Introduction
Hypoxic-ischemic brain damage (HIBD), a result of
asphyxia in the perinatal period, seriously threatens the health
and life of newborns. HIBD leads to apoptosis and necrosis of nerve
cells, with a high mortality rate and poor outcome. Long-term
nervous system sequelae, including disorders of recognition,
language, sensation, movement, vision and memory function affect
25–30% of patients with HIBD. To date, there is no specific remedy
for HIBD (1–4). Earlier interruption of the
pathological and physiological changes of HIBD is the key point for
decreasing case fatality and disability rate of newborn infants. In
spite of the great progresses that have been made in treatment, the
therapeutic efficacies remain unsatisfactory. Therefore, the
absence of effective therapies for HIBD has provoked an intensive
search for novel treatment strategies. A previous study
demonstrated that mNGF may have a potential therapeutic efficacy
for HIBD; however, little is known about its mechanism (5).
Glial fibrillary acidic protein (GFAP), a skeleton
protein generated from astrocytes, is crucial in the survival of
neurons and formation of synapses following brain injury (6). In this study, using a neonatal mouse
model of HIBD, we investigated the expression of GFAP in neonatal
rats with HIBD when injected with mouse nerve growth factor (mNGF)
and determined whether mNGF promotes the accrementition of
astrocytes. This study provides experimental evidence for the
treatment of HIBD and correlated diseases with mNGF.
Materials and methods
Animals, grouping and intervention
measures
A total of 60 Sprague-Dawley (SD) neonatal rats aged
7 days and weighing 10–12 g (specific pathogen-free) were purchased
from the Center for Laboratory Animals of Academy of Military
Medical Sciences (Beijing, China). The rats were randomly assigned
into 3 groups: control, HIBD and mNGF groups (n=20 per group).
According to the time of sacrifice, each group was divided into 2
subgroups (days 7 and 14). In the mNGF group, neonatal rats with
HIBD were injected intramuscularly into the buttocks with 20
ng/g/day mNGF for 5 days. In the HIBD group, neonatal rats with
HIBD received no treatment. In the control group, neonatal rats
were intramuscularly injected with 10 μl/g/day 0.1 M PBS solution
for 5 days. On days 7 and 14 post-treatment, animals were
sacrificed for further experiments (n=10 per time point).
Main reagents
Rabbit anti-rat GFAP polyclonal antibody was
purchased from Chemicon (Temecula, CA, USA). A
3,3′-diaminobenzidine (DAB) visualization kit, tetrazolium red,
cyanine-3 (cy3) and a streptavidin-biotin complex (SABC) kit were
purchased from Zhongshan Biotech Co. (Beijing, China) mNGF was
purchased from Wuhan Haite Biological Pharmaceutical Co. (Wuhan,
China). Other domestic reagents (analytically pure) were used in
this study.
Establishment of the HIBD rat model
The neonatal HIBD model was established as
previously described (7–9). The 7-day-old SD rats were
anesthetized with anhydrous diethyl ether for 0.5–1 min. Then, the
animals were placed in the left palm under a microscope. The
forefinger and middle finger were used to fix the head of the
animal and the thumb to fix the bilateral forelimbs. A midline
incision was made at the neck and the subcutaneous fat was
separated. The left carotid artery was isolated and ligated with
5-0 double suture. Hemostasis was performed using a gelatin sponge.
Following the procedures, the rats were placed into a transparent
plastic container, placed in a 37°C water bath and ventilated with
a constant flow of a humidified mixture of 8% oxygen and 92%
nitrogen for 2.5 h. Once the HIBD rat model was established, the
rats in the mNGF group were injected intramuscularly with mNGF.
Sample collection
At the different time points, 3 groups of rats were
sacrificed under anesthesia via inhalation of anhydrous diethyl
ether. The heart and aorta were exposed. Blood in the circulation
was removed by perfusion with 20 ml/kg cold normal saline via an
aortic cannula. The brain was collected, the macroscopic features
of which were recorded and was placed in a freezing microtome at
−200°C for 2 h. Frozen tissue sections (8 μm) were placed on slides
previously treated with polylysine, fixed with acetone for 20 min
and stored at −20°C on standby.
Tetrazolium red staining
One rat was sacrificed in each group and the brain
was collected and stained with 2% tetrazolium red. The white region
was defined as the ischemic area.
Detection of GFAP expression in the
hippocampus
The SABC immunohistochemical analysis was performed
to determine the GFAP expression in the hippocampus. After being
placed at room temperature for 20 min, the tissue sections were
treated as follows: treated with dimethyl benzene and a series of
increasing ethanol concentrations for deparaffinization and
dehydration; incubated with 0.6% methanol in
H2O2 for 20 min; washed in phosphate-buffered
saline (PBS) three times (5 min each); blocked with goat serum at
room temperature for 20 min; incubated at 37°C with rabbit anti-rat
GFAP polyclonal antibody (1:100) for 90 min; washed in PBS three
times (10 min each); incubated at 37°C with biotinylated goat
anti-rabbit IgG antibody for 30min; washed in PBS three times (10
min each); treated with SABC at 37°C for 30 min; washed in PBS four
times (5 min each) and visualized with DAB for 5 min. Then the
sections were observed under a light microscope. During lab
processing, if the biotinylated goat anti-rabbit IgG antibody was
replaced by goat anti-rabbit IgG antibody marked with cy3, the
sections were observed under a fluorescent microscope after
incubation for 30 min at 37°C with SABC and three washes with PBS
(10 min each). In the negative control group, the primary antibody
(rabbit anti-rat GFAP polyclonal antibody) and the secondary
antibody (biotinylated goat anti-rabbit IgG antibody) were replaced
with 0.01 mol/l PBS and goat serum, respectively. Positive cells
with GFAP expression in tissues colorated with DAB or cy3 were
bown-yellow or red, respectively. Positive cells were counted at a
magnification of ×200. Three sections were selected from each
sample and 4 visual fields were randomly selected from each
section. Positive cells in 12 visual fields were counted in each
sample and the number of GFAP-expressing cells was determined in
each subgroup.
Statistical analysis
Statistical analysis was performed with SPSS version
12.0 (SPSS Inc., Chicago, IL, USA). All data were shown as mean ±
standard deviation (SD) for normally distributed continuous
variables. One way analysis of variance was performed to determine
comparisons among different groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Behavior of rats with HIBD
After ligation of the left common carotid artery,
the following occurred at different hypoxic durations: hypoxia for
10 min induced dysphoria; hypoxia for 15–20 min caused cyanosis and
deep and rapid breathing; hypoxia for 20–30 min led to unstable
standing and a dragging step of the right hind limb during
creeping; hypoxia for 35–60 min significantly reduced the activity
and hypoxia for more than 1 h resulted in lethargy and irritability
in 90% of rats with HIBD. At 1 h after post-hypoxic re-oxygenation,
rats circled towards the left side. Abnormal behavior was not
observed in the rats receiving hypoxia alone.
Tetrazolium red staining
In the HIBD group and mNGF group, the ischemic and
intact hemispheres were white and red, respectively.
Morphological characteristics of
GFAP-expressing cells
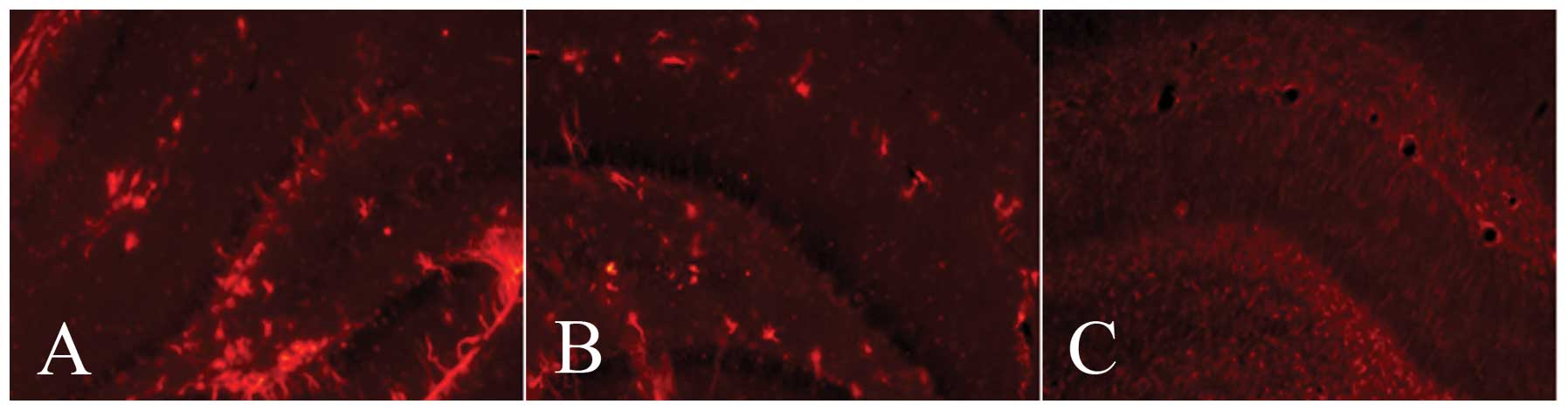
The GFAP protein was mainly found in the cytoplasm,
stained brown-yellow by DAB or red by cy3, respectively. The
expression levels of GFAP in the ischemic side of the hippocampus
in the mNGF and HIBD groups were higher than those in the control
group at days 7 and 14 after the intervention. GFAP-positive cells
in the mNGF and HIBD groups were mainly distributed in the ischemic
side of the hippocampal dentate gyrus (DG) and CA1 region,
respectively (Figs. 1 and 2).
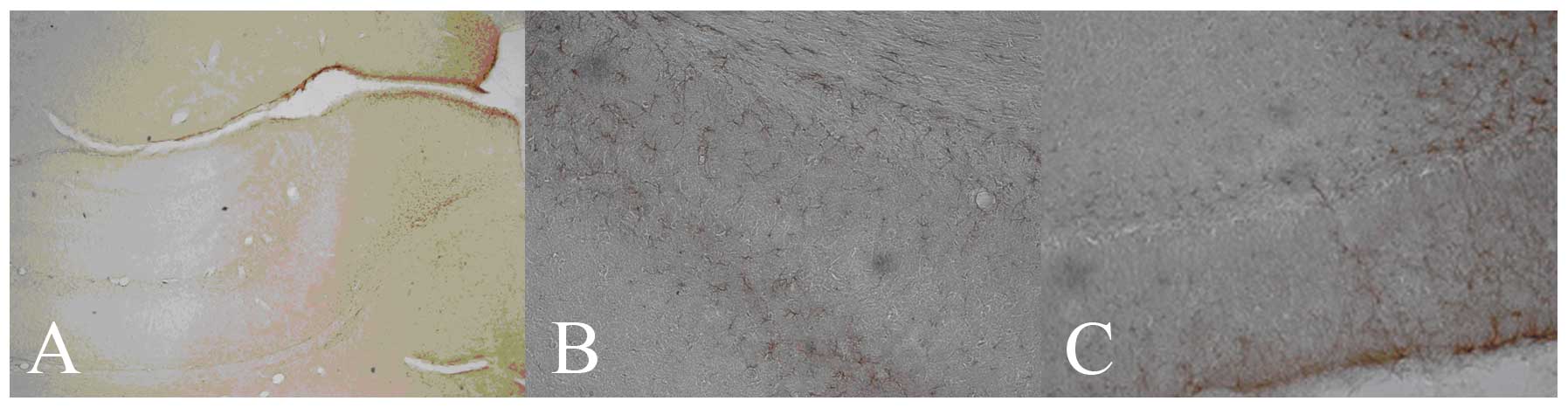 | Figure 1.GFAP protein expression in the
hippocampus of different groups at day 7 (DAB staining; positive
cells, brown-yellow). (A) Control group (hippocampus;
magnification, ×100); (B) HIBD group (CA1; magnification, ×200) and
(C) mNGF group (DG; magnification, ×200). GFAP, glial fibrillary
acidic protein; DAB, 3,3′-diaminobenzidine; HIBD, hypoxic-ischemic
brain damage; mNGF, mouse nerve growth factor; DG, dentate
gyrus. |
Comparison of GFAP-expressing cells in
the hippocampus among different groups
The number of GFAP-expressing cells and the extent
of staining were different in the 3 groups at day 7 (F=58.94,
P<0.01) and day 14 (F=79.57, P<0.01). The number of
GFAP-expressing cells in the mNGF group was higher than that in the
HIBD and control groups. In the HIBD group, the number of
GFAP-expressing cells was significantly increased when compared
with that of the controls. Compared with day 7, the number of
GFAP-expressing cells in the mNGF group significantly increased at
day 14 (P<0.01), but decreased in the HIBD group; however, this
was still lower than that in the mNGF group and higher than that in
the control group (P<0.01; Table
I).
 | Table I.Number of cells positive for GFAP in
the hippocampus of different groups. |
Table I.
Number of cells positive for GFAP in
the hippocampus of different groups.
| Group | Day 7 | Day 14 | P-value |
|---|
| Control | 19.01±3.75 | 20.50±1.12 | 0.0968 |
| HIBD | 43.52±3.05a | 25.53±1.09a | <0.01 |
| mNGF |
57.32±3.98a,b |
62.78±1.22a,b | <0.01 |
| F-value | 58.94 | 79.57 | |
| P-value | <0.01 | <0.01 | |
Discussion
Astrocytes, the most common cell type in the central
nervous system, play an important role in the association of
neurons and cerebral vessels, meninges and ventricles of the brain
(10). GFAP, a specific protein
expressed by mature astrocytes, is an important marker of colloid
cellular proliferation following a central lesion, which promotes
cariocinesis of astrocytes and differentiation from primitive
ancestral cells to mature astrocytes (11).
Newborn HIBD is a disease where hypoxia, ischemia
and a decrease in cerebral blood flow occur as a result of various
factors in the perinatal period. The hippocampus of newborns is
fragile and injures easily under exposure to an hypoxicischemic
condition. The amount of colloid cellular apoptosis is the maximum
total count of nerve cell death (12). In addition, effective treatment for
newborn hypoxic-ischemic brain damage is limited. To date, although
little is known about the mechanism of nerve injury and
regeneration, neurotrophic factors have been considered to be of
potential value for HIBD therapy. In this study, GFAP-expressing
cells increased compared with those of the control group following
cerebral anoxia and ischemia, which indicates that GFAP may be
involved in the recovery of HIBD.
NGF, the first type of typical neurotrophic factor
to be described and studied, exists commonly in tissues of animals.
It promotes activation of impaired nerves and has a close
association with the development, functional maintenance and
reparation of the nervous system (13). NGF, as a nutrition factor of the
central nervous system, facilitates regeneration of impaired
neurons for improvement of the pathological state and has the
ability to protect and repair nerves impaired by hypoxia and
ischemia. By enhancing the dendritic process generation and
expansion and survival of nerve cells, NGF increases the density of
nerve fibers in a dominant target region, particularly in the
hippocampus, basal forebrain and cholinergic neurons of the corpora
striata. NGF not only promotes the growth and maintains the
survival of sympathetic and sensory neurites, but also enhances the
karyokinesis and differentiation of nerve cells following an
increase in nerve cells. In addition, NGF determines the growth
direction of axons (14,15). Studies have suggested that
upregulation of NGF expression has a positive effect on the
protection of impaired neurons. The activities of free radical
scavengers, including hydrogen peroxidase, superoxide dismutase and
glutathion peroxidase, are intensified by NGF, which lessens the
degree of injury of nerve cells; neurons may be protected by the
rivalry of the neurotoxicity of excitatory amino acids and the
regulation of calcium ion levels in neural intracytoplasm by NGF
and the degree of nerve cell injury may be relieved by NGF through
inhibition of programmed cell death and increased cerebral blood
flow (16). Nakatomi et
al(17) suggested that the
infusion of NGF to cerebral ventricles following cerebral ischemia
significantly enhances the activation and proliferation of neural
stem cells (NSCs) and improves the symptoms of neurological
impairment. mNGF is a major molecule of the NGF family. A study has
suggested that mNGF has a high therapeutic efficacy for HIBD
patients; however, the mechanism of action remains unknown
(5).
In this study, the number of GFAP-expressing cells
increased following cerebral anoxia and ischemia; however, the
number of GFAP-expressing cells decreased with an extended
anoxia-ischemia time. mNGF promotes the expression of GFAP in the
hippocampus of HIBD. In addition, there was no trend in the
downregulation of GFAP expression with extending anoxia-ischemic
time. In conclusion, we confirm the potential of mNGF in a
repairing role for HIBD, which indicates that mNGF may be involved
in the recovery of HIBD by regulating the glial cell response and
enhancing the accrementition of astrocytes.
Acknowledgements
This study was supported by grants
from the China Postdoctoral Science Foundation (no.
20070410505).
References
|
1.
|
Barrett RD, Bennet L, Davidson J, Dean JM,
George S, Emerald BS and Gunn AJ: Destruction and reconstruction:
hypoxia and the developing brain. Birth Defects Res C Embryo Today.
81:163–176. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Kelen D and Robertson NJ: Experimental
treatments for hypoxic ischaemic encephalopathy. Early Hum Dev.
86:369–377. 2010. View Article : Google Scholar
|
|
3.
|
Lynch NE, Stevenson NJ, Livingstone V,
Murphy BP, Rennie JM and Boylan GB: The temporal evolution of
electrographic seizure burden in neonatal hypoxic ischemic
encephalopathy. Epilepsia. 53:549–557. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Tagin MA, Woolcott CG, Vincer MJ, Whyte RK
and Stinson DA: Hypothermia for neonatal hypoxic ischemic
encephalopathy: an updated systematic review and meta-analysis.
Arch Pediatr Adolesc Med. 166:558–566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Xue Y, Yin XJ and Yan L: Curative effect
of nerve growth factor on neonatal rat for hypoxic ischaemic
encephalopathy. Chongqing Yi Xue. 35:2030–2031. 2006.(In
Chinese).
|
|
6.
|
Mehler MF, Mabie PC, Zhang D and Kessler
JA: Bone morphogenetic proteins in the nervous system. Trends
Neurosci. 20:309–317. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Yin XJ, Liu DY, Luo FP, Long Q and Feng
ZC: Effect of basic fibroblast growth factor on expression of
protein and mRNA of bone morphogenetic protein 4 in
hypoxic-ischemic brain damage in newborn rats. Zhonghua Er Ke Za
Zhi. 47:856–861. 2009.(In Chinese).
|
|
8.
|
Yin XJ, Ju R and Feng ZC: Changes of
neural stem cells in neonatal rat model of hypoxic-ischemic
encephalopathy. Zhonghua Er Ke Za Zhi. 46:572–575. 2005.(In
Chinese).
|
|
9.
|
Rice JE, Vannucci RC and Brierley JB: The
influence of immaturity on hypoxic-ischemic brain damage in the
rat. Ann Neurol. 9:131–141. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Zhu ZM and Sun QY: Studying current
situation on biological function of star gliocyte. Physiology
Report. 40:14–15. 2005.(In Chinese).
|
|
11.
|
Karwacki Z, Kowiański P, Dziewiatkowski J,
et al: The influence of sevoflurane on the reactivity of astrocytes
in the course of the experimental intracerebral haemorrhage in rat.
J Physiol Pharmacol. 56:455–469. 2005.PubMed/NCBI
|
|
12.
|
Takuma K, Baba A and Matsuda T: Astrocyte
apoptosis: Implications for neuroprotection. Prog Neurobiol.
72:111–127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Zhu Y and Wang T: Biological effect of
nerve growth factor and its research progression in rehabilitation
of brain injuries. Zhongguo Kang Fu Yi Xue Za Zhi. 220:474–476.
2005.(In Chinese).
|
|
14.
|
Salama-Cohen P, Arévalo MA, Meier J,
Grantyn R and Rodríguez-Tébar A: NGF controls dendrite development
in hippocampal neurons by binding to p75NTR and modulating the
cellular targets of Notch. Mol Biol Cell. 16:339–347. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Oyesiku NM, Evans CO, Houslon S, et al:
Regional changes in the expression of neurotrophic factors and
their receptors following acute traumatic brain injury in the adult
rat brain. Brain Res. 833:161–172. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Philips MF, Mattiasson G, Wieloch T, et
al: Neuroprotective and behavioral efficacy of nerve growth
factor-transfected hippocampal progenitor cell transplants after
experimental traumatic brain iniury. J Neurosurg. 94:765–774. 2001.
View Article : Google Scholar
|
|
17.
|
Nakatomi H, Kuriu T, Okabe S, et al:
Regeneration of hippocampal pyramidal neurons after ischemic brain
injury by recruitment of endogenous neural progenitors. Cell.
110:429–441. 2002. View Article : Google Scholar : PubMed/NCBI
|