Introduction
Trauma-induced suppression of the cellular immune
function likely contributes to sepsis, multiple organ dysfunction
syndrome (MODS) and mortality. Hypertonic saline (HTS) is known to
have anti-inflammatory effects. After substantial of blood loss,
trauma patients often experience severe post-traumatic
complications, such as acute respiratory stress syndrome, multiple
organ failure and sepsis (1,2). HTS
resuscitation decreases the probability of sepsis following
hemorrhagic shock (3,4) and studies have shown that HTS is a
simple but effective tool for modulating immune function following
trauma (5–8). Ischemia and reperfusion primes
neutrophils and mononuclear cells to produce an excessive response
to inflammatory stimuli in post-traumatic patients (the ‘two-hit’
hypothesis) (9). Prevention of
exaggerated inflammation and immunosuppression has been a topic of
trauma research for a number of years. HTS has attracted attention
as a possible therapeutic approach for managing harmful immune
responses in trauma patients, particularly those associated with
neutrophil function (10–14). Macrophage migration inhibitory
factor (MIF) has been revealed to be central to several immune
responses, including the modulation of numerous cytokines and
monocyte, neutrophil and T cell activation. MIF may be a general
marker for systemic inflammation in septic acute critical illness.
By controlling immune and inflammatory responses, MIF is considered
to be important in the pathophysiology of septic shock and chronic
inflammatory diseases (15).
However, the role of MIF in trauma-like conditions is unknown.
Therefore, the present study was conducted to evaluate MIF in
macrophages or polymorphonuclear neutrophils (PMNs), in response to
early phase injury following stimulation with lipopolysaccharide
(LPS) to induce infection conditions or
N-formyl-methionyl-leucyl-phenylalanine (fMLP) to induce
trauma-like conditions, either in the presence or absence of
HTS.
Materials and methods
THP-1 cells
Culture and treatment of cells
THP-1 cells (American Type Culture Collection
TIB-202, Manassas, VA, USA), an immortalized human monocytic cell
line, were differentiated to macrophages as previously described
with a few modifications (16).
Cells were treated with 162 μmol/ml phorbol 12-myristate 13-acetate
(PMA; Sigma-Aldrich Co., St. Louis, MO, USA) for 72 h at 37°C, 5%
CO2. Differentiated cells were washed three times with
HBSS (Gibco, Carlsbad, CA, USA), removed from the plate with 0.25%
trypsin-EDTA (Gibco) and seeded in 96-well plates for an
enzyme-linked immunosorbent assay (ELISA) and 24-well tissue
culture plates for western blotting at 2x106/ml viable
cells per well in complete media. THP-1 monocyte-derived
macrophages were treated with LPS at different tonicities. The
effect of HTS on LPS-induced MIF was evaluated in macrophages with
1 μg/ml LPS. HTS at 10, 20 or 40 mmol/l above isotonicity (140
mmol/l) was added. This study was approved by the Institutional
Review Board from Korea University Guro Hospital (NO.KUGH –
10157).
Enzyme-linked immunosorbent assay for
MIF
Supernatants were collected after incubation for 2
or 20 h. The MIF concentration in the culture supernatants was
measured by sandwich ELISA. Briefly, 2 μg/ml of monoclonal capture
antibody (R&D Systems, Minneapolis, MN, USA) was added to a
96-well plate and incubated for one day at room temperature and
washed with buffer three times. After washing, the plates were
incubated in a blocking solution of phosphate-buffered saline (PBS)
containing 1% bovine serum albumin (BSA) and 0.05% Tween-20 for 1 h
at room temperature and washed with buffer three times. Test
samples and standard recombinant MIF (R&D Systems) were added
to the plates and incubated for 2 h at 4°C. Plates were washed
three times with PBS containing Tween-20, 200 ng/ml of biotinylated
detection monoclonal goat-antihuman antibodies (R&D Systems)
were added and the plates were incubated for 2 h at room
temperature. After washing three times,
streptavidin-alkaline-phosphatase (1:2000; Sigma-Aldrich Co.) was
added and the reaction was allowed to proceed for 20 min at room
temperature. The plates were washed three times and 1 mg/ml of
p-nitrophenylphosphate dissolved in diethanolamine (Sigma-Aldrich
Co.) was added to induce a color reaction which was stopped with 50
μl of 1 M NaOH. The optical density at 450 nm was measured on an
automated microplate reader (Bio-Rad Laboratories Inc., Hercules,
CA, USA). A standard curve was generated by plotting the optical
density vs. the log of the MIF concentration. Experiments were
conducted 10 times.
Protein extracts and western blot
analysis for MIF expression
Following incubation for 24 h at 37°C, cells were
washed twice in cold PBS and centrifuged for 10 min. Cell pellets
were resuspended in 10 μl per 2×106 cell/ml of
superlysis buffer [protease inhibitors, 1 M
4-(2-hydroxyethyl)-1-piper-azineethanesulfonic acid (HEPES), 5 M
NaCl, 0.5 M ethylenediaminetetraacetic acid (EDTA), 1 mM
Na3VO4, 20% Triton X-100, 50 mM
phenylmethylsulfonylfluoride], incubated on ice for 7 min and
centrifuged at 3,000 × g for 15 min at 4°C. The total protein
concentration was determined by the Bradford method using a
commercially available assay kit (Thermo Fisher Scientific,
Rockford, IL, USA) (8). Prepared
protein lysates were aliquoted and used for western blot analysis.
Proteins (5 μg/ml) were fractionated on a 15% sodium
dodecylsulfate-polyacrylamide gel (Bio-Rad Laboratories Inc.) and
transferred to a nitrocellulose membrane. Membranes were blocked
for 1 h in 5% BSA (Sigma-Aldrich Co.) and incubated with anti-human
MIF (1:250; R&D systems). After washing, membranes were
incubated with 1:2,000 horseradish peroxidase-labeled goat
anti-mouse antibody (R&D systems). Proteins were detected using
a SuperSignal (Thermo Fisher Scientific Inc.) chemiluminescence
kit.
PMN cells
Separation and stimulation of
PMNs
PMNs were separated using a modified Boyum method.
After obtaining consent, venous blood samples were collected
directly into a tube containing preserved EDTA from 10 healthy
volunteers. From each collected whole blood sample, 5 ml was
aliquoted into 15 ml test tubes with 5 ml of Polymorphprep (Nycomed
Pharma AS, Oslo, Norway), followed by centrifugation for 37 min at
500 x g. The PMN cell layer between the monocyte and red blood cell
layers was collected. To remove the remaining red blood cells,
samples were incubated with 0.2% saline solution for 30 sec, after
which a 1.8% saline solution was added to create 0.9% normal
osmotic pressure. Samples were centrifuged at 450 × g for 10 min,
followed by two washes with PBS. Separated PMNs were incubated in
RPMI-1640 medium containing penicillin and supplemented with fetal
bovine serum (FBS; 10%) and HEPES. A final concentration of
1×106 cell/ml with viability >95% was demonstrated
using trypan blue dye. fMLP (43.76 mg; Sigma-Aldrich Co.) was
dissolved in dimethyl sulfoxide (10 ml; DMSO, Sigma-Aldrich Co.) to
1 μM. PMNs were stimulated using fMLP. The prepared neutrophils
were divided into 5 groups. The control group received no
stimulation and isotonic conditions (140 mmol/l) were maintained.
Another group was stimulated with fMLP but maintained at isotonic
conditions. Three groups had hypertonic conditions of 10, 20 and 40
mmol/l above isotonicity with HTS added following stimulation with
fMLP. MIF concentrations in the supernatant were determined by the
ELISA, while cell lysates were used for western blotting and real
time-polymerase chain reaction (RT-PCR) to determine MIF
expression. The ELISA and western blotting for MIF were conducted
as described above.
RT-PCR
Expression of MIF mRNA was detected by quantitative
(q)RT-PCR. Total RNA was extracted from cells using TRIzol reagent
according to the manufacturer's instructions (RNeasy Mini Kit,
Qiagen, Hilden, Germany) and the concentration of the sample in
diethypirocarbonate-treated water was determined. Extracted RNA was
stored at −70°C and treated with DNase I prior to use. qRT-PCR was
employed to detect MIF mRNA. The total RNA (200 ng) from each
sample was reverse transcribed into complementary DNA (cDNA) using
a high-capacity cDNA reverse transcription kit. The final reaction
volume was 10 μl. After completion of the first-strand cDNA
synthesis, the MIF probe was used to analyze 34 cycles of 50°C for
2 min, 95°C for 10 min, 95°C 15 min and 60°C for 1 min, with an
RT-PCR system (AB7300, Applied Biosystems, Foster City, CA,
USA).
Data and statistical analysis
Measurements are presented as mean with standard
deviation. The Student's t-test and one-way ANOVA were used for the
statistical analysis. P<0.05 was considered to indicate
statistically significant differences.
Results
Effect of HTS on MIF concentration in
LPS-induced macrophage supernatants
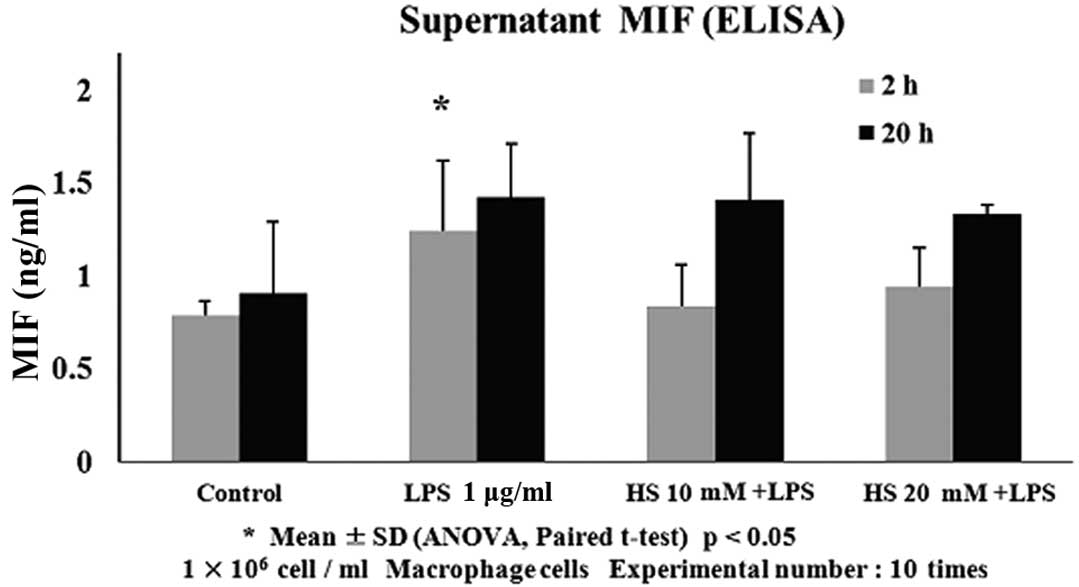
To understand the association between HTS and MIF in
LPS-induced macrophage cells, the MIF levels were measured in cell
supernatants (Fig. 1). The MIF
levels increased by 1.24±0.38 ng/ml in the supernatant of
LPS-stimulated cells compared with the control level of 0.79±0.07
ng/ml at 2 h incubation. HTS decreased the MIF levels to 0.84±0.22
ng/ml in LPS-stimulated macrophage cells at 10 mM above
hypertonicity (P<0.05). HTS decreased the MIF levels to
0.94±0.21 ng/ml in LPS-stimulated macrophage cells at 20 mM above
hypertonicity (P<0.05). MIF levels between the HTS10- and
HTS20-treated cells were not significantly different. The groups
incubated for 20 h were not significantly different from groups
incubated for 2 h. Although the MIF level of the 20-h stimulated
group increased to 1.42 ng/ml ± 0.29, compared to the control group
with 0.91 ng/ml ± 0.38, this was not a statistically significant.
In addition, there was no statistical difference between 1.42 ng/ml
± 0.35 at 10 mM above isotonicity and 1.33 ng/ml ± 0.05 at 20 mM
above isotonicity with added HTS.
Effect of HTS on MIF expression in
LPS-induced macrophages
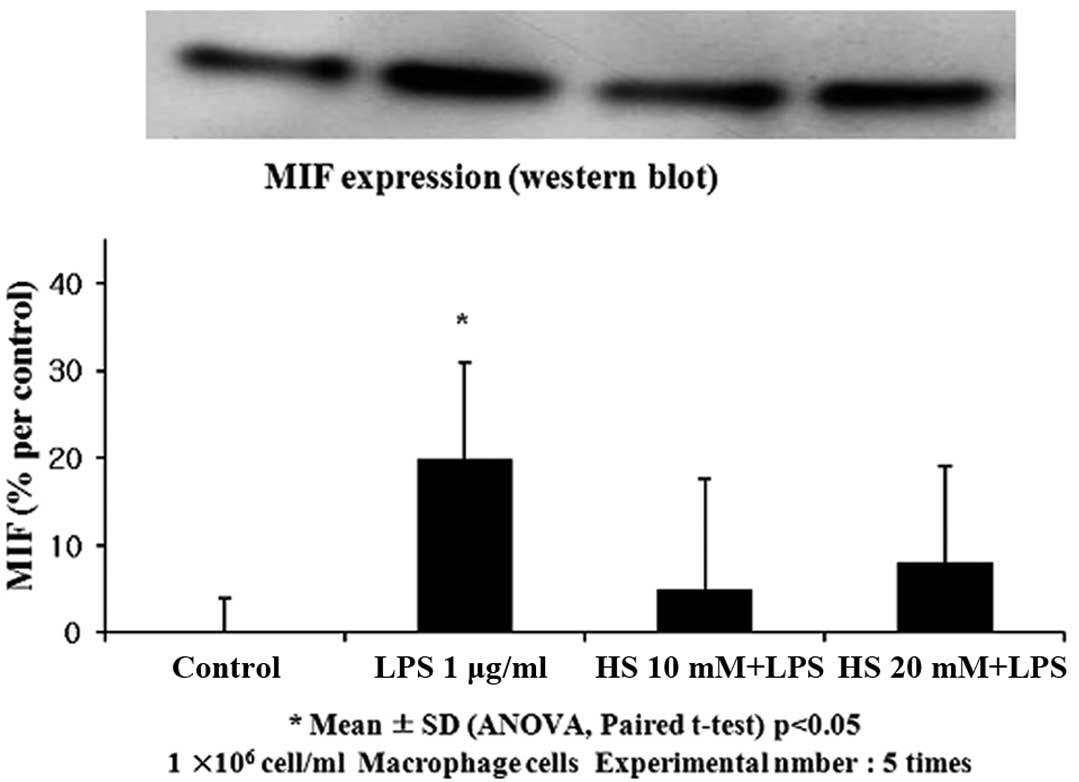
To determine the effect of HTS on MIF expression,
western blotting was performed (Fig.
2). Correlating with the ELISA results, the levels of MIF
protein were higher in the LPS-stimulated cells (20% increase in
band density; P<0.05). The addition of HTS to the LPS-stimulated
cells decreased the MIF protein at 10 mM and 20 mM above
isotonicity, However, MIF expession between the HTS10- and
HTS20-treated cells was not significantly different.
Effect of HTS on MIF concentration and
MIF expression in PMN cells with fMLP stimulation
PMN cells were plated on 96-well culture plates at a
concentration of 1x106 cell/ml in cell culture media
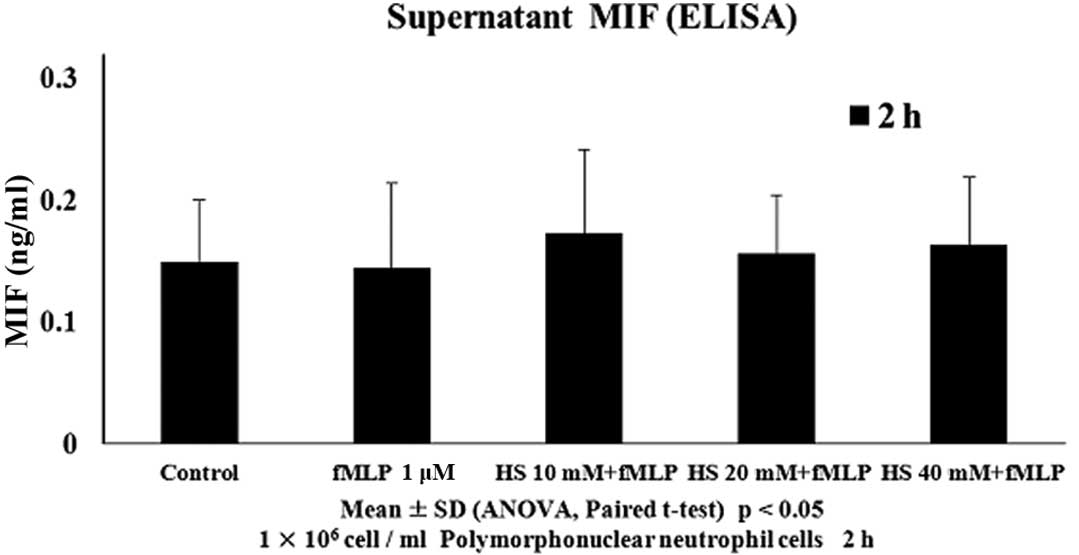
(Figs. 3–5). To determine the association between
HTS and MIF in fMLP-induced PMN cells, the MIF levels were measured
in cell supernatants. The MIF levels in fMLP-stimulated cells were
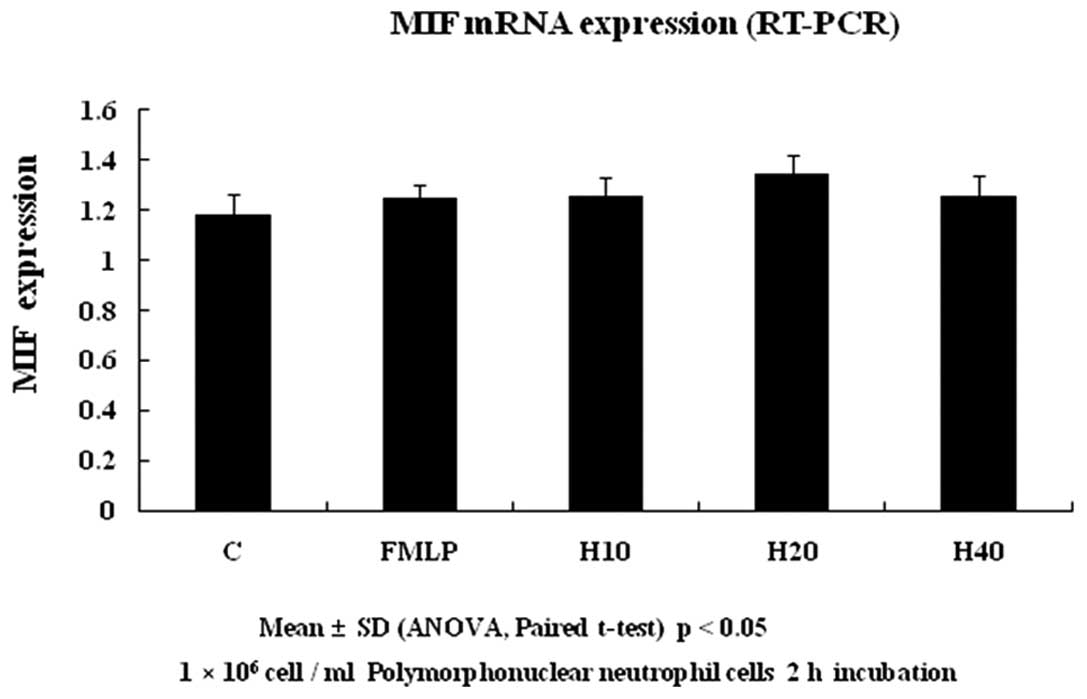
unchanged compared with the controls. In correlation with the ELISA
results, the levels of MIF expression were unchanged in the western
blotting and RT-PCR. Treatment with HTS had no effect.
Discussion
The present study demonstrated that MIF increased in
macrophages stimulated with LPS and decreased with HTS treatment.
It also demonstrated a lack of increase in MIF levels in
neutrophils stimulated with fMLP, unlike the MIF reaction in
macrophages stimulated with LPS. Few studies have examined the
association between the expression of MIF and HTS in cells.
A leading cause of late mortality in trauma patients
is MODS resulting from the deregulation of the immune-inflammatory
response and homeostasis. Following major trauma, a large number of
pro- and anti-inflammatory cytokines are released by activated
monocytes/macrophages and PMNs. In a healthy patient with a minor
injury, homeostasis is restored quickly and the inflammatory
response remains incomplete. In the case of a major trauma,
however, the inflammatory response may proliferate throughout the
whole body, leading to multiple organ injury, including acute
respiratory distress syndrome (ARDS), sepsis, septic shock and MODS
with a concomitant significant morbidity and mortality for the
injured patient (2,17).
Hemorrhagic shock is a leading cause of early
mortality in trauma patients. Resuscitation from traumatic blood
loss activates the innate immune system, potentially leading to
ARDS and MODS. HTS is a safe and efficient fluid for resuscitation
from hemorrhagic shock and reducing the intracranial pressure in
patients with brain injuries (18), with a number of potentially
beneficial immunomodulating effects (8,19).
HTS (7.5% saline) has been shown to modulate the entire systematic
immunoinflammatory response following injury (20), as well as decrease cytokine
production by monocytes and blunt neutrophil activation induced by
shock (21–24). The decrease in cytokine release may
be an additional beneficial effect of HTS on the immune function in
the resuscitation of post-traumatic patients (25).
Cytokines are important in modulating the host
immunoinflammatory responses to infection and trauma. They regulate
the first nonspecific phase of the host response by combining a
local inflammatory reaction and control the next specific immune
response. In 1966, historical experiments by Bloom and Bennett
(26) and David (27) first identified MIF as a
nondialyzable protein factor produced by sensitized lymphocytes
that was associated with delayed-type hypersensitivity. MIF was
characterized by the ability of crude extracts to inhibit the
random migration of guinea pig peritoneal exudate macrophages in
vitro(26,27) and subsequently activate macrophage
function (28,29). In spite of these observations
describing cytokine activity >30 years ago, a detailed view of
the biological function of MIF remained elusive until the classical
T cell cytokine macrophage MIF reemerged as a critical mediator of
the host immune and stress response (15).
LPS is the major component of the outer membrane of
Gram-negative bacteria, contributing to the structural integrity of
the bacteria and protecting the membrane from certain types of
chemical attack. LPS is important as mutation or removal results in
the death of Gram-negative bacteria. LPS is an endotoxin and
induces a strong response from normal animal immune systems. It has
also been implicated in nonpathogenic aspects of bacterial ecology,
including surface adhesion, bacteriophage sensitivity and
interactions with predators such as amoebae. LPS acts as a
prototypical endotoxin since it binds the CD14/TLR4/MD2 receptor
complex, which promotes the secretion of proinflammatory cytokines
in numerous cell types, particularly macrophages and B cells. In
immunology, the term ‘LPS challenge’ refers to the process of
exposing a subject to an LPS that may act as a toxin. LPS is also
an exogenous pyrogen (external fever-inducing substance) (30,31).
As an inflammation-triggering agent, the fMLP used
to stimulate PMNs cells in the present study is a synthetic
chemoattractant. It is a formyl peptide secreted in an area of
inflammation during bacterial infection that binds to its receptor
on the surface of neutrophils, stimulating cytokine secretion.
Thus, fMLP is generally accepted as inducing a reaction similar to
the inflammatory response (32).
In the results of the present study, MIF expression
was demonstrated to be increased in macrophages stimulated by LPS
and decreased by HTS. According to Choi et al(33), MIF expression changes in T cells
treated with HTS and this is associated with T cell dysfunction.
This is consistent with the results for MIF in macrophages in the
present study. However, no significant changes of MIF in PMN cells
were observed, regardless of stimulation or HTS. In confirming the
level of TNF-α by stimulating PMN cells with fMLP and LPS, Vulcano
et al(30), detected no
secretion when PMN cells were stimulated with fMLP, but secretion
when cells were stimulated with LPS. Vulcano et al(30) proposed that the difference was due
to dynamic processes at the LPS and fMLP binding sites. Studies
have described the independent actions of LPS and fMLP or a priming
effect exerted by LPS on a different agonist. However, in general,
the sequence of interaction between these two bacterial components
in the regulation of inflammation is not fully undersood (30). An increase in MIF was observed in
macrophages stimulated by LPS. Similar conditions were observed in
a study by Schmidt-Supprian et al(34), with processing with activated
protein C afterwards. The authors reported that activated protein C
reduces MIF levels, similar to HTS.
In the present study, RT-PCR data for macrophages
stimulated with LPS were not acquired, since MIF exhibited a
significant difference by ELISA and western blotting. RT-PCR was
used in the PMN-fMLP experimental group, since no significant
difference was observed by ELISA or western blotting.
As in the majority of studies that focus on trauma
and injury conditions, the present study was conducted by
stimulating PMN cells with fMLP and LPS was mainly used to study
infection conditions, respectively. Therefore, each experimental
group was considered to represent trauma and infection conditions.
The results of the present study suggested that MIF is not
expressed under simple trauma conditions but is expressed in
conjunction with infection. In this case, MIF should be considered
to be an important cytokine in the dividing stages and estimating
prognosis by the increase in infection rate, progression to sepsis
and MODS and mortality in cases of immune deficiency caused by
trauma. If the differences in the results reflect the differences
between infection, represented by macrophages and LPS, and trauma,
represented by PMNs and fMLP, no MIF expression is expected in
cases of trauma and only MIF would be expressed in cases where
trauma is combined with infection.
The limitations of the present study were, firstly,
the in vitro design may be different from complex situations
in vivo with various cytokines and a diverse environment.
Secondly, although the difference between the results of PMN cells
and macrophages was interpreted to be caused by differences in
stimulants, it may be attributable to a difference in the cells
themselves. As both cells and stimulants differed, a comparison
between the two results is expected to be insignificant. However,
since almost nothing is known concerning the expression of MIF in
trauma, infection, inflammation or other conditions, the importance
of the present study is in the results about the expression of MIF
caused by stimulation in macrophages and the findings of a reaction
with HTS and no expression of MIF in PMNs.
The present study demonstrated that MIF increased in
LPS-stimulated macrophages and decreased with HTS treatment.
However, it also demonstrated that the level of MIF was not
associated with stimulation or HTS for PMN cells stimulated with
fMLP. Inflammation and immune modulation by HTS occurs, at least in
part, by an MIF-mediated mechanism in LPS-stimulated macrophages
but not in fMLP-stimulated PMN cells. The present study provides
the first evidence supporting MIF as a mechanism for inflammation
and immune modulation by HTS in LPS-stimulated macrophages. MIF
appears to be a promising candidate for the treatment of sepsis in
traumatic conditions.
Acknowledgements
This study was partially supported by
a Korea University grant and Basic Science Research Program through
the National Research Foundation of Korea (NRF) funded by the
Ministry of Education, Science and Technology (R1009983).
References
|
1
|
Tsiotou AG, Sakorafas GH, Anagnostopoulos
G and Bramis J: Septic shock; current pathogenetic concepts from a
clinical perspective. Med Sci Monit. 11:RA76–85. 2005.PubMed/NCBI
|
|
2
|
Keel M and Trentz O: Pathophysiology of
polytrauma. Injury. 36:691–709. 2005. View Article : Google Scholar
|
|
3
|
Faist E, Baue AE, Dittmer H and Heberer G:
Multiple organ failure in polytrauma patients. J Trauma.
23:775–787. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moore FA and Moore EE: Evolving concepts
in the pathogenesis of postinjury multiple organ failure. Surg Clin
North Am. 75:257–277. 1995.PubMed/NCBI
|
|
5
|
Coimbra R, Hoyt DB, Junger WG, Angle N,
Wolf P, Loomis WH and Evers MF: Hypertonic saline resuscitation
decreases susceptibility to sepsis after hemorrhagic shock. J
Trauma. 42:602–607. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Junger WG, Coimbra R, Liu FC,
Herdon-Remelius C, Junger W, Junger H, Loomis WH, Hoyt DB and
Altman A: Hypertonic saline resuscitation: a tool to modulate
immune function in trauma patients? Shock. 8:235–241. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Angle N, Hoyt DB, Coimbra R, Liu F,
Herdon-Remelius C, Loomis W and Junger WG: Hypertonic saline
resuscitation diminishes lung injury by suppressing neutrophil
activation after hemorrhagic shock. Shock. 9:164–170. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Staudenmayer KL, Maier RV, Jelacic S and
Bulger EM: Hypertonic saline modulates innate immunity in a model
of systemic inflammation. Shock. 23:459–463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fan J, Marshall JC, Jimenez M, Shek PN,
Zagorski J and Rotstein OD: Hemorrhagic shock primes for increased
expression of cytokine-induced neutrophil chemoattractant in the
lung: role in pulmonary inflammation following lipopolysaccharide.
J Immunol. 161:440–447. 1998.
|
|
10
|
Botha AJ, Moore FA, Moore EE, Fontes B,
Banerjee A and Peterson VM: Postinjury neutrophil priming and
activation states: therapeutic challenges. Shock. 3:157–166. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuchkina NV, Orlov SN, Pokudin NI and
Chuchalin AG: Volume-dependent regulation of the respiratory burst
of activated human neutrophils. Experientia. 49:995–997. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hampton MB, Chambers ST, Vissers MC and
Winterbourn CC: Bacterial killing by neutrophils in hypertonic
environments. J Infect Dis. 169:839–846. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Davreux CJ, Soric I, Nathens AB, Watson
RW, McGilvray ID, Suntres ZE, Shek PN and Rotstein OD: N-acetyl
cysteine attenuates acute lung injury in the rat. Shock. 8:432–438.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Powers KA, Zurawska J, Szaszi K, Khadaroo
RG, Kapus A and Rotstein OD: Hypertonic resuscitation of
hemorrhagic shock prevents alveolar macrophage activation by
preventing systemic oxidative stress due to gut
ischemia/reperfusion. Surgery. 137:66–74. 2005. View Article : Google Scholar
|
|
15
|
Bernhagen J, Calandra T and Bucala R:
Regulation of the immune response by macrophage migration
inhibitory factor: biological and structural features. J Mol Med
(Berl). 76:151–161. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Daigneault M, Preston JA, Mariott HM,
Whyte MK and Dockrell DH: The identification of markers of
macrophage differentiation in PMA-stimulated THP-1 cells and
monocyte-derived macrophages. PLoS One. 5:e86682010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schaeffer V, Arbabi S, Garacia IA, Knoll
ML, Cuschieri J, Bulger EM and Maier RV: Role of the mTOR Pathway
in LPS-Activated Monocytes: influence of hypertonic saline. J Surg
Res. 171:769–776. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Himmelseher S: Hypertonic saline solutions
for treatment of intracranial hypertension. Curr Opin Anaesthesiol.
20:414–426. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bulger EM, Jurkovich GJ, Nathens AB, et
al: Hypertonic resuscitation of hypovolemic shock after blunt
trauma: a randomized controlled trial. Arch Surg. 143:139–148.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Poli-de-Figueiredo LF, Cruz RJ Jr,
Sannomiya P and Rocha-E-Silva M: Mechanisms of action of hypertonic
saline resuscitation in severe sepsis and septic shock. Endocr
Metab Immune Disord Drug Targets. 6:201–206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deitch EA, Shi HP, Feketeova E, et al:
Hypertonic saline resuscitation limits neutrophil activation after
trauma-hemorrhagic shock. Shock. 19:328–333. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y, Hashiguchi N, Yip L and Junger WG:
Hypertonic saline enhances neutrophil elastase release through
activation of P2 and A3 receptors. Am J Physiol Cell Physiol.
290:C1051–C1059. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi SH, Lee SW, Hong YS, et al: Selective
inhibition of polymorphonuclear neutrophils by resuscitative
concentration of hypertonic saline. Emerg Med J. 23:119–122. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hashiguchi N, Lum L, Romeril E, et al:
Hypertonic saline resuscitation: efficacy may require early
treatment in severely injured patients. J Trauma. 62:299–306. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hatanaka E, Shimomi FM, Curi R and Campa
A: Sodium chloride inhibits cytokine production by
lipopolysaccharide-stimulated human neutrophils and mononuclear
cells. Shock. 27:32–35. 2007. View Article : Google Scholar
|
|
26
|
Bloom BR and Bennett B: Mechanism of a
reaction in vitro associated with delayed-type hypersensitivity.
Science. 153:80–82. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
David JR: Delayed hypersensitivity in
vitro: its mediation by cell-free substances formed by lymphoid
cell-antigen interaction. Proc Natl Acad Sci USA. 56:72–77. 1966.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nathan CF, Karnovsky ML and David JR:
Alterations of macrophage functions by mediators from lymphocytes.
J Exp Med. 133:1356–1376. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nathan CF, Remold HG and David JR:
Characterization of a lymphocyte factor which alters macrophage
functions. J Exp Med. 137:275–290. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vulcano M, Alves Rosa MF, Minnucci FS,
Cherñavsky AC and Isturiz MA:
N-formyl-methionyl-leucyl-phenylalanine (fMLP) inhibits tumour
necrosis factor-alpha (TNF-alpha) production on lipopolysaccharide
(LPS)-stimulated human neutrophils. Clin Exp Immunol. 113:39–47.
1998. View Article : Google Scholar
|
|
31
|
Stewart I, Schluter PJ and Shaw GR:
Cyanobacterial lipopolysaccharides and human health - a review.
Environ Health. 5:72006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Snyderman R: Regulation of Leukocyte
Function. Pleman; New York, NY: 1984, View Article : Google Scholar
|
|
33
|
Yoon YH, Choi SH, Hong YS, Lee SW, Moon
SW, Cho HJ, et al: Effect of hypertonic saline and macrophage
migration inhibitory factor in restoration of T cell dysfunction. J
Korean Surg Soc. 81:229–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schmidt-Supprian M, Murphy C, While B,
Lawler M, Kapurniotu A, Voelter W, et al: Activated protein C
inhibits tumor necrosis factor and macrophage migration inhibitory
factor production in monocytes. Eur Cytokine Netw. 11:407–413.
2000.
|