Introduction
Burn wounds usually result in tissue ischemia and
inflammation, leading to increased numbers of mast cells (1,2).
Mast cells play a significant role not only in the acute
inflammatory phase but also in the late stage of wound healing
following burn injury (3). Chymase
is an enzyme that is mainly located in mast cell granules. The
enzyme has previously been demonstrated to be activated in tissue
fibrosis, including pulmonary fibrosis (4,5) and
cardiac fibrosis (6).
Significantly, the involvement of chymase in tissue matrix
remodeling has been suggested by its ability to activate
procollagenase (7) and degrade the
extracellular matrix (ECM) (8).
One of the major functions of chymase is to convert angiotensin I
(Ang I) to angiotensin II (Ang II). Moreover, chymase contributes
to the release of TGF-β1 from its precursor, human fibroblast
latent TGF-β1-binding protein (9).
Certain studies also showed that chymase is able to convert
inactive interleukin-1β (IL-1β), a proinflammatory cytokine, to its
active form (10,11).
Ang II is the major effector peptide in the
renin-angiotensin system (RAS). Besides being a physiological
mediator restoring circulatory integrity (12,13),
Ang II has been recognized as a growth factor that regulates cell
growth, angiogenesis, inflammation, tissue repair and remodeling
(2). Ang II in the human heart is
generated via two pathways, the angiotensin converting enzyme (ACE)
pathway and the chymase pathway. The chymase pathway accounts for
∼80% of Ang II formation in the heart (14). Similar pathways also exist in
hamsters (15,16).
A previous study showed that mast cell chymase
played a key role in the normal wound healing process by measuring
the size of the burn wounds, the density of the capillaries,
collagen accumulation, mast cell number and chymase activity in the
mouse dorsal skin prior to and 1, 3, 7 and 14 days subsequent to
burning (17). However, the role
of ACE-independent production of Ang II by the chymase enzyme in
burn injuries remains unclear.
In the present study, in order to investigate the
role of mast cell chymase in burn wounds, the mast cell membrane
stabilizer ketotifen was orally administered to hamsters with
partial-thickness burn injuries. The levels of Ang II in the mast
cells from the burn tissues were analyzed. Meanwhile, TGF-β1 and
IL-1β levels were also examined. Moreover, as collagen is the main
component of the ECM, the expression of this protein was also
investigated to assess the healing of burned tissues.
Materials and methods
Animal experiment
In total, 28 eight-week-old hamsters were purchased
from the Urumuqi Center for Disease Control and Prevention in the
Xinjiang Uyghur Autonomous Region of China. Animals were housed in
individual stainless steel cages in a temperature-controlled
environment (25–30°C) with 12 h light-dark cycles. Food pellets and
water were available ad libitum. Animals were acclimatized
for a minimum of 2 weeks prior to thermal injury. All animal care
and experimental protocols were approved by the Animal Ethics
Committee of the First Affiliated Hospital of Xinjiang Medical
University and were in accordance with institution guidelines.
Burn wounds in hamsters
The 28 eight-week-old hamsters were randomly divided
into two groups. Half of the hamsters (n=14) formed the control
group and the other half (n=14) formed the ketotifen group. The
animals were weighed and then anesthetized with ketamine (0.7 g/kg)
i.p. Diazepam and atropine were added to maintain adequate
anesthesia. Once anesthetized, the dorsal torso of each animal was
shaved and a commercial depilating agent was applied to fully
remove the hair in an ∼3 cm diameter area. The control and
ketotifen groups were orally administered physiological saline (1
ml) and ketotifen (4 mg/kg), respectively, once daily for two days
prior to burning and for two days subsequent to burning.
A scald template was fashioned from the caudal end
of a plastic 50 ml syringe without the plunger. The caudal end was
placed on the dorsal torso skin of hamster with a gentle pressure
that just kept the water in the syringe. Then 20 ml of 75°C water
was put into the 50 ml syringe without the plunger to create a
scald wound of ∼3 cm in diameter with a contact time of 12 sec. The
burn area covered ∼5% of the total body surface. A deep
partial-thickness burn injury was made on the back skin in this
pattern with high reproducibility. Every hamster from the two
groups survived the process. The hamsters were sacrificed and the
back skin was harvested on day 3 subsequent to burning.
Measurement of Ang II
The quantitative measurement of Ang II in burn
tissues was measured by a radio-immunity kit (Beijing North
Institute of Biological Technology, Beijing, China). A total of 0.1
g burn tissues was minced and homogenized subsequent to being
washed in cold saline. The burn tissue was transferred into a tube
containing 1 ml NaCl (0.9%) and centrifuged at 12,000 × g for 15
min. The supernatant was used to measure Ang II levels via a
radioimmunoassay (RIA). The RIA for Ang II was performed using
125I-angiotensin II and rabbit anti-ANG II antibody (Beijing North
Institute of Biological Technology) in accordance with the
instructions of the radio-immunity kit. The ratio of B/Bo (B,
experimental condition; Bo, control condition) was corrected for
non-specific binding, presented as a percentage of maximal binding
and read against a standard curve (log-logit transformation).
Flow cytometry
A section of the burn tissue was cut into 1x1-mm
samples subsequent to being rinsed and was then homogenized with
cold Hanks’ balanced salt solution. The mixed tissue fluid was
placed on a filter of 300 mesh and then the filtered liquid was
centrifuged at 2,000 × g for 5 min. Cells were collected and
suspended in Hanks’ balanced salt solution, counted with a
hemocytometer and adjusted to a concentration of 106
cells/ml. A quantitative assessment of apoptosis was performed
using the Annexin V-FITC apoptosis detection kit as described by
the manufacturer’s instructions (Kaiji Bio Co., Nanjing, China;
Cat. #, KGA108). Briefly, cells were treated with 5 μl
Annexin V-FITC and 5 μl propidium iodide (PI) and placed in
the dark at room temperature for 15 min prior to being run through
the flow cytometer. Data were acquired on a Beckman Coulter XL
(Beckman Coulter, Fullerton, CA, USA).
Western blotting analysis
Total protein from the burn tissues of the hamsters
was extracted with protein lysate buffer containing PMSF (1 mmol/l)
following homogenization. Tissue lysates were centrifuged at 12,000
× g for 5 min at 4°C. Protein concentrations in the supernatant
from each group were determined by using a BCA protein quantitative
analysis kit (BMD Biomed Tech, Beijing, China). An equal amount (4
μl) of protein from each supernatant was subjected to 8–10%
gradient sodium dodecylsulfate-polyacrylamide gel electrophoresis.
Following electrophoresis, proteins were transferred onto a
polyvinylidene fluoride (PVDF) membrane (Invitrogen, Carlsbad, CA,
USA). The PVDF membrane was then incubated for 1 h at 4°C with the
primary antibody following block solution treatment. The primary
antibodies used in this study were mouse anti-TGF-β1, mouse
anti-collagens I and III, mouse anti-IL-1β and mouse anti-β-actin
(1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Following
incubation with the primary antibody, the membranes were probed
with the appropriate alkaline phosphatase-conjugated secondary
antibody (anti-mouse or anti-rabbit, 1:20,000; Invitrogen) and then
incubated with a solution of BCIP/NBT substrate for alkaline
phosphatase until the appearance of the purple band. An efficient
transfer was confirmed by staining the membrane with Ponceau S. The
relative intensity of the immunoreactive bands was quantified using
a computer-assisted densitometry program (BioRad Tech, Hercules,
CA, USA).
Statistical analysis
The results are shown as mean ± standard deviation
(mean ± SD). An analysis of variance and Dunnett’s t-test were
performed to evaluate the differences between groups, using SPSS
10.0 software (Madison, WI, USA). Statistical differences were
considered significant if P<0.05.
Results
Ang II levels were significantly
decreased in the ketotifen group
There was a significant difference in Ang II levels
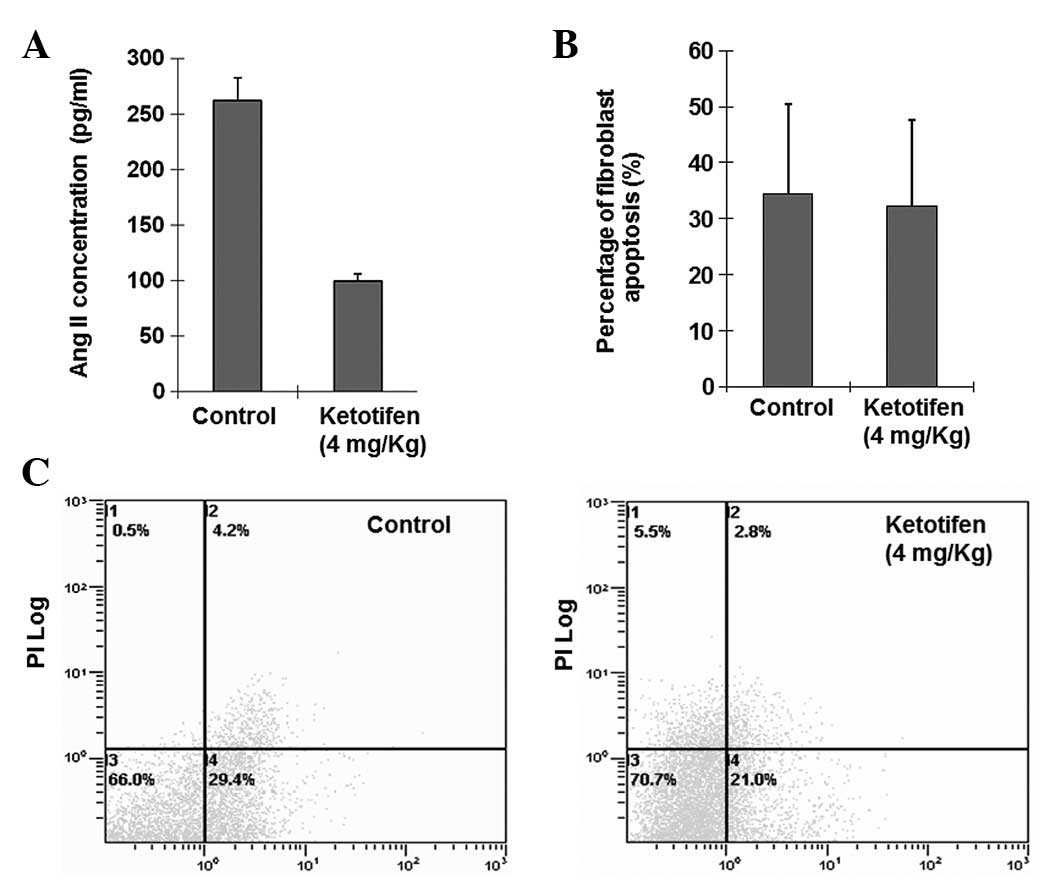
in burn tissues between the two groups (P<0.05). Fig. 1A shows that Ang II levels in burn
tissues were significantly decreased in the ketotifen group
(100.1142±6.0702 pg/ml) when compared with the control group
(261.8450±20.8356 pg/ml). Therefore, the results suggest that
chymase converted Ang I to Ang II in burn wound tissues.
Fibroblast apoptosis rates were similar
between the ketotifen group and the control group
Alterations of the plasma membrane, with
translocation of phosphatidylserine from the inner side of the
plasma membrane to the external surface, are the hallmark of
apoptosis. The Annexin V-FITC/PI-stained fluorescence-activated
cell sorter (FACS) analysis of fibroblast apoptosis in the burn
tissues indicated that the percentage of apoptotic cells
(34.4±16.05 versus 32.32±0.1534%) were similar (P>0.05; Fig. 1B and C) in the two groups.
Ketotifen significantly suppresses
collagen accumulation in the burn wound
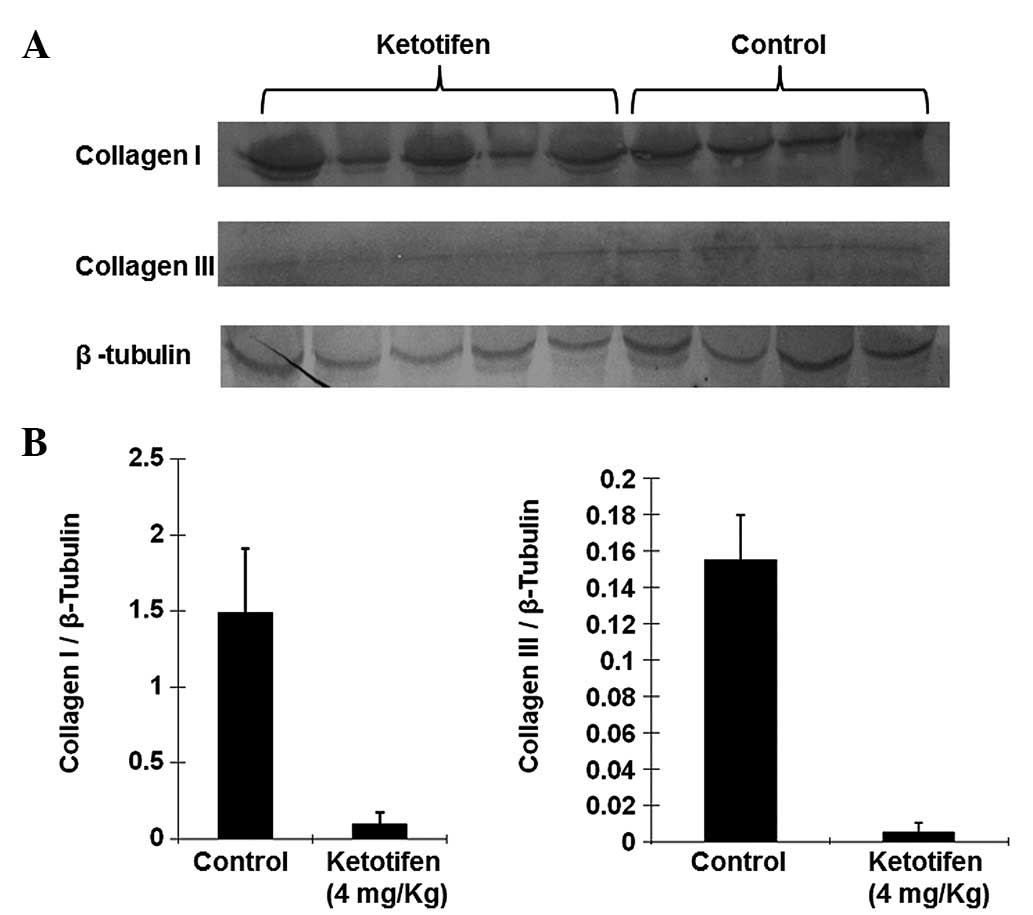
Collagen is the main component of the ECM. Levels of
collagen I and III in the burn tissues of the two groups were
markedly different. Fig. 2 shows
that the expression levels of collagen I and III relative to those
of b-tubulin in burn tissues from the ketotifen group (collagen I,
0.1013±0.0755; collagen III, 0.0054±0.0051) were significantly
lower than those of the control group (collagen I, 1.4903±0.4230;
collagen III, 0.1548±0.0248; P<0.01). This result suggested that
ketotifen was able to significantly suppress collagen accumulation
in the burn wound.
Ketotifen inhibits the chymase-induced
generation of mature TGF-β1 in burn wounds
TGF-β1 is thought to be one of the major cytokines
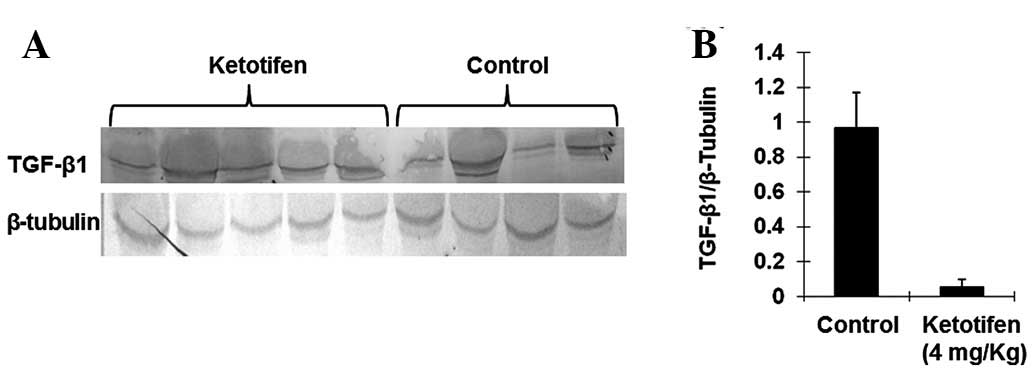
involved in organ fibrosis. The level of TGF-β1 was therefore also
investigated by western blotting. As shown in Fig. 3, there was a significant difference
in the TGF-β1 expression levels between the ketotifen group
(0.0518±0.0449) and the control group (0.9645±0.2046). The
expression of TGF-β1 was significantly decreased in the ketotifen
group (P<0.01). This result suggested that ketotifen inhibited
the chymase-induced generation of mature TGF-β1 in burn wounds.
Thus, chymase may contribute to TGF-β1 activation.
Ketotifen treatment markedly decreased
the expression of IL-1β
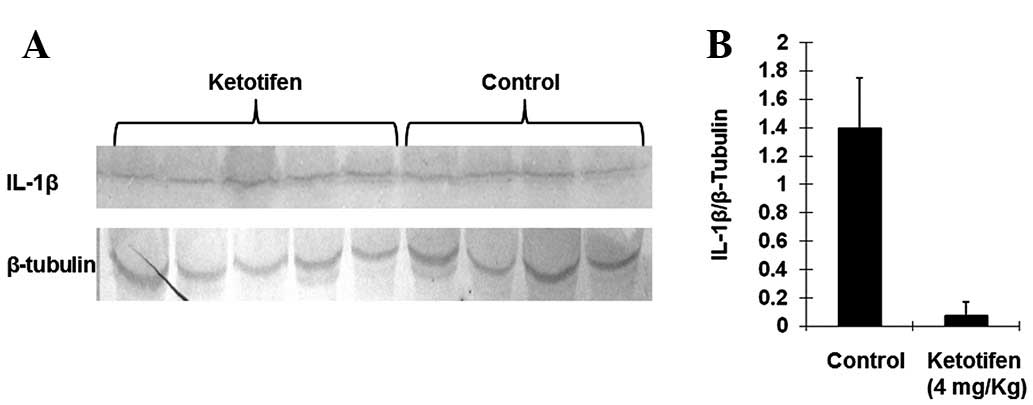
IL-1β is a significant proinflammatory factor. Mast
cell chymase is able to induce specific conversion of the IL-1β
precursor to an active IL-1 species in humans (18). As shown in Fig. 4, ketotifen treatment markedly
decreased the expression of IL-1β (0.0740±0.0945) as compared with
the control group (1.3913±0.3853), which indicated that mast cell
chymase may be involved in the activation of IL-1β in burn tissues
(P<0.05).
Discussion
It has previously been shown that mast cell chymase
is the Ang II forming enzyme in the major non-ACE pathway in the
heart (19). Such pathways were
later identified not only in the human heart, but also in the
thoracic artery, saphenous vein (20), radial artery (21), gastroepiploic artery (22), bleomycin-induced pulmonary fibrosis
(4,5,23)
and cardiac fibrosis (6).
In the present study, we used ketotifen, a mast cell
membrane stabilizer, to investigate the production of Ang II in
burn tissues in hamsters. The results showed that the production of
Ang II in the ketotifen group was significantly decreased. This
suggests that mast cell chymase has the same effects on the
conversion of Ang I to Ang II in burn tissues as it does in other
tissues or organs, including the heart (6).
Ang II is the major effector peptide in the RAS.
Besides being a physiological mediator restoring circulatory
integrity (12,13), Ang II is now recognized as a growth
factor that regulates cell growth, angiogenesis, inflammation,
tissue repair and remodeling (2).
Ang II contributes greatly to tissue fibrosis, including hepatic
(24), renal (25) and cardiac fibrosis (26). Ang II combines with Ang II type-1
receptor to increase the expression of TGF-β1 (27,28).
In addition, chymase also contributes to the release of TGF-β1 from
its precursor (9). TGF-β1 has been
identified as the most significant profibrotic cytokine (29), it induces an increase in collagen
production and secretion and enhances the abundance of mRNA levels
for collagen types I and III (30). Ang II also activates collagen I
gene expression, but would require activation of the MAPK/ERK and
TGF-β signaling pathways (31).
The present study showed that ketotifen treatment
significantly reduced the production of TGF-β1 and collagens I and
III. These results indicate that greatly decreased Ang II levels
cannot induce excessive expression of TGF-β or collagen I and III
genes due to the deficiency in activated mast cell chymase.
Ang II is able to stimulate not only cardiac
fibroblast proliferation (32) but
also skin fibroblast proliferation (33). Certain studies have indicated that
mast cell chymase is able to induce smooth muscle cell and
endothelial cell apoptosis (34–36).
However, no studies have reported whether or not Ang II and chymase
are able to induce fibroblast apoptosis. The present study showed
that there was no significant difference in fibro-blast apoptosis
between the ketotifen group and the control group. This result
indicates that mast cell chymase may have no effect on fibroblast
apoptosis.
According to previous studies Ang II had no effect
on the the activation of IL-1β. However, human mast cell chymase is
able to induce the accumulation of neutrophils, eosinophils and
other inflammatory cells in vivo(37,38),
as well as the rapid and specific conversion of precursor IL-1β to
an active IL-1 species (39). In
the present study, in comparison to the control group, IL-1β was
greatly reduced in the ketotifen group, suggesting that chymase may
be involved in the activation of IL-1β in burn tissues.
Wound healing subsequent to burn injuries is an
inevitable result of tissue repair involving the interaction of
fibroblasts, the ECM and cytokines. Increased vascular permeability
and inflammation following burn injury may cause an increase in
mast cells and stimulate the release of mast cell chymase from
secretory granules (1,40). Results from the present study
showed that in burn tissues, mast cell chymase contributed to the
conversion of Ang II, the activation of TGF-β1 and the production
of collagens I and III. Mast cell chymase is able to induce skin
fibroblast proliferation (33,41),
but the present study showed that mast cell chymase had no effect
on fibroblast apotosis. The study indicated that mast cell chymase
is conducive to wound healing.
In conclusion, in a hamster model of burn injuries,
ketotifen, a mast cell membrane stabilizer, decreased the local
concentration of Ang II, the expression levels of TGF-β1 and
collagens I and III and the concentration of inflammatory marker
IL-1β. These results suggest that mast cell chymase contributes to
burn wound healing. Thus, chymase activity provides a promising
future therapeutic target to accelerate wound healing.
Acknowledgements
The authors thank Dr Tao Liu and Dr
Chuanshan Zhang at the laboratory of The First Affiliated Hospital
of Xinjiang Medical University for their technical assistance.
References
|
1.
|
Räntfors J and Cassuto J: Role of
histamine receptors in the regulation of edema and circulation
postburn. Burns. 29:769–777. 2003.PubMed/NCBI
|
|
2.
|
Chen K, Chen J, Li D, Zhang X and Mehta
JL: Angiotensin II regulation of collagen type I expression in
cardiac fibroblasts: modulation by PPAR-gamma ligand pioglitazone.
Hypertension. 44:655–661. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Persinger MA, Lepage P, Simard JP and
Parker GH: Mast cell numbers in incisional wounds in rat skin as a
function of distance, time and treatment. Br J Dermatol.
108:179–187. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Lang YD, Chang SF, Wang LF and Chen CM:
Chymase mediates paraquat-induced collagen production in human lung
fibroblasts. Toxicol Lett. 193:19–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Tomimori Y, Muto T, Saito K, et al:
Involvement of mast cell chymase in bleomycin-induced pulmonary
fibrosis in mice. Eur J Pharmacol. 478:179–185. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Akgul A: Can cardiac fibrosis be
prevented? Mast cell inhibition versus anti-chymase activity. Eur J
Cardiothorac Surg. 35:553–554. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Saarinen J, Kalkkinen N, Welgus HG and
Kovanen PT: Activation of human interstitial procollagenase through
direct cleavage of the Leu83-Thr84 bond by mast cell chymase. J
Biol Chem. 269:18134–18140. 1994.
|
|
8.
|
Vartio T, Seppä H and Vaheri A:
Susceptibility of soluble and matrix fibronectins to degradation by
tissue proteinases, mast cell chymase and cathepsin G. J Biol Chem.
256:471–477. 1981.PubMed/NCBI
|
|
9.
|
Takai S, Jin D, Sakaguchi M, et al: A
novel chymase inhibitor,
4-[1-([bis-(4-methyl-phenyl)-methyl]-carbamoyl)3-
(2-ethoxy-benzyl)-4-oxo-azetidine-2-yloxy]-benzoic acid (BCEAB),
suppressed cardiac fibrosis in cardiomyopathic hamsters. J
Pharmacol Exp Ther. 305:17–23. 2003.
|
|
10.
|
Doggrell SA and Wanstall JC: Vascular
chymase: pathophysiological role and therapeutic potential of
inhibition. Cardiovasc Res. 61:653–662. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Takai S and Miyazaki M: A novel
therapeutic strategy against vascular disorders with chymase
inhibitor. Curr Vasc Pharmacol. 1:217–224. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Le Noble FA, Hekking JW, Van Straaten HW,
Slaaf DW and Struyker Boudier HA: Angiotensin II stimulates
angiogenesis in the chorio-allantoic membrane of the chick embryo.
Eur J Pharmacol. 195:305–306. 1991.PubMed/NCBI
|
|
13.
|
Suzuki Y, Ruiz-Ortega M, Lorenzo O,
Ruperez M, Esteban V and Egido J: Inflammation and angiotensin II.
Int J Biochem Cell Biol. 35:881–900. 2003. View Article : Google Scholar
|
|
14.
|
Urata H and Ganten D: Cardiac angiotensin
II formation: the angiotensin-I converting enzyme and human
chymase. Eur Heart J. 14(Suppl 1): 177–182. 1993.PubMed/NCBI
|
|
15.
|
Shiota N, Fukamizu A, Takai S, Okunishi H,
Murakami K and Miyazaki M: Activation of angiotensin II-forming
chymase in the cardiomyopathic hamster heart. J Hypertens.
15:431–440. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Takai S, Shiota N, Yamamoto D, Okunishi H
and Miyazaki M: Purification and characterization of angiotensin
II-generating chymase from hamster cheek pouch. Life Sci.
58:591–597. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Nishikori Y, Kakizoe E, Kobayashi Y,
Shimoura K, Okunishi H and Dekio S: Skin mast cell promotion of
matrix remodeling in burn wound healing in mice: relevance of
chymase. Arch Dermatol Res. 290:553–560. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Wu Q, Kuo HC and Deng GG: Serine proteases
and cardiac function. Biochim Biophys Acta. 1751:82–94. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Urata H, Kinoshita A, Misono KS, Bumpus FM
and Husain A: Identification of a highly specific chymase as the
major angiotensin II-forming enzyme in the human heart. J Biol
Chem. 265:22348–22357. 1990.PubMed/NCBI
|
|
20.
|
Chester AH and Borland JA:
Chymase-dependent angiotensin II formation in human blood vessels.
J Hum Hypertens. 14:373–376. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Okunishi H, Miyazaki M, Okamura T and Toda
N: Different distribution of two types of angiotensin II-generating
enzymes in the aortic wall. Biochem Biophys Res Commun.
149:1186–1192. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Voors AA, Pinto YM, Buikema H, et al: Dual
pathway for angiotensin II formation in human internal mammary
arteries. Br J Pharmacol. 125:1028–1032. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Sakaguchi M, Takai S, Jin D, et al: A
specific chymase inhibitor, NK3201, suppresses bleomycin-induced
pulmonary fibrosis in hamsters. Eur J Pharmacol. 493:173–176. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Okamoto K, Tajima H, Ohta T, et al:
Angiotensin II induces tumor progression and fibrosis in
intrahepatic cholangiocarcinoma through an interaction with hepatic
stellate cells. Int J Oncol. 37:1251–1259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Schulman IH, Zhou MS, Treuer AV,
Chadipiralla K, Hare JM and Raij L: Altered renal expression of
angiotensin II receptors, renin receptor, and ACE-2 precede the
development of renal fibrosis in aging rats. Am J Nephrol.
32:249–261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Schellings MW, Vanhoutte D, Van Almen GC,
et al: Syndecan-1 amplifies angiotensin II-induced cardiac
fibrosis. Hypertension. 55:249–256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Sun Y, Zhang J, Zhang JQ and Ramires FJ:
Local angiotensin II and transforming growth factor-beta1 in renal
fibrosis of rats. Hypertension. 35:1078–1084. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Yoshiji H, Kuriyama S, Yoshii J, et al:
Angiotensin-II type 1 receptor interaction is a major regulator for
liver fibrosis development in rats. Hepatology. 34:745–750. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Pulichino AM, Wang IM, Caron A, et al:
Identification of transforming growth factor beta1-driven genetic
programs of acute lung fibrosis. Am J Respir Cell Mol Biol.
39:324–336. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Lijnen PJ, Petrov VV and Fagard RH:
Induction of cardiac fibrosis by transforming growth
factor-beta(1). Mol Genet Metab. 71:418–435. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Tharaux PL, Chatziantoniou C, Fakhouri F
and Dussaule JC: Angiotensin II activates collagen I gene through a
mechanism involving the MAP/ER kinase pathway. Hypertension.
36:330–336. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Weber KT and Brilla CG: Pathological
hypertrophy and cardiac interstitium. Fibrosis and
renin-angiotensin-aldosterone system. Circulation. 83:1849–1865.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Kawaguchi Y, Takagi K, Hara M, et al:
Angiotensin II in the lesional skin of systemic sclerosis patients
contributes to tissue fibrosis via angiotensin II type 1 receptors.
Arthritis Rheum. 50:216–226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Heikkilä HM, Lätti S, Leskinen MJ, Hakala
JK, Kovanen PT and Lindstedt KA: Activated mast cells induce
endothelial cell apoptosis by a combined action of chymase and
tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol.
28:309–314. 2008.PubMed/NCBI
|
|
35.
|
Leskinen MJ, Heikkilä HM, Speer MY, Hakala
JK, Laine M, Kovanen PT and Lindstedt KA: Mast cell chymase induces
smooth muscle cell apoptosis by disrupting NF-kappaB-mediated
survival signaling. Exp Cell Res. 312:1289–1298. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Sun J, Zhang J, Lindholt JS, et al:
Critical role of mast cell chymase in mouse abdominal aortic
aneurysm formation. Circulation. 120:973–982. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
He S and Walls AF: Human mast cell chymase
induces the accumulation of neutrophils, eosinophils and other
inflammatory cells in vivo. Br J Pharmacol. 125:1491–1500. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Ishida K, Takai S, Murano M, et al: Role
of chymase-dependent matrix metalloproteinase-9 activation in mice
with dextran sodium sulfate-induced colitis. J Pharmacol Exp Ther.
324:422–426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Mizutani H, Schechter N, Lazarus G, Black
RA and Kupper TS: Rapid and specific conversion of precursor
interleukin 1 beta (IL-1 beta) to an active IL-1 species by human
mast cell chymase. J Exp Med. 174:821–825. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Santos FX, Arroyo C, Garcia I, et al: Role
of mast cells in the pathogenesis of postburn inflammatory
response: reactive oxygen species as mast cell stimulators. Burns.
26:145–147. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Dong X, Chen J, Zhang Y and Cen Y: Mast
cell chymase promotes cell proliferation and expression of certain
cytokines in a dose-dependent manner. Mol Med Rep. 5:1487–1490.
2012.PubMed/NCBI
|