoglobin improved.
(C, D and E) The white cell and platelet count, PT, APTT and Fg
index remained at normal levels. PAMBA, p-aminomethylbenzoic acid;
FDP, fibrin degradation product; ESR, erythrocyte sedimentation
rate; PT, prothrombin time; APTT, activated partial thromboplastin
time; Fg, fibrinogen. |
On July 12th, a CT scan of the abdomen revealed an
increase in left kidney volume, a renal hemorrhage and a small
number of blood clots around the left kidney. PT was 13.5 sec, APTT
was 25.5 sec and fibrinogen (Fg) levels were 4.6 g/l, all within
normal ranges. However, the D-dimer assay result was 10.18 mg/l and
the FDP level was 40 mg/l, both of which had notably increased.
Considering the existence of hyperfibrinolysis, we treated the
patient with 0.5 g p-aminomethylbenzoic acid (PAMBA) per day from
July 13th. The patient’s Hb levels steadily increased and reached
72 g/l (Table I) in two days. The
patient was consequently discharged from hospital in a good
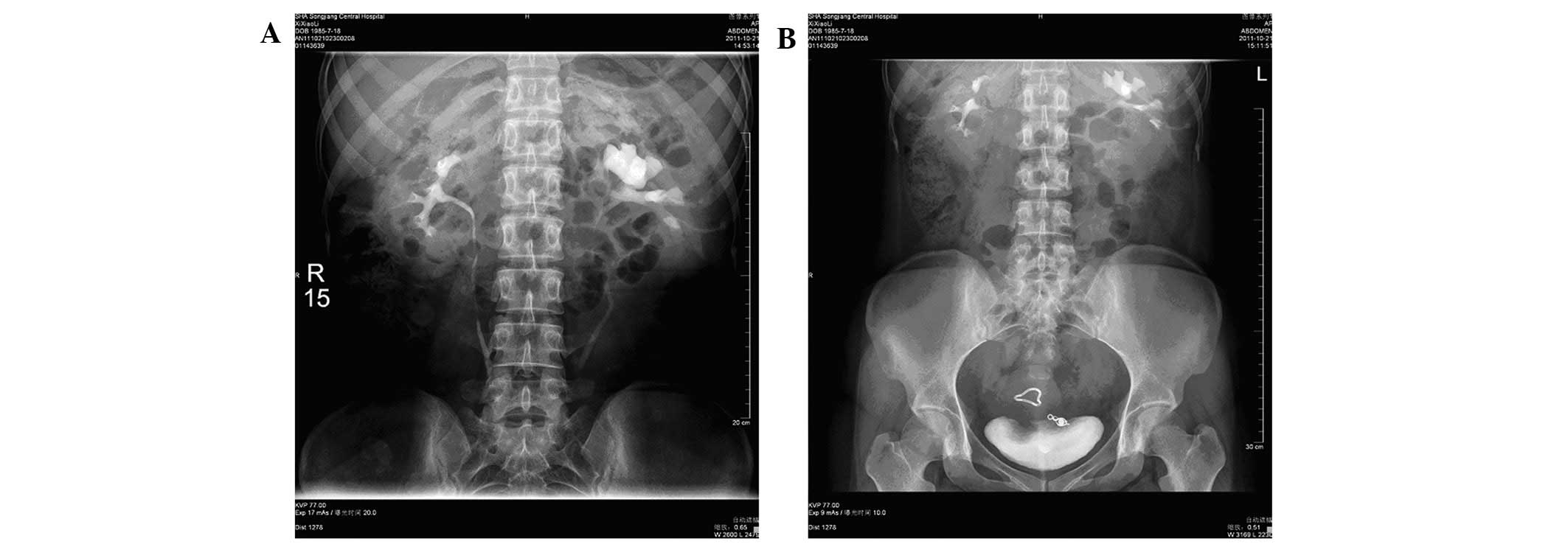
condition 10 days later. A follow-up IVP examination was performed
three months post-surgery (Fig.
4); complete blood cell counts, blood coagulation and
fibrinolytic function measurements were all within normal ranges.
This case report was approved by the ethics committee of the
Songjiang Hospital Affliated to First Hospital of Shanghai, and
informed patient consent was obtained.
 | Table ILaboratory test indices and
therapy. |
Table I
Laboratory test indices and
therapy.
|
Date
(day/month)
|
|---|
| Index | 15/06 | 07/07 | 08/07 | 09/07 | 10/07 | 11/07 | 12/07 | 13/07 | 14/07 | 15/07 | 16/07 | 18/07 | 22/07 | 21/10 |
|---|
| WBC
(×109) | 6.9 | 11.4 | 22.3 | 13.5 | 13.5 | 12.8 | 14.4 | 16.4 | 13.1 | 11.4 | 10.9 | 8.0 | 6.5 | 7.0 |
| ANC% | 53 | 81 | 92.5 | 77.2 | 77.1 | 73.2 | 72.7 | 72.7 | 73.2 | 75.1 | 75.9 | 77.1 | 66.1 | 58.8 |
| RBC
(×1012) | 4.6 | 2.14 | 2.36 | 1.93 | 2.17 | 1.86 | 2.1 | 2.03 | 2.22 | 2.49 | 2.4 | 2.54 | 2.83 | 4.83 |
| Hb (g/l) | 135 | 62.2 | 69.2 | 53.2 | 63.2 | 55.2 | 62.2 | 59.2 | 64.2 | 72.2 | 71.2 | 75.2 | 86 | 144 |
| PLT
(×109) | 188 | 135 | 159 | 183 | 196 | 217 | 258 | 264 | 297 | 335 | 330 | 304 | 253 | 245 |
| PT (sec) | | | 13.6 | | | | 13.5 | | | 13.2 | | | 12.8 | 13.2 |
| Fg (g/l) | | | 3.48 | | | | 4.6 | | | 4.32 | | | 3.63 | 3.45 |
| APTT (sec) | | | 24.4 | | | | 25.5 | | | 26.8 | | | 26.4 | 27.2 |
| D-D (mg/l) | | | 0.3 | | | | 10.18 | | | 16 | | | 0.5 | neg |
| FDP (mg/l) | | | 8 | | | | 40 | | | 60 | | | 12 | neg |
| ESR (mm/h) | | | 38 | | | | 39 | | | 30 | | | 18 | 3 |
| RBC suspension
transfusion (units) | | 2 | | 2 | | 2 | | | | | | | | |
| PAMBA (g) | | | | | | | | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | | |
Discussion
Activation and functions of
fibrinolysis
The main functions of the fibrinolytic system in the
body are to remove fibrin deposition, dissolve blood clots in the
vessel walls and maintain a smooth bloodstream. The system mainly
consists of plasminogen (PLG), plasminogen activator (PA),
plasminogen activator-specific inhibitors (PAIs), plasmin and
plasmin inhibitor. The fibrinolytic system may be activated by the
coagulation process or directly by fibrinolytic enzymes which are
induced by certain factors. A key factor in the triggering of the
fibrinolytic process, tissue-type PA (t-PA), exists in a variety of
organs, including the uterus, ovary, prostate, lung and kidney. The
stress response, shock, malignancies, organ transplantation, trauma
and thrombolytic drugs also affect the t-PA activity.
Types of hyperfibrinolysis
Hyperfibrinolysis is abnormally enhanced activity in
the fibrinolytic system, resulting in premature fibrin, excessive
damage and/or the degradation of blood coagulation factors,
including fibrinogen, thus causing bleeding. There are two kinds of
hyperfibrinolysis, congenital and acquired. Congenital
hyperfibrinolysis is extremely rare. Only 10 cases have been
reported in the literature to date. Clinical hyperfibrinolysis
cases are mainly acquired (1).
Acquired hyperfibrinolysis has two types, primary and secondary.
Primary acquired hyperfibrinolysis is a hemorrhagic syndrome caused
by certain primary diseases which induce the increase of t-PA,
kallikrein and activating factor XII (FXIIa), or the decrease of
fibrinolysis system inhibitors such as PAI. Secondary acquired
hyperfibrinolysis is a thrombosis-hemorrhage syndrome induced by
certain primary diseases which cause local or disseminated
intravascular coagulation (DIC) or hypercoagulative states,
intravascular fibrin precipitates and the release of t-PA into the
blood circulation.
Diagnosis of hyperfibrinolysis
The main clinical manifestation of hyperfibrinolysis
is hemorrhage but there is a lack of other specific symptoms.
Patients may exhibit spontaneous skin ecchymosis, mucosal bleeding,
surgery or post-traumatic wound site and injection site bleeding
and bleeding of joints and muscles, GI tract, urinary tract and
catheter insertion sites. In addition, certain patients have the
symptoms of the primary disease.
FDP and D-dimer assays are the essential indices of
fibrinolytic activity (2). FDP
assays count the total amount of X, Y, D and E fragments. These are
the products of plasmin-induced fibrin and reflect the overall
level of fibrinolytic activity, which increases in both primary and
secondary hyperfibrinolysis. A D-dimer is a specific degradation
product formed by FXIIa from cross-linked fibrin monomers, followed
by plasmin hydrolysis. Healthy individuals have D-dimer levels
<500 ng/ml, while the D-dimer levels of patients with different
types of thrombotic diseases or hypercoagulative states (following
surgery or pregnancy) can reach several thousand nanograms per
milliliter, depending on the activity of fibrinolysis. Monitoring
the D-dimer level aids the identification of primary and secondary
hyperfibrinolysis. The direct detection of PA, PAI, Fg and PLG may
reflect the cause of fibrinolysis more concretely, however, few
hospitals to date have carried out such a comprehensive
examination.
Secondary hyperfibrinolysis is mostly caused by DIC,
which is always accompanied by increased FDP and D-dimer levels,
although these two indices rise slightly in a variety of
physiological hypercoagulation situations. According to the British
Journal of Hematology DIC Diagnosis and Treatment Guidelines
(3), abnormal DIC laboratory test
results are obtained with decreasing probability in this order; PLT
decreased, FDP increased, PT prolonged, APTT prolonged and Fg
decreased. APTT and PT, the conventional indicators of coagulation
factors, measure the intrinsic and extrinsic coagulation function,
respectively, although they are not sensitive and only work with
sufficient coagulation factor consumption (>20–30%). However, if
PLT count, Fg level and plasma protamine paracoagualtion tests (3P
tests) are taken into consideration at the same time, it should not
be difficult to diagnose DIC at an early stage.
Therapy of hyperfibrinolysis
Currently, fibrinolytic therapy includes the
application of a range of fibrinolytic inhibitors and blood
transfusion products. ε-aminocaproic acid (EACA), tranexamic acid
and PAMBA are drugs that are able to competitively inhibit the
combination of plasminogen and fibrin, so that plasminogen is not
activated to become plasmin, thus achieving an anti-fibrinolytic
function. These drugs are effective in patients suffering from
hemorrhage caused by severe liver disease or surgery. Tranexamic
acid and PAMBA are ∼10 and 4–5 times more potent, respectively,
than EACA. The CRASH-2 Joint Working Group (4) holds the opinion that such drugs
should be used as soon as possible in patients with severe
trauma.
Aprotinin is a natural serine protease inhibitor
that is able to irreversibly combine and inactivate a variety of
serine proteases, including fibrinolytic enzymes and kallikrein.
Aprotinin is also able to affect multiple intermediates, thus
resulting in the attenuation of inflammatory responses, the
inhibition of primary and secondary fibrinolysis and thrombin
generation. There have been a number of studies (5–7) on
the application of aprotinin in liver transplants and
cardiothoracic surgery. The normal intake of this drug is
80,000–100,000 units every day, in 2 to 3 doses. The main
side-effects are anaphylaxis and renal dysfunction, which are
particularly likely to occur in patients who receive a second
application within six months.
Fibrinogen is a protein synthesized by the liver
which has functions in blood coagulation and is also the precursor
of fibrin. Thrombin converts fibrinogen into fibrin monomers. When
these monomers cross-link with each other, they become a stable
insoluble protein fiber clot to complete the coagulation process.
Patients with severe liver function disorders or severe trauma,
whose plasma fibrinogen concentrations have decreased, may have a
hemorrhagic tendency. Generally, a 2 g Fg infusion increases the
blood plasma Fg level by 0.5 g/l. When Fg levels reach 1.25 g/l or
above, the hemorrhaging stops (8).
A new type of dynamic analytical instrument, the
thromboelastography (TEG) coagulation analyzer, is now in clinical
application, and being used to monitor the blood coagulation
process. The Rotational Thrombosis Elastic Measurement (ROTEM)
derived from the TEG is able to fully reflect the process in
patients, from blood coagulation to fibrinolysis, and has achieved
good results on guiding the treatment of trauma patients (9,10).
Experience of diagnosis and
treatment
Our patient exhibited symptoms of shock 48 h
post-surgery. PLT count, PT, APTT and Fg tests were performed
multiple times and the results were all within the normal range;
the cause of the symptoms was therefore considered to be chronic
blood loss, which may be controlled by routine hemostasis and
erythrocyte suspension transfusions. However, after nearly one week
of active treatment, the patient’s Hb level demonstrated no
improvement. The FDP and D-dimer levels progressively increased,
while PLT count, PT and APTT remained within the normal ranges.
With no evidence of DIC, we believed that the hypercoagulative
state of the patient was due to chronic blood loss and t-PA
released into the blood circulation from the kidney following
surgery. This activated the fibrinolytic enzymes directly and thus
induced the secondary hyperfibrinolysis and caused a hemorrhage
syndrome. While FDP has a strong anticoagulant effect, it
aggravated the hemorrhage and worsened the patient’s situation.
As an established drug that is safe and effective,
PAMBA was administered promptly as the antifibrinolytic treatment.
A good result was achieved as the patient recovered quickly.
Conclusion
The main lessons we have learnt from this case arose
during diagnosis. The patient had hemorrhage symptoms 48 h
post-surgery, and following consideration of the PLT count, PT,
APTT, FDP, D-dimer assay and Fg test results, which were were all
within the normal ranges at the time, we only administered a blood
transfusion and anti-hypovolemia treatment to the patient. We did
not realize that a severe hypercoagulative state would induce
hyperfibrinolysis symptoms, so an FDP or D-dimer assay test was not
performed in time. This was, therefore, the main reason that an
effective treatment result was not obtained at the beginning of
treatment. We did not identify the rising FDP and D-dimer levels
until 6 days had passed since the hemorrhage symptoms occurred. We
were fortunate that there were no serious consequences.
The key to curing hyperfibrinolysis patients lies in
whether the primary disease or predisposing factors can be dealt
with quickly. In this case, the hyperfibrinolysis was induced
subsequent to surgery, without evidence of therioma or DIC. An
effective result was achieved by antifibrinolytic treatment.
In conclusion, for future patients with delayed
hemorrhage after surgery or non-effective blood transfusion, we
shall consider the possibility of hyperfibrinolysis. We will
monitor the FDP, PT and APTT levels to ensure that we diagnose the
disease in time, prior to using a safe and effective therapy to
cure the patient.
References
|
1
|
Wikkelsø AJ, Afshari A, Stensballe J and
Johansson PI: Hyperfibrinolysis as the cause of haemorrhage and
increased mortality in trauma patients. Ugeskr Laeger. 2:1284–1287.
2011.(In Danish).
|
|
2
|
Zhang ZN, Yang TY and Hao YS: Hematology
People’s Medical Publishing House; Shanghai: pp. 1789–1791.
2003
|
|
3
|
Levi M, Toh CH, Thachil J and Watson HG:
Guidelines for the diagnosis and management of disseminated
intravascular coagulation. British Committee for Standards in
Haematology. Br J Haematol. 145:24–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
CRASH-2 collaborators; Roberts I, Shakur
H, et al: The importance of early treatment with tranexamic acid in
bleeding trauma patients: an exploratory analysis of the CRASH-2
randomised controlled trial. Lancet. 377:1096–1101. 1101.e1–e2.
2011.PubMed/NCBI
|
|
5
|
Henry D, Carless P, Fergusson D and
Laupacis A: The safety of aprotinin and lysine-derived
antifibrinolytic drugs in cardiac surgery: a meta-analysis. CMAJ.
180:183–193. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brown JR, Birkmeyer NJ and O’Connor GT:
Meta-analysis comparing the effectiveness and adverse outcomes of
antifibrinolytic agents in cardiac surgery. Circulation.
115:2801–2813. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gurusamy KS, Pissanou T, Pikhart H, et al:
Methods to decrease blood loss and transfusion requirements for
liver transplantation. Cochrane Database Syst Rev.
12:CD0090522011.PubMed/NCBI
|
|
8
|
Fries D and Martini WZ: Role of fibrinogen
in trauma-induced coagulopathy. Br J Anaesth. 105:116–121. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schöchl H, Frietsch T, Pavelka M and
Jámbor C: Hyperfibrinolysis after major trauma: differential
diagnosis of lysis patterns and prognostic value of
thrombelastometry. J Trauma. 67:125–131. 2009.PubMed/NCBI
|
|
10
|
Tauber H, Innerhofer P, Breitkopf R, et
al: Prevalence and impact of abnormal ROTEM(R) assays in severe
blunt trauma: results of the ‘Diagnosis and Treatment of
Trauma-Induced Coagulopathy (DIA-TRE-TIC) study’. Br J Anaesth.
107:378–387. 2011.PubMed/NCBI
|