Introduction
Haloperidol (HP) is a typical incisive antipsychotic
drug. Chemically, it belongs to the butyrophenone series of
antipsychotic compounds. Due to its marked central antidopaminergic
action, HP is classified as a highly potent neuroleptic agent
(1). It is prescribed in several
diagnoses, such as psychosis, manic phases, hyperactivity,
aggressiveness and acute delirium, and in certain cases, it is
employed in long-term treatment. However, the use of typical
neuroleptic drugs is limited by their side-effects and toxicity
(2–6). Despite significant advantages
provided by these remedies, patients using these drugs have to cope
with the residual symptomatology which interferes significantly
with their social and occupational life (7). Certain patients may develop
disfiguring, disabling and potentially life-threatening adverse
effects, including parkinsonian symptoms, tardive dyskinesia and
neuroleptic malignant syndrome (8,9),
whereas others are completely resistant to the treatment.
HP may have a direct cytotoxic effect via the
production of toxic metabolites (10,11).
Forsman et al reported the presence of reduced HP (RHP) as a
major metabolite in the plasma of patients (12). The formation of these compounds is
NADPH-dependent (12). Reduced HP
is oxidized to a toxic pyridinium metabolite (RHPP+) by
the specific isozymes CYP 450 and CYP 3A4 (13,14).
HP is metabolically reduced in humans, but not in rats and the
majority of other experimental animals, with the exception of
guinea pigs (Cavia porcellus) (15). Thus, the molecular mechanisms of HP
reduction may be studied using guinea pigs as a model for human HP
metabolism. The main pathways of reactive oxygen species (ROS)
production by HP treatment and the antioxidant system of defense
are presented in Fig. 1.
Burkhardt et al observed that neuroleptics
are able to inhibit NADH/ubiquinone oxidoreductase (complex-I)
through their metabolites such as RHPP+(16). Complex-I inhibition is associated
with the excessive generation of ROS. Chronic treatment with HP is
known to induce oxidative stress due to the increased turnover of
dopamine (17). Behl et al
demonstrated that amyloid beta resistant cells were resistant to HP
toxicity (18). This suggests a
role for free radicals in HP-induced cell damage. Moreover, lipid
peroxidation has been implicated to be a causal factor in the
development of tardive dyskinesia and other movement disorders
(19). Other evidence supporting
this hypothesis includes elevated levels of lipid peroxidation in
HP-treated rats (17), as well as
in psychotic patients (20).
It appears to be clear that ROS are crucial in the
generation of adverse HP side-effects. However, it is not known
which of the main antioxidant enzymes have the greatest activity in
the removal of HP-induced ROS. Thus, the determination of the
oxidative stress and ROS-associated enzymes in an animal model was
the aim of the present study. The level of oxidative stress was
measured and compared in the plasma of 17 guinea pigs (10
HP-treated and 7 untreated). Furthermore, the superoxide dismutase
(SOD), glutathione reductase (GR) and
glutathione-S-transferase (GST) activity detection was
recorded, as well as the glucose levels and reduced and oxidized
glutathione (GSH/GSSG) ratio.
Materials and methods
Animals
All animal experiments in the present study were
performed inaccordance with the recommendations of the European
Community Guide for the Care and Use of Laboratory Animals and
followed the guidelines for animal treatment approved by local
authorities.
Four-month-old guinea pigs were obtained from Velaz
(Prague, Czech Republic) and during the study they were kept in the
Animal Facility of Masaryk University (Brno, Czech Republic). A
total of 10 animals were treated with HP and 7 with a physiological
solution (saline) applied intraperitoneally for 21 successive days.
The total dose of HP was 4,200 μg per 100 g of body mass.
Determination of low-molecular-mass
thiols and HP
The high performance liquid chromatography with
electrochemical detection (HPLC-ED) system consisted of two solvent
delivery pumps operating in the range of 0.001–9.999 ml/min (Model
582; ESA Inc., Chelmsford, MA, USA), Zorbax Eclipse AAA Column
(4.6x150 mm 3.5-micron particle size; Varian Inc., Paulo Alto, CA,
USA) and a CoulArray electrochemical detector (Model 5600A, ESA
Inc.). The sample (30 μl) was injected using an autosampler (Model
542; ESA Inc.). The HPLC-ED experimental conditions were as
follows: the compositions of the mobile phases were 80 mM
trifluoroacetic acid (A) and methanol (B). The mobile phases were
mixed in a gradient from 3% B in the 1st min, 10% B between the 2nd
and 6th minute and 98% B from the 7th minute of the separation. The
flow of the mobile phase was 0.8 ml/min, the temperature of the
separation was 40°C, the working electrode potential was 900 mV,
the detector temperature was 30°C and each measurement was
performed in triplicate. The signals of GSH, GSSG and HP were
quantified as the sum of the current responses from all working
electrodes. For the real sample measurements, the shift of the
retention time was of ∼±2%.
Spectrometric measurement
Spectrophotometric measurements were carried out
using an automated chemical analyzer BS-400 (Mindray, Shenzhen,
China). The analyzer was composed of a cuvette space (37±1°C),
reagent space with a carousel for reagents (4±1°C), sample space
with a carousel for the preparation of samples and an optical
detector. The transfer of samples and reagents was performed by a
robotic arm equipped with a dosing needle (dosage error ≤5% of
volume). The cuvette contents were mixed by an automatic mixer,
including a stirrer, immediately after the addition of reagents or
samples. Contamination was reduced by a rinsing system which
included rinsing of the dosing needle and stirrer with MilliQ
water. For detection, the following wavelengths were usable: 340,
380, 412, 450, 505, 546, 570, 605, 660, 700, 740 and 800 nm.
Determination of SOD
Kit 19160 SOD (Sigma Aldrich, St. Louis, MO, USA)
was used for the assay of SOD (EC 1.15.1.1.). First, 200 μl R1
reagent (WTS solution diluted 20-fold with buffer) was pipetted
into a plastic cuvette and incubated at 37°C for 108 sec.
Subsequently, 20 μl of sample was added and in 378 sec, the
reaction was started by adding 20 μl R2 reagent (enzyme solution
diluted 167-fold with buffer). The reaction was incubated for 72
sec and then absorbance was measured at λ=450 nm. The kinetic
reaction was measured for 108 sec and the absorbance was recorded
every 9 sec.
Determination of GR
A GR Assay Kit (Sigma Aldrich) was used for the GR
activity determination. Reagents R1 and R2 were prepared by
dissolving in assay buffer (100 mM potassium phosphate buffer, pH
7.5, with 1 mM EDTA). The R1 reagent (260 μl; 1.15 mM oxidized GSH
in the assay buffer) was added with 10 μl of sample and 30 μl R2
reagent (1 mM NADPH in GR assay buffer) into a plastic cuvette. The
decrease in absorbance was measured at 340 nm using a kinetic
program for 1,260 sec.
Determination of GST
The method used was based on the GST-catalyzed
reaction between GSH and the GST substrate,
1-chloro-2,4-dinitrobenzene (CDNB). GST substrate has the broadest
range of isozyme detectability (e.g., α, μ, π and other GST
isoforms). Under certain conditions, the interaction between GSH
and CDNB is dependent on the presence of active GST. The
GST-catalyzed formation of GS-DNB produces a dinitrophenylthioether
which may be detected spectrophotometrically at 340 nm. A 180-μl
volume of reactants consisting of 2 mM CDNB and PBS (1.4 mM
NaH2PO4 and 4.3 mM
Na2HPO4, pH 7.4; 1:19, v/v, 37°C) was added
to the sample in a plastic microtube. Furthermore, 12.5 mM GSH (30
μl) in 0.1 M phosphate buffer (pH 7.4) was added. A wavelength of
340 nm was used to determine the GST activity.
Determination of antioxidant activity by
the ferric reducing antioxidant power (FRAP) method
The FRAP method is based on the reduction of
complexes of 2,4,6-tripyridyl-s-triazine (TPTZ) with ferric
chloride hexahydrate (FeCl3·6H2O); these
substances are almost colorless and eventually slightly brown.
Following the reduction, blue ferrous complexes are formed. The
reagents were prepared as follows: solution 1 contained 10 mmol/l
TPTZ in 40 mmol/l hydrochloric acid. Solution 2 contained 20 mmol/l
ferric chloride hexahydrate in ACS water. Solution 3 contained 20
mmol/l acetate buffer, pH 3.6. These three solutions (TPTZ,
FeCl3 and acetate buffer) are mixed in a 1:1:10
ratio.
The procedure for the determination was taken from
the study by Sochor et al(21). After 150 μl of reagent was injected
into a plastic cuvette with the subsequent addition of 3 μl sample,
the absorbance was measured at 605 nm for 12 min. The difference
between absorbance at the last (12th) and the 2nd minute of the
assay procedure was used to calculate the antioxidant activity.
Determination of antioxidant activity by
the free radicals (FR) method
This method is based on ability of chlorophyllin
(the sodium-copper salt of chlorophyl) to accept and donate
electrons with a stable change of maximum absorption. This effect
requires an alkaline environment and the addition of a
catalyst.
The procedure for the determination was taken from
the study by Sochor et al(21). Reagent (150 μl) was injected into a
plastic cuvette with the subsequent addition of a 6 μl sample. The
absorbance was measured at 450 nm in the second and last (12th)
minute of the assay. The difference between two absorbances was
considered to be the output value.
Determination of glucose
First, 200 μl of the reagent (0.1 M phosphate
buffer, pH 7.5, 0.75 mM phenol, 0.25 mM 4-amino-antipyrine (4-AAP),
glucose oxidase ≥15 kU/l, peroxidase ≥1.5 U/l) was pipetted into a
plastic cuvette with 20 μl of the sample. The absorbance was then
measured for 10 min at λ=505 nm. To calculate the absorbance, the
values of the sample, reagents and reaction mixture after 10 min of
incubation with the sample were used.
Statistical analysis
Software Statistica 10 (StatSoft Inc., Tulsa, OK,
USA) was used for the statistical analysis. The Shapiro-Wilk test
was used to assess normality. Mann-Whitney U tests were used to
evaluate the differences between the groups. Simple linear
correlations were performed to reveal the associations between the
variables. Tree clustering was used to visualize the distribution
of variables and K-means clustering was used to divide the cases
into clusters. Unless noted otherwise, P<0.05 was considered to
indicate statistically significant differences.
Results
A total of 10 guinea pigs were treated with HP and
seven with saline. The oxidative stress and enzyme activity levels
in plasma were measured. The SOD, GR and GST activity levels were
detected and the glucose levels and the GSH/GSSG ratio were
measured. HP was present in the plasma of treated animals, while it
was undetectable in the untreated animals. Animals treated with HP
exhibited significantly increased activity of GST (P= 0.007). The
elevation of SOD and GR activity levels and an elevated level of
GSH in HP-treated animals were observed but not significant. Also,
the GSH/GSSG ratio was not shifted due to the oxidative state and
no significant differences were observed in the glucose levels
between the control and HP-treated animals (Fig. 2). The present study demonstrates
that the administration of HP causes significant oxidative stress,
measurable by spectrometric FR and FRAP assays (P=0.02 and P=0.05,
respectively).
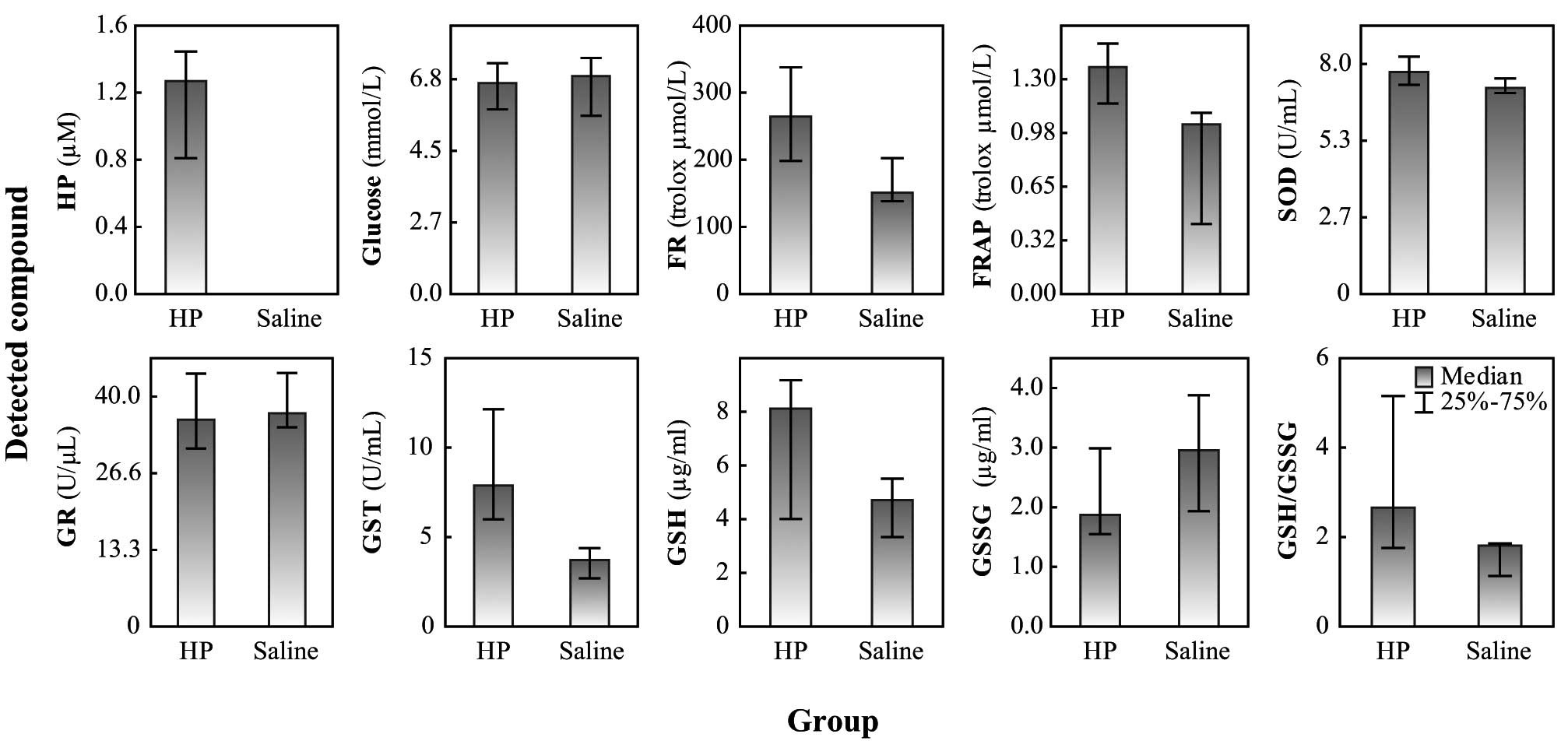 | Figure 2.Levels of oxidative stress and enzyme
activities in blood plasma of guinea pigs. HP, haloperidol; saline,
solution of 0.90% w/v of NaCl; FR, free radicals method was used
for the determination of oxidative stress; FRAP, ferric reducing
antioxidant power method was used for the determination of
oxidative stress; SOD, superoxide dismutase; Glc, glucose; GR,
glutathione reductase; GST, glutathione-S-transferase; GSH,
glutathione; GSSG, oxidized glutathione; GSH/GSSG, ratio between
reduced and oxidized glutathione. |
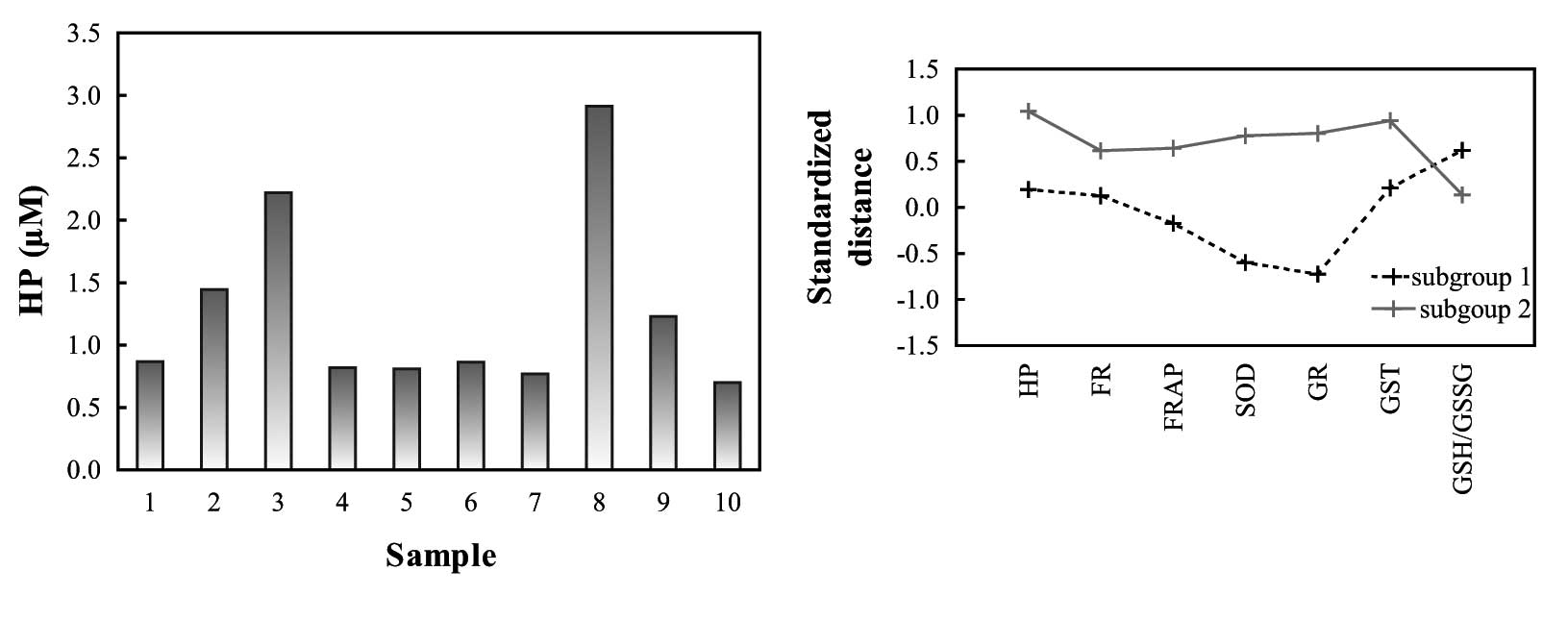
The plasma levels of HP in the treated animals
varied considerably (range, 0.7–2.9 μM), although all animals
received the same dose of HP according to their body mass (Fig. 3A). Using K-means clustering, the
HP-treated guinea pigs were divided into two clusters according to
levels of HP, oxidative stress and ROS-enzymes (Fig. 3B and Table I). Two characteristic contrasting
subgroups of animals were observed: the first subgroup had higher
HP, FR, FRAP, SOD, GR and GST values and lower GSH/GSSG ratios,
while the second had lower HP, FR, FRAP, SOD, GR and GST values and
higher GSH/GSSG ratios.
 | Table I.Concentrations of HP, oxidative stress
parameters and oxidative stress enzymes in the placebo and
treatment groups and two subgroups (clusters) of treated animals
(shown as mean ± 1 SD). |
Table I.
Concentrations of HP, oxidative stress
parameters and oxidative stress enzymes in the placebo and
treatment groups and two subgroups (clusters) of treated animals
(shown as mean ± 1 SD).
| Group | Number | HP (μM) | Glc (mM) | FR (trolox μM) | FRAP (trolox μM) | SOD (U/l) | GR (U/l) | GST (U/l) | GSH/GSSG |
|---|
| Placebo | 7 | 0±0 | 6.70±1.03 | 194±42 | 1.17±1.07 | 7.94±0.38 | 35.0±5.66 | 4.90±2.38 | 1.8±1.0 |
| Treatment | 10 | 1.26±0.74 | 6.13±0.94 | 287±81 | 1.36±1.49 | 7.97±0.85 | 32.7±8.99 | 9.33±3.16 | 3.4±2.2 |
| Subgroup 1 | 4 | 0.90±0.21 | 6.42±0.92 | 272±94 | 0.71±1.34 | 7.80±0.50 | 29.0±8.76 | 8.89±2.99 | 4.0±2.5 |
| Subroup 2 | 5 | 1.62±0.93 | 5.83±1.07 | 276±70 | 1.68±1.24 | 7.78±0.86 | 36.8±9.04 | 10.53±3.02 | 3.1±2.0 |
The greatest difference in activity (U/μl) between
the two groups of animals was observed for GR. In the placebo
group, significantly positive correlations were observed between
oxidative stress detected by the FR method (r=0.88, P=0.008) and
GST activity, as well as between oxidative stress detected by the
FR method (r= 0.86, P= 0.01) and SOD activity. A significant
negative correlation was observed between the level of plasma
glucose and oxidative stress detected by the FRAP method (r=−0.78,
P=0.04). There was also positive correlation between SOD and GST
activity (r=0.80, P=0.03) in the plasma of untreated animals. By
contrast, no similar significant correlations were observed in the
HP-treated animals (Fig. 4).
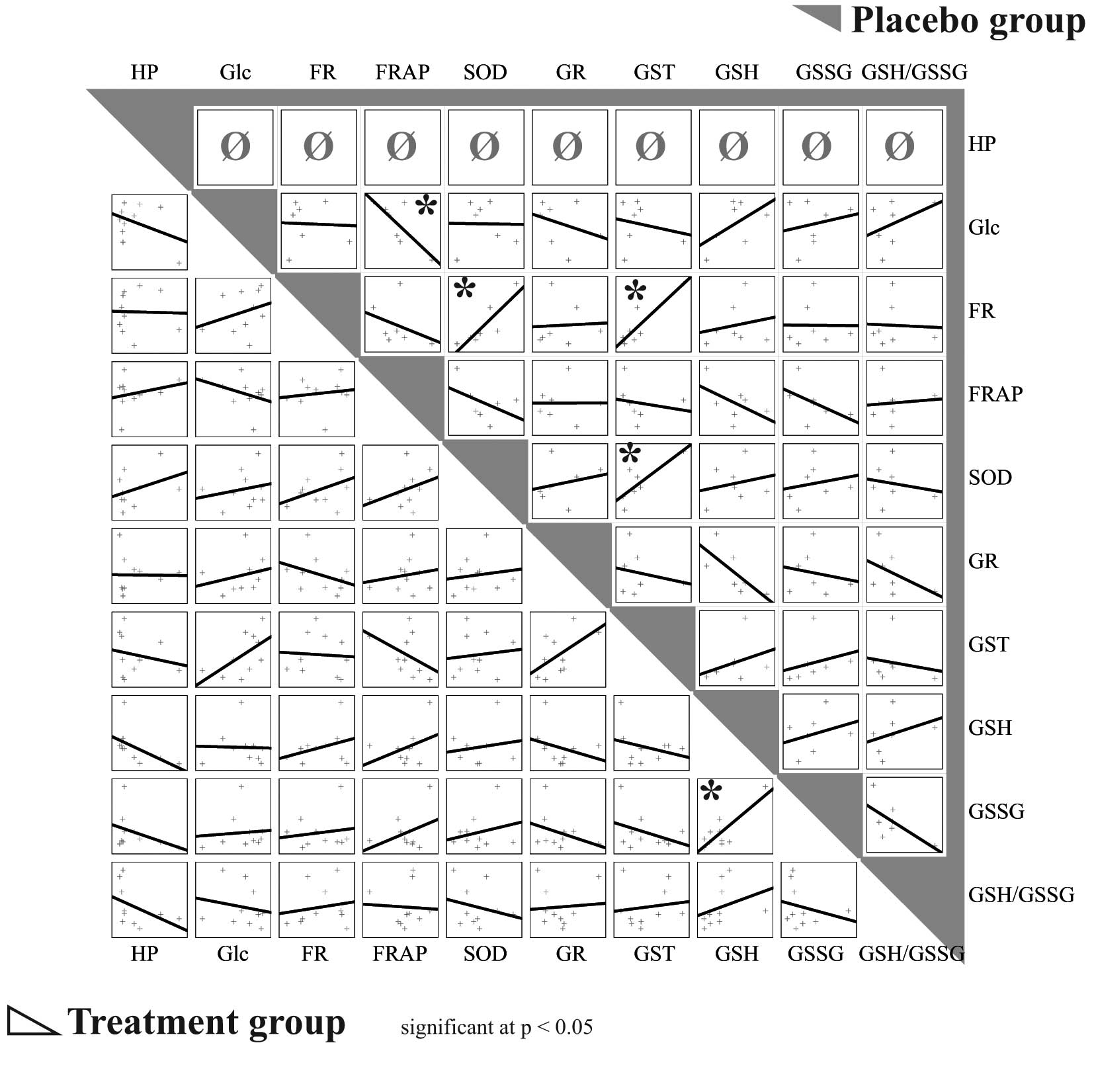 | Figure 4.Correlations between observed
parameters in treated and placebo (saline) groups. Treatment group
bottom left, placebo group top right (gray). *P<0.05.
HP, haloperidol; FR, oxidative stress measured by free radicals
method; FRAP, oxidative stress measured by ferric reducing
antioxidant power method; SOD, superoxide dismutase; Glc, glucose;
GR, glutathione reductase; GST, glutathione-S-transferase;
GSH, glutathione; GSSG, oxidized glutathione; GSH/GSSG, ratio
between reduced and oxidized glutathione. |
Discussion
Adult guinea pigs were used to study the molecular
mechanisms of HP metabolism. Since the observation of the initial
activity of the antioxidant enzymes was being investigated, the
animals were treated for a relatively short period (3 weeks).
According to Lawler et al, increased levels of the
antioxidant enzymes may be detected immediately after ROS
production (22). Cells respond to
acute oxidative stress by the induction of the expression of genes
products which protect the cell. However, chronic oxidative stress
causing long-term increased production of these enzymes is
extremely burdensome for the cell. As a result, although ROS
exposure remains present, the production of enzymes gradually
decreases (23).
Although all the experimental animals were treated
with a total dose of 4,200 μg of HP per 100 g of body mass, the
levels of HP in their plasma varied considerably. This may indicate
a high interindividual variability in the activity of the enzymes
involved in the metabolism of HP in guinea pigs.
When the activity of the antioxidant enzymes was
compared between the treated and placebo groups, only one
statistically significant difference was identified. In animals
treated with HP, significantly increased activity of GST was
observed. GSTs are evolutionarily conserved enzymes important in
the detoxification of numerous xenobiotic compounds. These enzymes
catalyze the conjugation of GSH to electrophilic substrates, thus
producing compounds that are generally less reactive and more
soluble. This facilitates the removal of these compounds from the
cell via membrane-based GSH conjugate pumps. The broad substrate
specificity of GSTs allows them to protect cells against a wide
range of toxic chemicals (24).
The GSH peroxidase activity of a number of GST proteins also
suggests that they may be important in organic peroxide
detoxification (25). GSTs are
able to conjugate GSH to these toxic reactive compounds, forming
4-hydroxynonenal and cholesterol α-oxide which are generated during
the oxidation of membranes (26).
GSTs may have a wider role in the response to cellular stress
beyond their enzymatic activity. In particular, GSTs have been
shown to act as stress-sensitive inhibitors of the mammalian
stress-activated protein kinase c-Jun NH2-terminal
kinase. This helps to maintain c-Jun NH2-terminal kinase
in an inactive form in unstressed cells (27). Based on the increased activity of
GST and in accordance with the studies of Shivakumar and
Ravindranath and Pai et al(17,20),
peroxidation of membrane lipids was proposed to be the main
mechanism of HP adverse effects. This hypothesis is be further
supported by the observation that HP tends to decrease the
permeability of a number of biological membranes to various
inorganic and organic molecules, including water, and that it
exerts this effect at minute concentrations (28).
The present study demonstrates that the
administration of HP causes significant oxidative stress which is
measurable by spectrometric FR and FRAP assays but not by the
GSH/GSSG ratio. This ratio was not changed relative to the
oxidative state. It may indicate that 3 weeks of HP treatment are
not long enough to deplete the GSH supplies of healthy guinea pigs.
This is in agreement with Pai et al who demonstrated no
changes in GSH levels after the first two weeks of HP
administration in psychotic patients (20).
In the placebo group, significant positive
correlations were observed between oxidative stress detected by the
FR method and GST and SOD activity levels, respectively, which is
in compliance with activation of antioxidant enzymes by oxidative
stress (22). This correlation was
observed in the untreated group only, although oxidative stress was
significantly higher in the treated group. A significant negative
correlation was observed between the level of plasma glucose and
oxidative stress detected by the FRAP method, but only in the
placebo group. In the treated group, no significant correlations
were observed. It appears that the mechanisms of defense against
small, relatively-stable daily oxidative stress are different from
those activated by acute high stress.
Two groups of animals were identified according to
how they responded to oxidative stress (high plasma HP and high
oxidative parameters group and low plasma HP and low oxidative
parameters group). This appeared to be the reason for the lack of
significance of the correlations between oxidative stress detected
by the FR method and GST and SOD activity levels, respectively, in
the treated animals. However, these two sub-groups were too small
to conduct the same further statistical assessments as for all the
groups together. These results demonstrate the great variability in
the activation of antioxidant enzymes by HP detoxification in
guinea pigs.
Acknowledgements
The present study was supported by
MUNI/A/0846/2011, NanoBioMetalNet CZ.1.07/2.4.0 0/31.0 023, MSMT
6215712402 and CEITEC CZ.1.05/1.1.00/02.0068. The authors wish to
thank Mrs. Sarka Lakoma and Ms. Martina Stankova for their
excellent technical assistance.
References
|
1.
|
Janssen PA, Soudijn W, van Wijngaarden I
and Dresse A: Pimozide, a chemically novel highly potent and orally
long-acting neuroleptic drug. 3. Regional distribution of pimozide
and of haloperidol in dog brain. Arzneimittelforschung. 18:282–287.
1968.PubMed/NCBI
|
|
2.
|
Ikemura M, Nakagawa Y, Shinone K, Inoue H
and Nata M: The blood concentration and organ distribution of
haloperidol at therapeutic and toxic doses in severe fatty liver
disease. Leg Med (Tokyo). 14:147–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Maxa JL, Taleghani AM, Ogu CC and Tanzi M:
Possible toxic encephalopathy following high-dose intravenous
haloperidol. Ann Pharmacother. 31:736–737. 1997.PubMed/NCBI
|
|
4.
|
Tsujimoto A, Tsujimoto G, Ishizaki T,
Nakazawa S and Ichihashi Y: Toxic haloperidol reactions with
observation of serum haloperidol concentration in two children. Dev
Pharmacol Ther. 4:12–17. 1982.PubMed/NCBI
|
|
5.
|
Engel N and Mahlknecht U: Aging and
anti-aging: Unexpected side effects of everyday medication through
sirtuin1 modulation. Int J Mol Med. 21:223–232. 2008.PubMed/NCBI
|
|
6.
|
Huang QY, Li XF and Liu SP: E-cadherin and
caveolin-1 alterations in the heart of rats having undergone
chlorpromazine-induced toxicity. Mol Med Rep. 5:705–709.
2012.PubMed/NCBI
|
|
7.
|
Breier A, Schreiber JL, Dyer J and Pickar
D: National Institute of Mental Health longitudinal study of
chronic schizophrenia. Prognosis and predictors of outcome. Arch
Gen Psychiatry. 48:239–246. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Baldessarini RJ, Cohen BM and Teicher MH:
Significance of neuroleptic dose and plasma level in the
pharmacological treatment of psychoses. Arch Gen Psychiatry.
45:79–91. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Levenson JL: Neuroleptic malignant
syndrome. Am J Psychiatry. 142:1137–1145. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Gorrod JW and Fang J: On the metabolism of
haloperidol. Xenobiotica. 23:495–508. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Wright AM, Bempong J, Kirby ML, Barlow RL
and Bloomquist JR: Effects of haloperidol metabolites on
neurotransmitter uptake and release: possible role in neurotoxicity
and tardive dyskinesia. Brain Res. 788:215–222. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Forsman A and Larsson M: Metabolism of
haloperidol. Curr Ther Res Clin Exp. 24:567–568. 1978.
|
|
13.
|
Fang J, Baker GB, Silverstone PH and
Coutts RT: Involvement of CYP3A4 and CYP2D6 in the metabolism of
haloperidol. Cell Mol Neurobiol. 17:227–233. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Eyles DW, McGrath JJ and Pond SM:
Formation of pyridinium species of haloperidol in human liver and
brain. Psychopharmacology (Berl). 125:214–219. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Korpi ER, Costakos DT and Wyatt RJ: Rapid
formation of reduced haloperidol in guinea pigs following
haloperidol administration. Acta Pharmacol Toxicol (Copenh).
56:94–98. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Burkhardt C, Kelly JP, Lim YH, Filley CM
and Parker WD: Neuroleptic medications inhibit complex I of the
electron transport chain. Ann Neurol. 33:512–517. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Shivakumar BR and Ravindranath V:
Oxidative stress and thiol modification induced by chronic
administration of haloperidol. J Pharmacol Exp Ther. 265:1137–1141.
1993.PubMed/NCBI
|
|
18.
|
Behl C, Lezoualc’h F, Widmann M, Rupprecht
R and Holsboer F: Oxidative stress-resistant cells are protected
against haloperidol toxicity. Brain Res. 717:193–195. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Peet M, Laugharne J, Rangarajan N and
Reynolds GP: Tardive dyskinesia, lipid peroxidation, and sustained
amelioration with vitamin E treatment. Int Clin Psychopharmacol.
8:151–153. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Pai BN, Janakiramaiah N, Gangadhar BN and
Ravindranath V: Depletion of glutathione and enhanced lipid
peroxidation in the CSF of acute psychotics following haloperidol
administration. Biol Psychiatry. 36:489–491. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Sochor J, Ryvolova M, Krystofova O, et al:
Fully automated spectrometric protocols for determination of
antioxidant activity: advantages and disadvantages. Molecules.
15:8618–8640. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Lawler JM and Powers SK: Oxidative stress,
antioxidant status, and the contracting diaphragm. Can J Appl
Physiol. 23:23–55. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Vaziri ND, Dicus M, Ho ND, Boroujerdi-Rad
L and Sindhu RK: Oxidative stress and dysregulation of superoxide
dismutase and NADPH oxidase in renal insufficiency. Kidney Int.
63:179–185. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Salinas AE and Wong MG: Glutathione
S-transferases - a review. Curr Med Chem. 6:279–309. 1999.
|
|
25.
|
Tan KL and Board PG: Purification and
characterization of a recombinant human Theta-class glutathione
transferase (GSTT2-2). Biochem J. 315:727–732. 1996.PubMed/NCBI
|
|
26.
|
Hubatsch I, Ridderström M and Mannervik B:
Human glutathione transferase A4-4: an alpha class enzyme with high
catalytic efficiency in the conjugation of 4-hydroxynonenal and
other genotoxic products of lipid peroxidation. Biochem J.
330:175–179. 1998.
|
|
27.
|
Adler V, Yin ZM, Fuchs SY, et al:
Regulation of JNK signaling by GSTp. EMBO J. 18:1321–1334. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Seeman PM and Bialy HS: The surface
activity of tranquilizers. Biochem Pharmacol. 12:1181–1191. 1963.
View Article : Google Scholar : PubMed/NCBI
|