Introduction
Human corneal endothelial cells (HCECs) form a
homogeneous single layer of flat hexagonal cells, which are
attached to the basement membrane (Descemet’s membrane). HCECs are
essential for maintaining corneal transparency, which is dependent
upon endothelial regulation of stromal hydration, including the
barrier and pump functions of the aqueous humor (1). Damage to HCECs caused by intraocular
surgery, glaucoma, trauma or diseases, including Fuchs’ corneal
dystrophy, may result in irreversible corneal edema, since there is
little or no mitotic activity in the HCECs after birth (2). Despite several successful, recently
developed HCEC replacement surgeries, including Descemet’s membrane
endothelial keratoplasty (DMEK) and Descemet’s stripping automated
endothelial keratoplasty (DSAEK), owing to the aging population,
increased requirements for corneal transplants and an insufficient
quantity of available donor tissues has led researchers to explore
alternative ways of preparing in vitro corneal endothelial
cell monolayers (3). However,
proliferation of functional adult HCECs is difficult to achieve
using standard cell culture techniques (4). HCECs were originally believed to be
incapable of dividing in vitro, but have been successfully
isolated and cultured with epidermal growth factor (EGF),
platelet-derived growth factor (PDGF), bovine pituitary extract and
fetal bovine serum (FBS). However, the number of cells with
proliferative activity and the ability to respond to such agents is
relatively low and much variation in morphology and function exists
after several cell passages (4–6).
Thus, there is a need to develop a stable and effective cell
culture system to maximize cell proliferation and maintain
physiological function. In this study, we successfully developed a
HCEC cell culture system which maintains cell functions.
Materials and methods
Primary HCEC culture
Human donor corneas were obtained according to the
principles set out in the Declaration of Helsinki. Whole donor
corneas (n=6) with a corneal endothelial cell density of
1500–2000/mm2 and corneal scleral rims (n=6), following
penetrating keratoplasty, were obtained from the Tongji University
Hospital eye bank (Shanghai, China), following ethical approval and
specific consent for research use. The mean donor age was 67.2±11.1
and ranged from 56 to 76 years. Under a dissecting microscope, the
Descemet’s membrane with the endothelium attached was carefully
peeled off. The removed membrane was cut into small pieces, ∼1–2 mm
in diameter and cultured in a cell culture dish covered with type
IV collagen in Dulbecco’s modified Eagle’s medium [DMEM/nutrient
mixture F12 (1:1)] with high glucose, supplemented with 15% FBS,
insulin-transferrin-selenium and minimal essential
medium-non-essential amino acid (MEM-NEAA; Gibco, Carlsbad, CA,
USA). Cells were subcultured after reaching confluency by treating
with trypsin/ethylenediaminetetraacetic acid (EDTA) and then seeded
at a density of 5×105 cells/well in 6-well dishes.
Second or third passage cells were used for further
experiments.
HCEC sphere-forming cultures
Cell culturing followed the method previously
reported (7) with certain
modifications. Briefly, cells were plated in 24-well super
hydrophilic plates at densities ranging from 1×104 to
1×105 cells/plate, then further cultured in DMEM/F12
(1:1; Gibco) supplemented with B27 (Invitrogen, Carlsbad, CA, USA),
40 ng/ml basic fibroblast growth factor (bFGF; R&D Systems,
Minneapolis, MN, USA), 20 ng/ml EGF (Sigma-Aldrich, St. Louis, MO,
USA) and N2 supplement (Gibco). Basal medium containing
methylcellulose gel matrix (1.5%, Wako Pure Chemical Industries,
Osaka, Japan) was used to prevent the reaggregation of cells.
Primary sphere-forming cells cultured for 7 days were used in these
studies. For the secondary sphere formation assays, primary spheres
were incubated in 0.05% trypsin and 0.02% EDTA and the dissociated
cells were plated at 5×104 cells/well, following the
same culture method as described above.
Treatment with Y-27632
HCECs from sphere-forming cultures were incubated
with medium containing Y-27632 (30 μM, for 1 h at 37°C; Shanghai
Biochempartner Co, China). The treated cells were harvested for
further experiments.
Homotypic adhesion assay
The homotypic adhesion assay was performed as
reported previously (8). Briefly,
monolayer HCECs, incubated as described above, on 24-well plates
were gently washed three times with phosphate-buffered saline
(PBS). Cells (1×105) in 1 ml medium with or without
Y-27632 at 30 μM were seeded into each well. The 24-well plate was
then placed in a horizontal shaker and agitated at 70 rpm at 37°C.
The unattached cells were removed prior to calculating cell number
under a microscope, after incubation for 10, 30 and 60 min. The
number of attached cells was calculated using the following
formula: number of adherent cells = 1×105 – number of
unattached cells.
Immunocytochemistry
Immunostaining of cells was used to localize ZO-1,
Na+/K+-ATPase, β3-tubulin and nestin
proteins. The primary antibodies used were: mouse anti-human ZO-1
mAb (clone 1, isotype IgG; BD Biosciences, San Jose, CA, USA;
dilution 1:200 in PBS, incubation time, 45 min), mouse anti-human
Na+/K+-ATPase mAb (clone 9-A5, isotype IgG1;
Abcam, Cambridge, UK; 1:200, 45 min), mouse anti-nestin mAb (BD
Pharmingen, San Diego, CA, USA; 1:200, 45 min) and rabbit
anti-β3-tubulin polyclonal antibody (Covance Research Products,
Danvers, PA, USA; 1:2000, 45 min). Following treatment with the
primary antibody, 1% (w/w) bovine serum albumin (BSA) was added to
the PBS solution to remove unbound antibodies. In all cases the
secondary antibody was Alexa Fluor 546 goat anti-mouse IgG (γ1;
Invitrogen; 1:200, 30 min). Cells were fixed with 4%
paraformaldehyde in PBS for 10 min then incubated with 0.5% Triton
X-100 and 1% (w/w) BSA in PBS for 10 min, prior to exposure to the
primary and secondary antibodies. Following these steps, the
samples were rinsed twice in PBS.
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from cultured HCECs using
the RNeasy plus mini kit (Qiagen, Beijing, China) according to the
manufacturer’s instructions and quantitated at 260 nm. Total RNA
was then reverse transcribed into cDNA using Superscript III
reverse transcriptase (Invitrogen) with oligo random hexamers. The
cDNAs of each component were amplified by PCR using specific
primers and DNA polymerase. The reaction was first incubated at
95°C for 10 min, followed by 39 cycles at 98°C for 30 sec, 58°C for
30 sec and 74°C for 30 sec. RT-PCR primers are listed as follows:
Na+/K+-ATPase, forward, 5′-CCC AGG ACT CAT
GGT TTT TC-3′, reverse, 5′-GGA GCA AAG CTG ACC TGA AC-3′;
β-catenin, forward, 5′-TAC CTC CCA AGT CCT GTA TGA G-3′, reverse,
5′-TGA GCA GCA TCA AAC TGT GTA G-3′; ZO-1, forward, 5′-AGT CCC TTA
CCT TTC GCC TGA-3′, reverse, 5′-TCT CTT AGC ATT ATG TGA GCT
GC-3′.
Flow cytometry analyses
For Ki67 studies, HCECs prepared from sphere-forming
colonies with or without Y-27632 treatment were passaged in 1:4
dilutions and dissociated into single cells by 0.25% trypsin
digestion, fixed in 70% (w/v) ethanol, then washed and incubated
for 20 min with 1% BSA. The HCECs were incubated with a 1:20
dilution of anti-mouse Ki67, then washed and incubated with 1:1000
diluted Alexa Fluor 488 conjugated goat anti-mouse IgG
(Invitrogen), according to the manufacturer’s instructions. Flow
cytometric analyses were then performed using a FACSCalibur flow
cytometer (BD Biosciences).
Measurement of corneal endothelial cell
pump function
The pump function of confluent monolayers of HCECs
was measured using an Ussing chamber as described previously
(9). Cells cultured on Snapwell
inserts coated with type IV collagen were placed in the Ussing
chamber with the endothelial cell surface side in contact with one
chamber and the Snapwell membrane side in contact with another
chamber. The chambers were carefully filled with Krebs-Ringer
bicarbonate and maintained at 37°C using an attached heater. The
short circuit current was measured with narrow polyethylene tubes
positioned close to either side of the Snapwell insert and filled
with 3 M potassium chloride (KCl) and 4% agar gel connected to
silver electrodes. These electrodes were connected to the computer
through the Ussing system VCC-MC2 (Physiologic Instruments, San
Diego, CA, USA) and an iWorx 118 Research Grade Recorder (iWorx
Systems, Dover, NH, USA). After the short circuit current had
reached a steady state for 10 min, ouabain (1 mM), a
Na+/K+-ATPase inhibitor, was added to the
chamber and the short circuit current was re-measured.
Statistical analysis
All experimental results were analyzed by one-way
analysis of variance using SPSS version 12.0 software (SPSS Inc.,
Chicago, IL, USA). Summary statistics were expressed as mean ±
standard deviation (SD). P<0.01 was considered to indicate a
statistically significant difference and all p-values were
two-sided.
Results
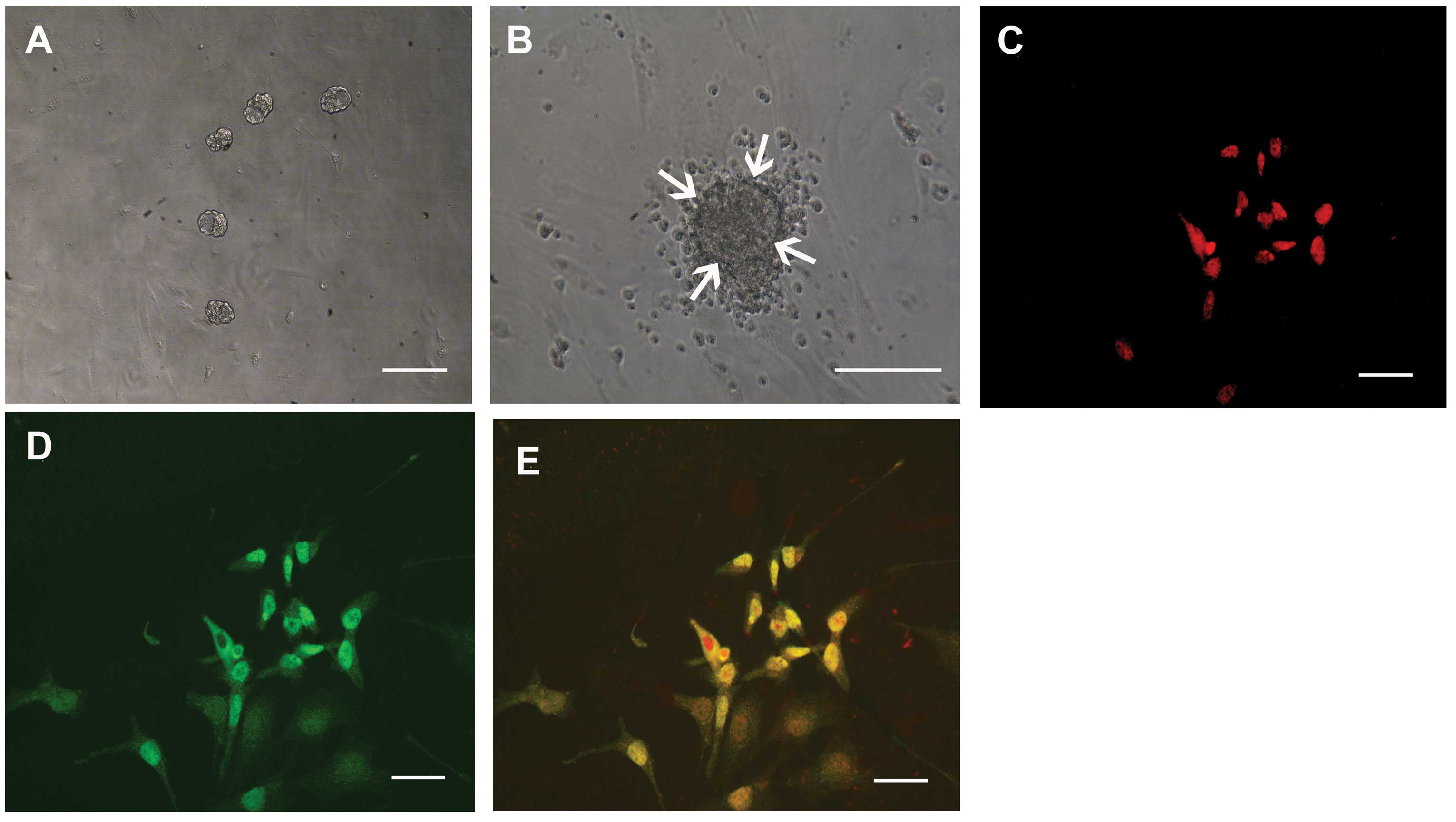
Isolation of sphere colonies from
HCECs
Spheres developed from the single cell suspensions
on day 7 (Fig. 1A) and grew larger
by day 10 (Fig. 1B). A number of
cells migrated from the sphere colonies (Fig. 1B) and the progeny from sphere
colonies could be passaged twice, whereas the non-proliferating
cells died. The number of spheres was counted after 10 days of
culture, revealing that 51.2±8.1 spheres (mean ± SD, n=8) were
generated per 10,000 cells. When the primary sphere colonies from
HCECs were dissociated into single cells and were cultured in the
presence of the methylcellulose gel matrix, secondary and tertiary
sphere colonies were generated. This suggests that HCECs had the
capacity for self-renewal of sphere colonies, although this
capacity was limited. The cells released from the spheres were
immunostained for nestin (as a marker of immature cells, Fig. 1C) and also immunostained positive
for an immature neuronal marker, β3-tubulin (Fig. 1D).
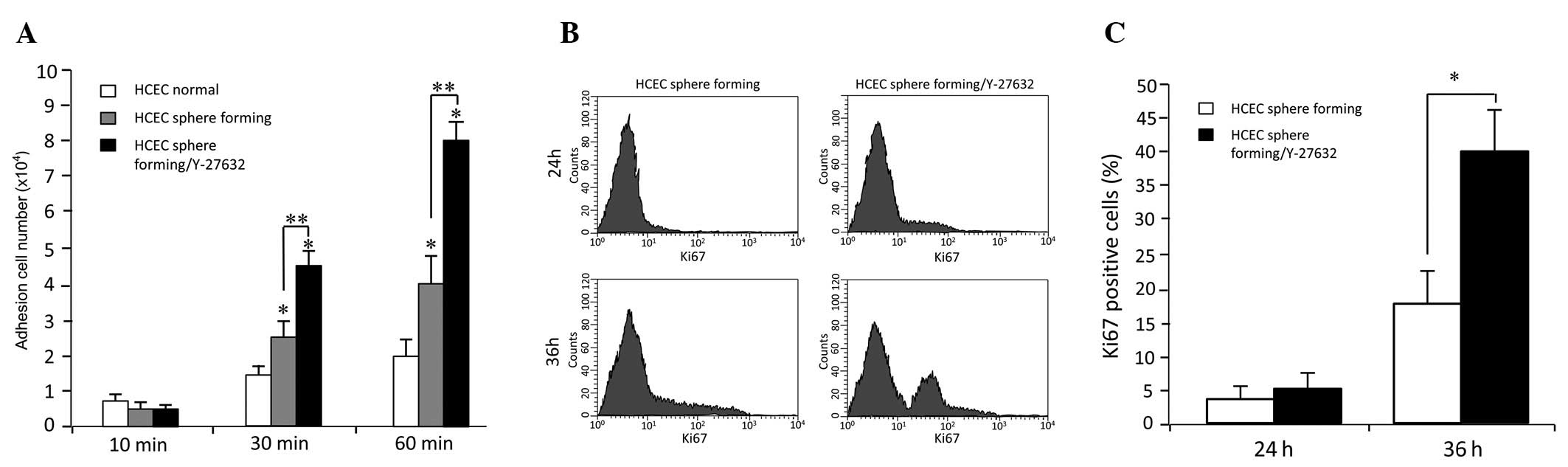
Effects of Y-27632 on adhesion capacity
and cell cycle progression of HCECs
To investigate the effect of the Rho-associated
protein kinase (ROCK) signalling pathway on adhesion of HCECs, a
homotypic adhesion assay was performed. There were statistical
differences between the untreated cells and cells treated with 30
μM Y-27632, after 30- and 60-min incubations (p<0.05), however
not at 10 min. The increase of adhesion cells was time-dependent
(Fig. 2A).
Quantitative flow cytometric analysis revealed a
significantly higher presence of Ki67-positive cells in HCECs
cultured with Y-27632, 36 h after subculture (Fig. 2B and C), thus demonstrating that
Y-27632 alters HCEC proliferation.
Effects of Y-27632 on gene expression and
density of Na+/K+-ATPase-positive cells
Expression of genes involved in active transmembrane
transporter activity (Na+/K+-ATPase) or cell
adhesion (ZO-1 and β-catenin), were assessed by semi-quantitative
RT-PCR (Fig. 3A). No significant
difference was observed among the three cell lines in the
expression of genes associated with several cell adhesion or ion
transporter channel proteins, which are characteristically
expressed by HCECs. Na+/K+-ATPase, a key HCEC
transmembrane protein, demonstrated positive staining at the
intercellular junction in HCECs (Fig.
3C, E and G). Although hexagonal morphology was identified by
phase contrast microscopy and immunocytochemistry, differences
among the three cell lines were not observed. Immunostaining
revealed a high density of Na+/K+-ATPase
located between cell nuclei in the HCECs (sphere-forming) and HCECs
(sphere-forming/Y-27632), however not in HCECs (normal; Fig. 3C, E and G).
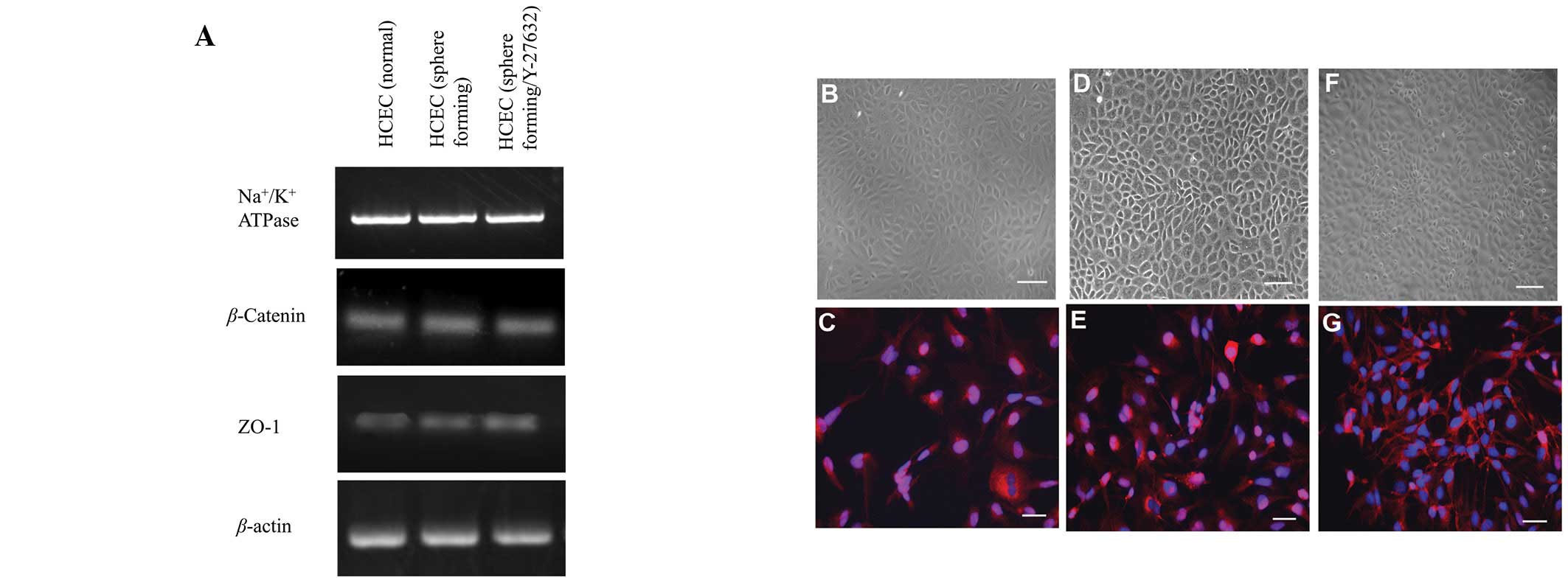 | Figure 3.HCEC-associated genes and cell
localization of junctional components expressed by cell lines. (A)
Semi-quantitative reverse transcription-polymerase chain reaction
for HCEC-associated genes. Total RNA was prepared from cultured
cells 7 days after reaching confluency. No significant difference
in mRNA expression was observed among the three cell lines.
Compared with phase contrast micrographs (B, D and F),
immunostaining demonstrated that
Na+/K+-ATPase was localized between cell
nuclei (blue, nuclei; red, Na+/K+-ATPase)
with differences shown in the three cell lines (C, E and G). B, D
and F, scale bar = 100 μm; C, E and G, scale bar = 50 μm. HCEC,
human corneal endothelial cell. |
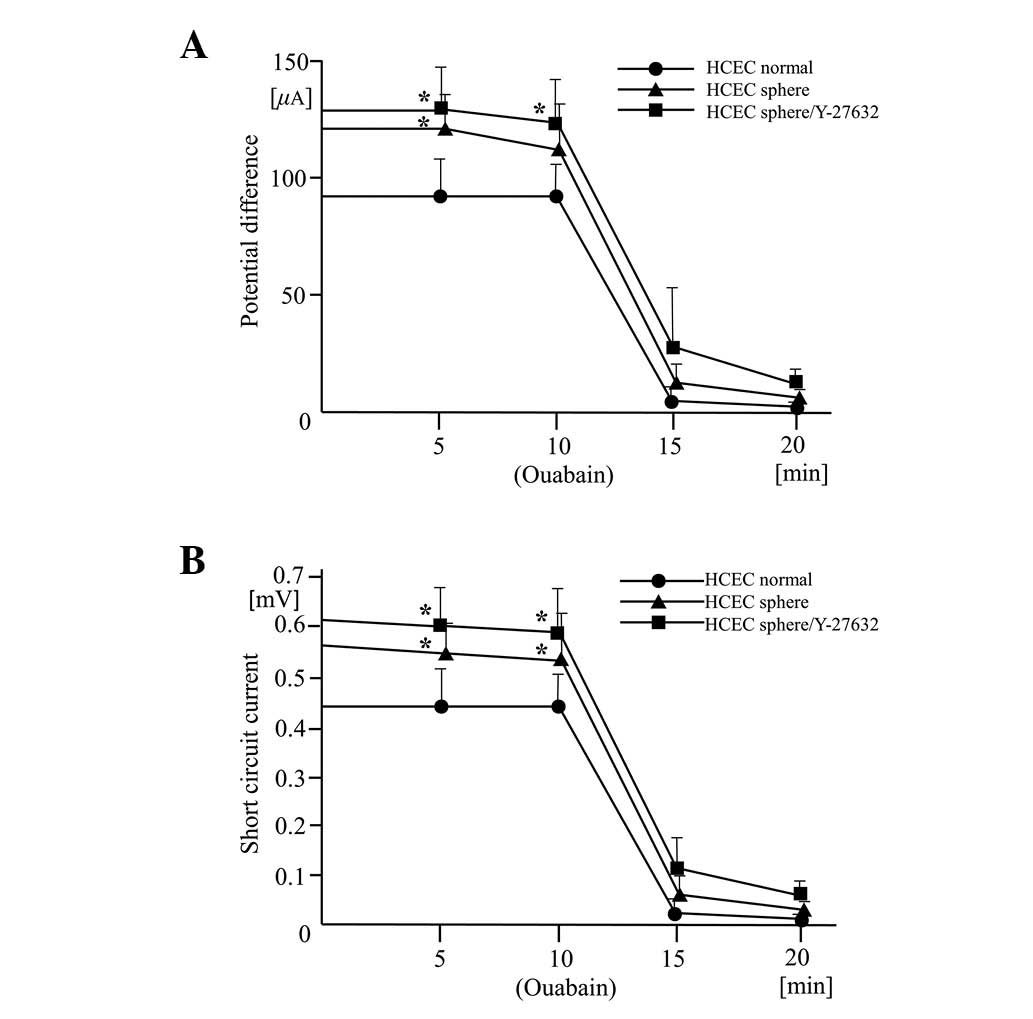
Effects of Y-27632 on potential
difference and short circuit current driven by
Na+/K+-ATPase
The traces of the potential difference and short
circuit current driven by the Na+/K+-ATPase
were of similar shapes in the three cell lines compared with normal
HCECs. However, there were higher resting potential differences and
resting short circuit measurements in the HCEC sphere and HCEC
sphere/Y-27632 cell lines (Fig. 4A and
B). The potential difference and short circuit currents
maintain corneal transparency and levels in all the cell lines were
clearly reduced by the presence of the
Na+/K+-ATPase inhibitor, ouabain. This
confirms that the origin of the current is
Na+/K+-ATPase. The pump function in normal
HCECs, detected in earlier and later passages of cells, was more
variable than in the other two cell lines. This possibly indicates
incomplete Na+/K+-ATPase activity or the
presence of an intercellular barrier that regulates ion
permeability.
Discussion
A necessary prerequisite for successful tissue
engineering of the human corneal endothelium is to ensure effective
isolation, preservation and expansion from a small number of HCECs
(4,6). The present study revealed that
spheres derived from HCECs demonstrate a high proliferative
capacity and cells directly derived from the spheres expressed
markers of neural (β3-tubulin and nestin) lineages. We were not
able to demonstrate directly that isolated spheres gave rise to
HCECs due to lack of specific markers (10). However, the characteristic
hexagonal morphology and transport activity of HCECs in the Ussing
chamber system suggests that the cultures largely give rise to
cells that have features of HCECs (11). These findings indicate that
HCEC-derived spheres have the characteristics of HCEC precursor
cells.
ROCK is one of the main downstream effectors of the
Ras-homologous (Rho) family of GTPases, which are involved in a
number of cellular functions, including cell proliferation,
apoptosis, invasion and metastasis (12). Overexpression of ROCK promotes
invasion and metastasis in a number of solid tumors, including
liver, breast and colon cancers (13). Although the HCECs tend to have a
cell senescence phenotype after only a few passages, we
successfully cultivated and passaged HCEC spheres with or without
the presence of the ROCK inhibitor Y-27632. We further demonstrated
elevated cell-cell adhesion by the homotypic adhesion assay and the
adhesion of HCECs was dose- and time-dependent with Y-27632.
Quantitative flow cytometric analyses revealed the increased
presence of Ki67-positive cells in HCECs cultured with Y-27632 for
only 36 h. This suggests that Y-27632 promotes HCEC proliferation.
Although further studies are required, the increased cell adhesion
and motility observed in our study, which was enhanced by ROCK
inhibition, may also have a positive effect on HCEC proliferation.
Further investigations are necessary to determine whether the
increase in cell proliferation observed in our study may be
attributed to an effect on the molecular components regulating
cell-cycle progression (14).
Corneal endothelial cells accumulate
Na+/K+-ATPase at intercellular contacts along
the lateral cell membrane to maintain a bicarbonate gradient across
the cell layer to sustain a constant flow of water out of the
stroma (11). We detected
Na+/K+-ATPase in the cells and at the lateral
cell contacts. The presence of this protein in our HCEC populations
indicates that HCEC cells from direct HCEC culture and from
sphere-forming culture have this pump function. However, the number
of Na+/K+-ATPase-positive cells was
significantly higher in the HCECs (sphere-forming/Y-27632) than
those observed in HCECs (sphere-forming) and HCECs (normal
culture). We used the Ussing chamber assay to detect the cell pump
function evaluated from cell electrophysiological measurements.
Prior to the addition of the Na+/K+-ATPase
inhibitor ouabain, potential difference and short circuit current
were detected in the three cell lines. Compared with the values of
the normal HCECs, HCEC (sphere) and HCEC (sphere/Y-27632) had a
higher potential difference and higher short circuit current, which
may have been caused by increased
Na+/K+-ATPase activity. These results are
consistent with the observation that relatively mature
intercellular adhesion allows regular intercellular ion transport
with differences in cellular density (11,15).
The results of this study demonstrated that Y-27632
promoted the HCEC adhesion and proliferation ability and increased
the HCEC cell pump function. In addition, it was demonstrated that
Y-27632 does not alter important morphological features of HCECs.
These are all important characteristics required for ex vivo
cultured HCEC transplantation.
Acknowledgements
The authors thank Dr Hui Qi for the
technical advice and assistance with the Ussing chamber system, Dr
Dan Song for the donor tissue preparation and Dr Lixia Lu for use
of important instruments. Funding for this study was obtained from
the Natural Science Foundation of China (NSFC: 30973247/C170601)
and Shanghai Excellent University Teacher Foundation (1500144019).
Mingfeng Wu and Fei Du were partially supported by a stem cell
traineeship from the Huadong Stem Cell Bank of China.
References
|
1.
|
Abib FC, Holzchuh R, Schaefer A, Schaefer
T and Godois R: The endothelial sample size analysis in corneal
specular microscopy clinical examinations. Cornea. 31:546–550.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Koizumi N, Okumura N and Kinoshita S:
Development of new therapeutic modalities for corneal endothelial
disease focused on the proliferation of corneal endothelial cells
using animal models. Exp Eye Res. 95:60–67. 2012. View Article : Google Scholar
|
|
3.
|
Patel SV: Graft survival and endothelial
outcomes in the new era of endothelial keratoplasty. Exp Eye Res.
95:40–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Peh GS, Beuerman RW, Colman A, Tan DT and
Mehta JS: Human corneal endothelial cell expansion for corneal
endothelium transplantation: an overview. Transplantation.
91:811–819. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Gao Y, Zhou Q, Qu M, Yang L, Wang Y and
Shi W: In vitro culture of human fetal corneal endothelial
cells. Graefes Arch Clin Exp Ophthalmol. 249:663–669. 2011.
View Article : Google Scholar
|
|
6.
|
Jäckel T, Knels L, Valtink M, Funk RH and
Engelmann K: Serum-free corneal organ culture medium (SFM) but not
conventional minimal essential organ culture medium (MEM) protects
human corneal endothelial cells from apoptotic and necrotic cell
death. Br J Ophthalmol. 95:123–130. 2011.
|
|
7.
|
Hitani K, Yokoo S, Honda N, Usui T,
Yamagami S and Amano S: Transplantation of a sheet of human corneal
endothelial cell in a rabbit model. Mol Vis. 14:1–9.
2008.PubMed/NCBI
|
|
8.
|
Park TY, Park MH, Shin WC, et al:
Anti-metastatic potential of ginsenoside Rp1, a novel ginsenoside
derivative. Biol Pharm Bull. 31:1802–1805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Mimura T, Yamagami S, Yokoo S, et al:
Cultured human corneal endothelial cell transplantation with a
collagen sheet in a rabbit model. Invest Ophthalmol Vis Sci.
45:2992–2997. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Peh GS, Toh KP, Wu FY, Tan DT and Mehta
JS: Cultivation of human corneal endothelial cells isolated from
paired donor corneas. PLoS One. 6:e283102011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Hatou S, Yamada M, Akune Y, et al: Role of
insulin in regulation of Na+-/K+-dependent
ATPase activity and pump function in corneal endothelial cells.
Invest Ophthalmol Vis Sci. 51:3935–3942. 2010.PubMed/NCBI
|
|
12.
|
Gallo RM, Khan MA, Shi J, et al:
Regulation of the actin cytoskeleton by rho kinase controls antigen
presentation by CD1d. J Immunol. July 13–2012.(Epub ahead of
print).
|
|
13.
|
Matsuoka T, Yashiro M, Kato Y, Shinto O,
Kashiwagi S and Hirakawa K: RhoA/ROCK signaling mediates plasticity
of scirrhous gastric carcinoma motility. Clin Exp Metastasis.
28:627–636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Joyce NC and Harris DL: Decreasing
expression of the G1-phase inhibitors, p21Cip1 and p16INK4a,
promotes division of corneal endothelial cells from older donors.
Mol Vis. 16:897–906. 2010.PubMed/NCBI
|
|
15.
|
Yokoo S, Yamagami S, Yanagi Y, et al:
Human corneal endothelial cell precursors isolated by
sphere-forming assay. Invest Ophthalmol Vis Sci. 46:1626–1631.
2005. View Article : Google Scholar : PubMed/NCBI
|