Introduction
The contractibility of blood vessels depends on
their normal structure and the availability of calcium ions; it
changes under the influence of a number of contracting [e.g.,
angiotensin II (ANG II) and endothelin-1] and relaxing [e.g.,
nitric oxide (NO) and prostacyclin] factors, which control the
activities of various pathways of intracellular and intercellular
signaling (1–3). The endothelium lines the inside of
the vessel and is not only an integral part of the vessel
structure, but also actively participates in its normal
functioning. Endothelial cells produce substances which regulate
contraction-relaxation activity, but the action of the internal
layer of the vessel is subject to the influence of various factors,
acting via receptors (1,4). Smooth muscle contraction, stimulated
by a number of physiological factors, may take place with the
participation of calcium ions released from the intracellular
reserve, which flow from the extracellular space through the
channels in the cellular membrane. However, an excessive increase
in calcium ion concentration may promote cell death as a result of
apoptosis, ischemia/reperfusion (I/R) and excitotoxicity (5–7). The
smooth muscle is also important in the pathogenesis of vascular
diseases, including sclerosis, arterial hypertension and restenosis
(8,9).
I/R and oxidative stress are pathological phenomena,
which may change the reactivity of the vessels and modulate the
effect of substances which control the vascular smooth muscle tone
(10,11). Tissue damage caused by hypoxia is
mediated by mechanisms associated with oxygen (reactive oxygen
species, ROS) and nitrogen (reactive nitrogen species, RNS)
released in large amounts after reperfusion, which promote
inflammatory processes, cell death and organ failure. Such
phenomena are responsible for the failure of bypass graft surgery
and organ transplantation surgery, as well as complications of
myocardial, cerebral and renal hypoxia syndromes (12–15).
The purpose of the present study was to establish
the modulating effect of I/R on contraction triggered by ANG II (an
agonist of the metabotropic AT1 receptor) and Bay K8644 (an agonist
of calcium channels located in the cellular membrane) as well as to
investigate the role of the signaling pathway associated with NO
and cGMP in these reactions.
Material and methods
Preparation of arteries
In the experiment, Guiding Principles for the Care
and Use of Animals in the Field of Physiological Sciences as well
as specific national laws were followed. The Ethical Committee for
the Affairs of Experiments on Animals in Bydgoszcz approved the
experiments undertaken (No. 1/2008-4). All reagents were purchased
from Sigma-Aldrich (Poland, Poznań). Studies were performed on
isolated and perfused Wistar rats' tail arteries. Animals, with
body weights ranging from 250 to 350 g, were anesthetized with
urethane administered intraperitoneally at a dose of 120 mg/kg of
body weight. In order to establish the effect of I/R on the
reactivity of the vascular smooth muscle triggered by ANG II (30
nM/l) and Bay K8644 (30 μM/l), a clamp was placed on the
proximal segment of the prepared artery for 30 or 60 min and the
artery was then removed. Then, a cannula was inserted into the
proximal segment of the detached fragment of the rat's caudal
artery measuring 2.5–3 cm in length, which was subsequently
connected to the perfusion system and the equipment to allow
constant measurement and recording of the perfusion pressure. The
distal end of the prepared artery was loaded with a weight of 500
mg, and the preparation was placed in a vertical position in a
thermostatic dish intended for isolated organs with a volume of 20
ml, oxygenated with saline at a temperature of 37°C. The flow of
the perfusion fluid was gradually increased until it reached 1
ml/min. During the next stage, the contraction of the arteries was
evaluated after 30, 60 and 120 min of reperfusion.
Assessment of role of calcium ion
pools
In order to evaluate the involvement of
intracellular and extracellular calcium ion pools in reactions
triggered by the studied agonists under control conditions,
following I/R and in the presence of sodium nitroprusside (SNP, 100
μM/l) as a donor of NO, 8Br-cGMP (100
μM/l), an endothelial NO synthase (NOSe) inhibitor
(L-NG-nitroarginine methyl ester; L-NAME, 300 μM/l) or ODQ
[inhibitor of soluble guanylyl cyclase (GC), 100 μM/l], the
experiments were conducted using two types of Krebs fluid: i) Fluid
without Ca2+-EGTA [Krebs (no calcium); calcium-free
physiological salt solution (FPSS)], with the following
composition: NaCl (71.8 mM/l), KCl (4.7 mM/l), NaHCO3
(28.4 mM/l), MgSO4 (2.4 mM/l),
KH2PO4 (1.2 mM/l) and glucose (11.1 mM/l)
with the addition of EGTA (30 μM/l); ii) Fluid with
Ca2+-EGTA [Krebs (normal); physiological salt solution
(PSS)] with the the same composition as FPSS with CaCl2
(1.7 mM/l), after emptying the intracellular pool of calcium
ions.
Evidence for the vessel contraction in the conducted
experiments included an increase in pressure of the perfusate in an
experimental system, at a preset flow of the perfusion fluid (∼1
ml/min).
Statistical analysis
Results are presented as average values and standard
deviation. Statistical differences were evaluated using the
Student's t-test. P<0.05 was considered to indicate a
statistically significant result. Calculations were conducted with
Statistica 6.0PL software.
Results
Effects of ANG II and Bay K8644
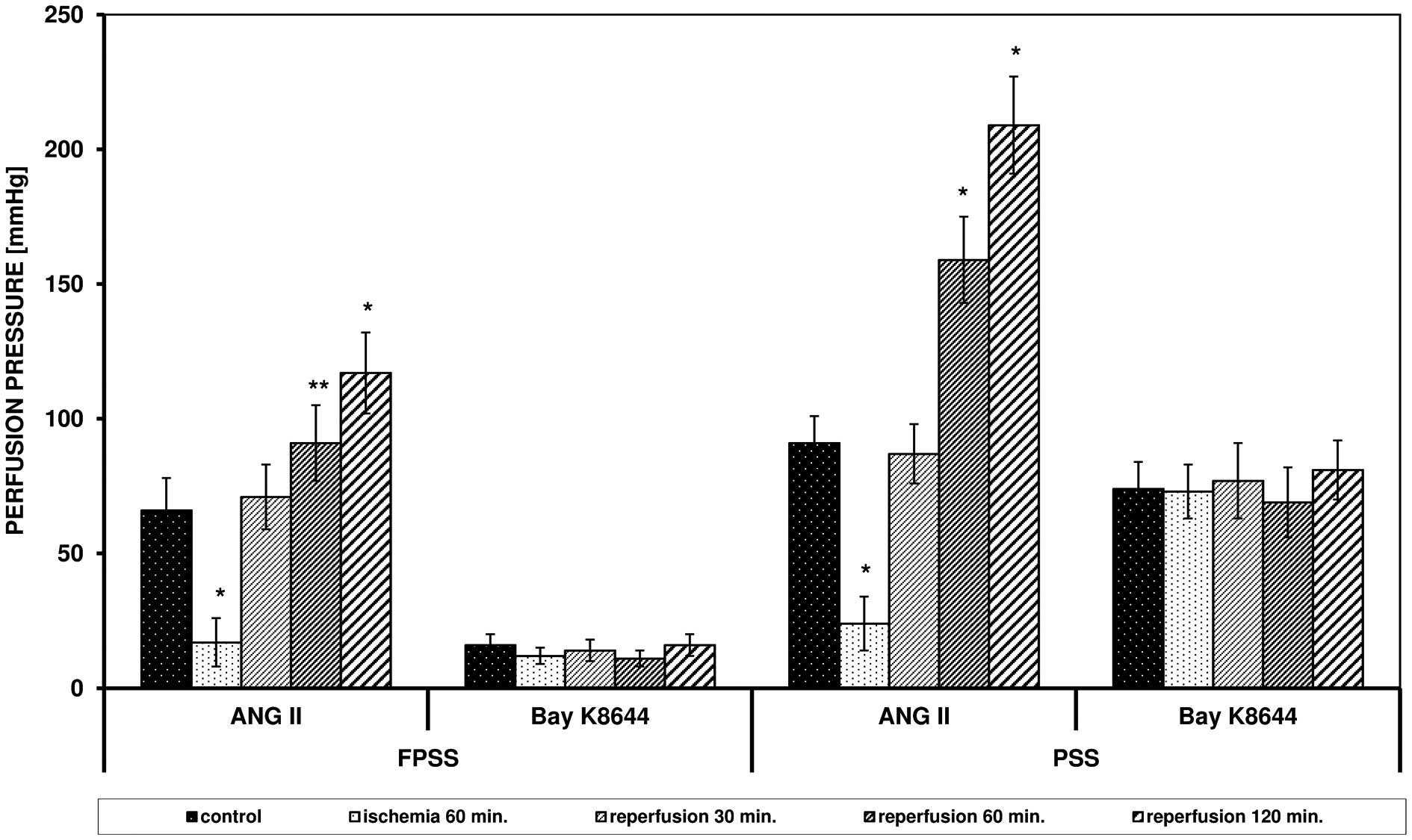
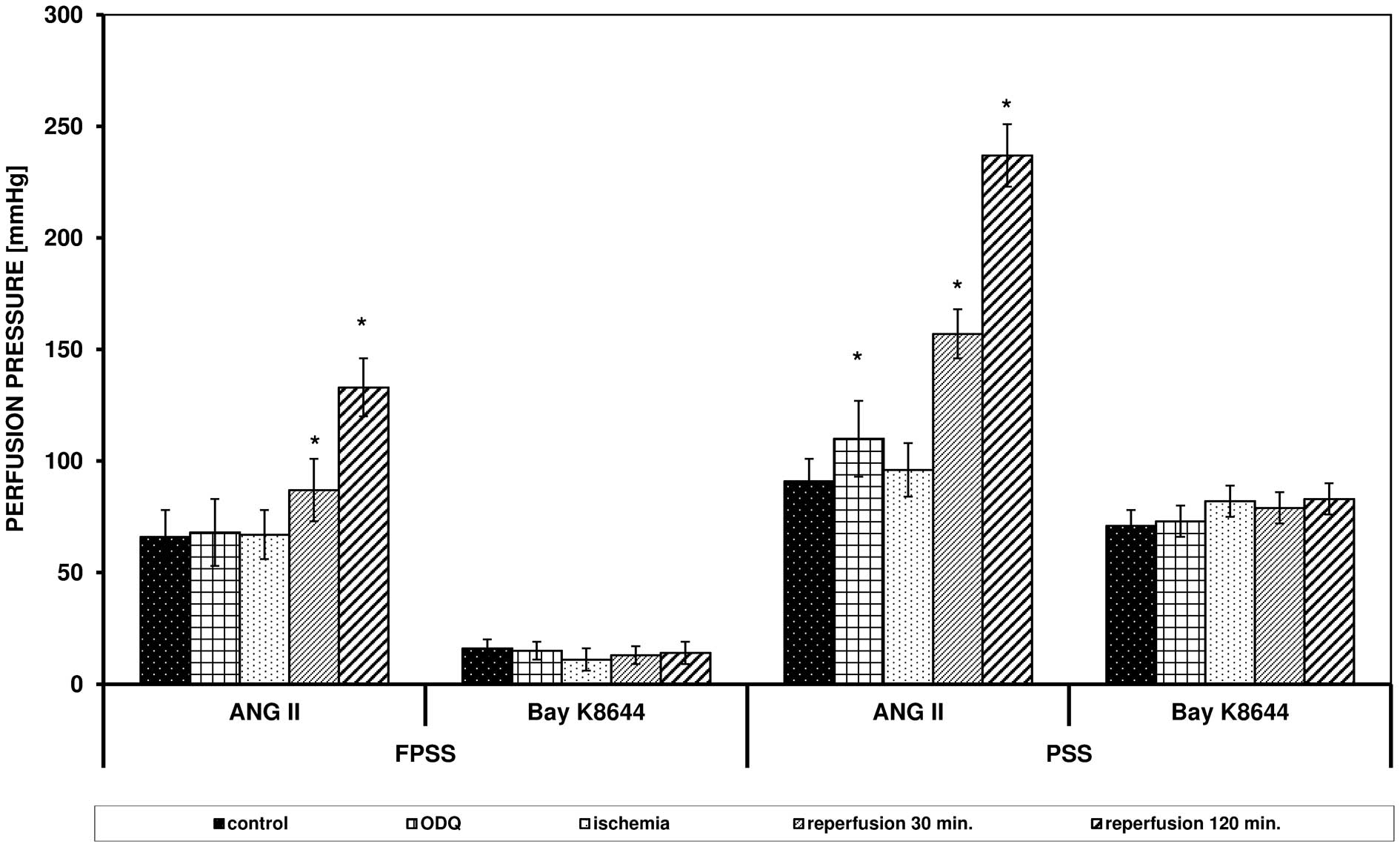
ANG II triggered an increase in perfusion pressure
in FPSS and PSS, but the values were higher in PSS (Fig. 1). After 60 min of ischemia, a
reduced response of the arteries to ANG II was observed, but after
60 and 120 min of reperfusion, the maximum effects were
significantly elevated in FPSS and PSS (in PSS after 60 and 120 min
P<0.0001 vs. control; in FPSS after 60 min of reperfusion
0.05>P>0.0001). Bay K8644 triggered contraction only with the
use of an extracellular pool of calcium ions, which, in contrast to
the experiments with ANG II, was not modulated by I/R. The obtained
values of reperfusion pressure triggered by ANG II and Bay K8644
following I/R in FPSS and PSS are presented in Fig. 1.
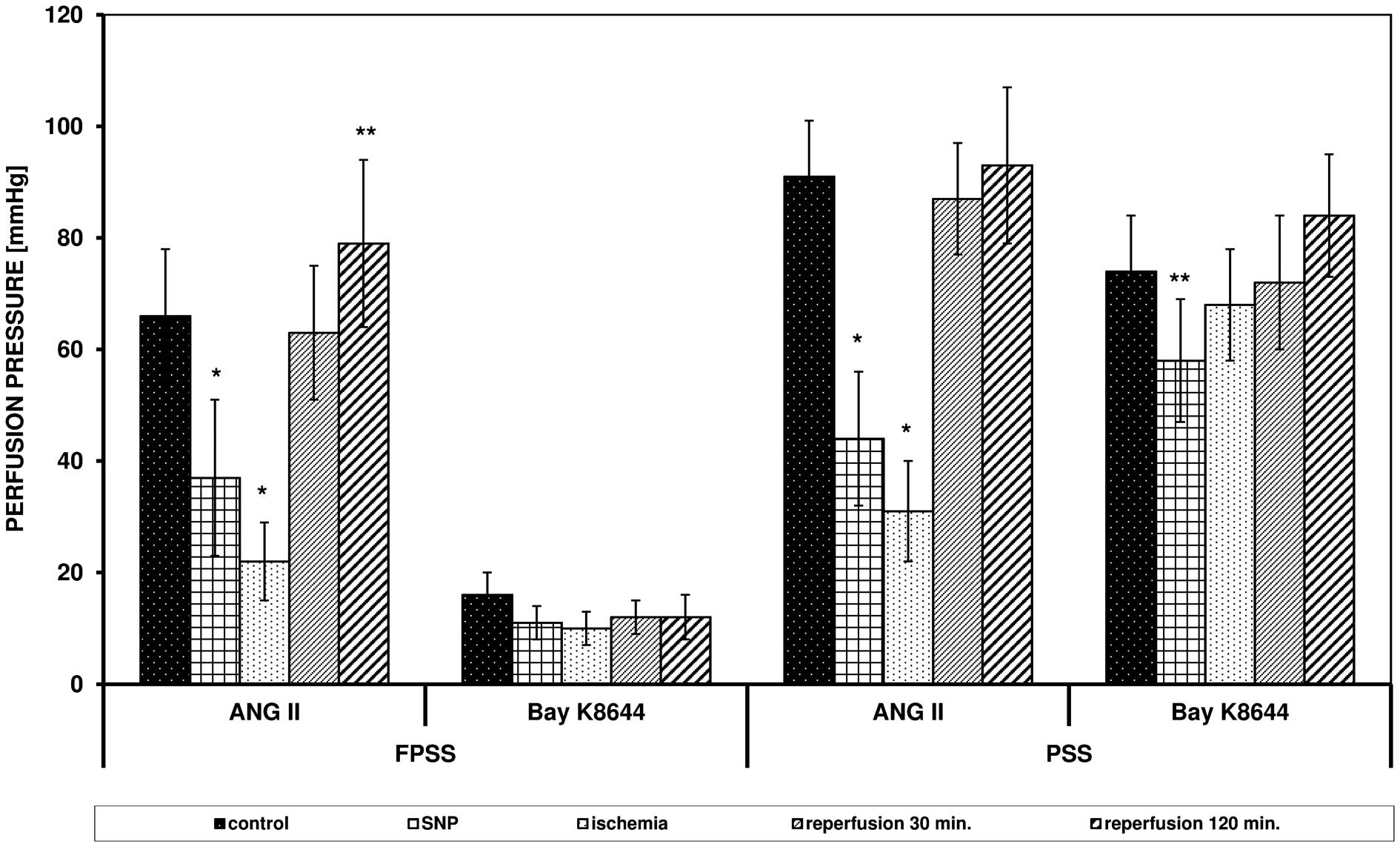
Effects of SNP and
8Br-cGMP
The addition of SNP (100 μM/l) reduced the
control response of the arteries to ANG II with involvement of
intra- and extracellular pools of calcium ions and reduced the
contraction triggered by Bay K8644 in PSS (Fig. 2).
SNP did not alter the effect of ischemia on
contraction triggered by ANG II. In FPSS, the perfusion pressure
was 22 mmHg (vs. 17 mmHg without SNP, P=0.24), and in PSS it was 31
mmHg (vs. 24 mmHg without SNP, P= 0.16). The presence of SNP
significantly reduced the intensifying effect of reperfusion on the
response of the arteries triggered by ANG II. In FPSS, the pressure
after 120 min of reperfusion was 79 mmHg (vs. 117 mmHg without SNP,
P=0.0002), and in PSS it was 93 mmHg (vs. 209 mmHg without SNP,
P<0.0001). SNP reduced the contraction triggered by Bay K8644 in
PSS (P= 0.0088).
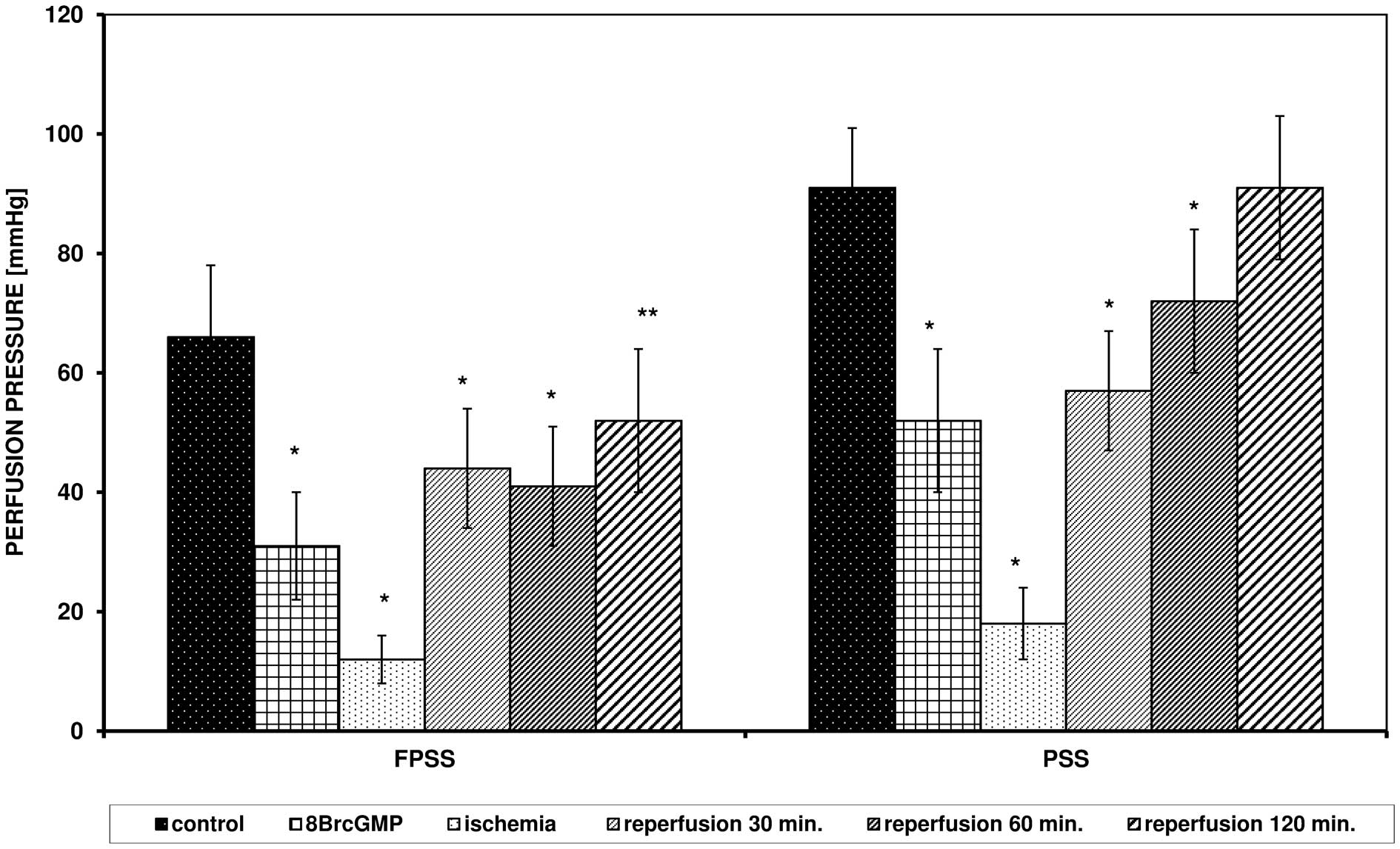
8Br-cGMP (100 μM/l) changed the
response of the arteries to ANG II after I/R in a manner similar to
SNP (Fig. 3).
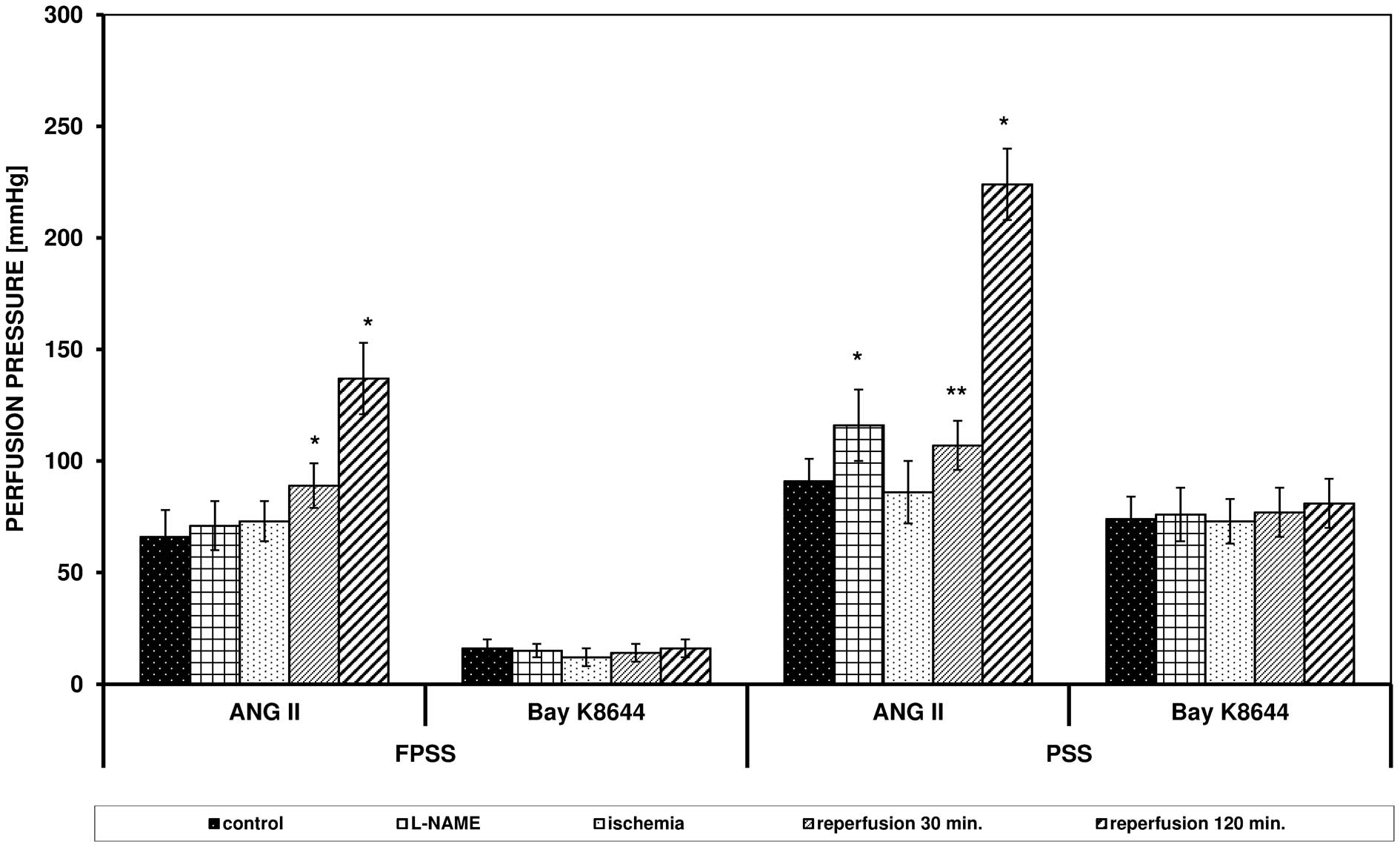
Effects of the inhibitors L-NAME and
ODQ
L-NAME (an NOS inhibitor; 300 μM/l) and ODQ
(a cG inhibitor; 100 μM/l) did not significantly influence
the perfusion pressure triggered by ANG II with participation of an
intracellular pool of Ca2+, but in PSS, an increased
reaction was observed (91 vs. 116 mmHg in the presence of L-NAME,
P= 0.0004; 91 vs. 110 mmHg in the presence of ODQ, P= 0.0022;
Figs. 4 and 5).
The two inhibitors eliminated the inhibitory effect
of ischemia on the response of the arteries triggered by ANG II and
increased the contraction resultant from reperfusion in FPSS and
PSS (Figs. 4 and 5). After ischemia, in the presence of
L-NAME, the perfusion pressure in PSS was 86 mmHg (vs. 24 mmHg
without the inhibitor, P<0.0001), after 30 min of reperfusion
the pressure reached 107 mmHg (vs. 87 mmHg without the inhibitor,
P= 0.0027). In PSS, following the addition of ODQ, the perfusion
pressure after ischemia was 96 mmHg (vs. 24 mmHg without the
inhibitor, P<0.0001) and after 30 min of reperfusion it was 157
mmHg (vs. 87 mmHg without the inhibitor, P<0.0001).
L-NAME and ODQ did not influence the action of Bay
K8644.
Discussion
Surgical procedures, including bypass grafting and
transplantations, are associated with temporary ischemia in organs
and their reperfusion, which results in local contraction of the
smooth muscles (13,16). Elevated vascular muscle contractile
tension following I/R triggers an excessive increase in calcium ion
concentration as well as damage of the endothelial cells and the
smooth muscles, which disturbs the balance between factors which
stimulate the contraction and relaxation of the vessels, and may
lead to total elimination of the blood flow (11,17).
In the present study, the effect of I/R on
contraction triggered by ANG II (an agonist of metabotropic
angiotensin receptor AT1) and Bay K8644 (an agonist of calcium
channels) was analyzed. In order to establish the significance of
calcium ions (from intracellular reserves and the extracellular
fluid), the experiments were conducted in fluid without calcium
ions (for evaluation of the significance of the intracellular pool)
and in standard Krebs fluid, after emptying the cellular reserves
of calcium (for evaluation of the significance of the extracellular
pool). Then, the effects of signaling pathways associated with NO
and cGMP on the aforementioned reactions were evaluated.
The presented results reveal that in the case of
contraction triggered by ANG II, the two pools of calcium ions
mediate the response, but the response in PSS was more intense, and
Bay K8644 led to an increase of the perfusion pressure only in the
presence of calcium ions entering the cell from outside, since the
action of Bay K8644 results from the direct activation of
dihydropyridinic calcium channels located in the cellular membrane.
Similar effects were also observed in studies which evaluated human
mesenteric arteries (18,19).
It is known that NO is a basic substance of
endothelial origin, which triggers effects via cGMP (20,21).
Cyclic nucleotides, such as cAMP and cGMP, act contrary to calcium
ions in smooth muscles. By lowering the level of
[Ca2+]i and the smooth muscle sensitivity to
calcium ions, these nucleotides cause the vessel to relax (22–24).
Besides the modulation of the smooth muscle, the signaling cascade
NO → cG → cGMP inhibits artery proliferation and prevents
aggregation and inflammatory effects, which indicates the potential
suitability of this pathway, and the opportunity for the use of
drugs administered in circulatory diseases (25–28).
Results of assays evaluating the response of the
arteries to ANG II following I/R revealed that ischemia reduces and
reperfusion intensifies the action of this peptide. The results of
previous experiments have indicated that inhibiting the effect of
ischemia on the contraction of arteries is associated with the
presence of endothelium, NO synthesis and cGMP activation. The
experiments which were conducted on rat tail arteries with removed
endothelium revealed no reduction in response to ANG II following
ischemia. However, intensification of the contraction following
reperfusion was observed under these conditions (21). Studies on human mesenteric arteries
revealed a modulating effect of NO, thromboxane A2 and guanylate
cyclase on the reactivity of the vessels following I/R (29,30).
Finally, studies of the effects of catalase and aminotriazole on
the contraction of the caudal artery in rats stimulated by ANG II
after I/R revealed that an antioxidative system modulates the
responses to ANG II, and reperfusion disturbs the balance between
antioxidants and the production of ROS (31).
A series of experiments conducted on the influence
of Bay K8644 revealed no effect of I/R on responses to direct
activation of the dihydropyridinic calcium channels. Similarly,
experiments using a depolarizing concentration of KCl revealed that
ischemia does not influence the response of the arteries to KCl
(27). It should be noted that in
the present study SNP reduced the contraction triggered by Bay
K8644 in PSS, but L-NAME and ODQ did not change the response
mediated by the entry of calcium ions through channels located in
the cellular membrane. Similar results have been achieved in
experiments on human mesenteric arteries, and in addition, it has
been revealed that an increasing concentration of acetylcholine
reduces the contraction stimulated by Bay K8644, but does not
change the reaction in the presence of L-NNA and ODQ (19).
In conclusion, I/R modulate the contraction of
arteries triggered by ANG II, but do not influence the reactions
induced by Bay K8644. Intra- and extracellular pools of calcium
ions mediate the action of ANG II, but Bay K8644 stimulates
contraction only with the participation of calcium ions entering
the cell. Regulation of the vascular smooth muscle tone associated
with the action of NO and cGMP is subject to modulation under
conditions of I/R.
References
|
1.
|
Lüscher TF and Barton M: Biology of the
endothelium. Clin Cardiol. 20(Suppl 2): II-3-II-101997.
|
|
2.
|
Karaki H, Ozaki H, Hori M, et al: Calcium
movements, distribution, and functions in smooth muscle. Pharmacol
Rev. 49:157–230. 1997.PubMed/NCBI
|
|
3.
|
Touyz RM and Schiffrin EL: Signal
transduction mechanisms mediating the physiological and
pathophysiological actions of angiotensin II in vascular smooth
muscle cells. Pharmacol Rev. 52:639–672. 2000.PubMed/NCBI
|
|
4.
|
Rubanyi GB: Endothelium-derived relaxing
and contracting factors. J Cell Biochem. 46:27–36. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Choi DW: Calcium-mediated neurotoxicity:
relationship to specific channel types and role in ischemic damage.
Trends Neurosci. 11:465–469. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Fryer HJ, Knox RJ, Strittmatter SM and
Kalb R: Excitotoxic death of a subset of embryonic rat motor
neurons in vitro. J Neurochem. 72:500–513. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Schäfer M, Bahde D, Bosche B, Ladilov Y,
Schäfer C, Piper HM and Noll T: Modulation of early
[Ca2+]i rise in metabolically inhibited
endothelial cells by xestospongin C. Am J Physiol Heart Circ
Physiol. 280:H1002–H1010. 2001.
|
|
8.
|
Bornfeldt KE: Intracellular signaling in
arterial smooth muscle migration versus proliferation. Trends
Cardiovasc Med. 6:143–151. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Katoh Y and Periasamy M: Growth and
differentiation of smooth muscle cells during vascular development.
Trends Cardiovasc Med. 6:100–106. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Gutierrez J, Ballinger SW, Darley-Usmar VM
and Landar A: Free radicals, mitochondria, and oxidized lipids: the
emerging role in signal transduction in vascular cells. Circ Res.
99:924–932. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Hashimoto K, Pearson PJ, Schaff HV and
Cartier R: Endothelial cell dysfunction after ischemia arrest and
reperfusion: a possible mechanism of myocardial injury during
reflow. J Thorac Cardiovasc Surg. 102:688–694. 1991.PubMed/NCBI
|
|
12.
|
Horner RL and Bradley TD: Update in sleep
and control of ventilation. Am J Respir Crit Care Med. 175:426–431.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Szabo A and Heemann U: Ischemia
reperfusion injury and chronic allograft rejection. Transplant
Proc. 30:4281–4284. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Hangaishi M, Nakajima H, Taguchi J, et al:
Lecithinized Cu, Zn-superoxide dismutase limits the infarct size
following ischemia-reperfusion injury in rat hearts in vivo.
Biochem Biophys Res Commun. 285:1220–1225. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zakynthinos S, Katsaounou P, Karatza MH,
Roussos C and Vassilakopoulos T: Antioxidants increase the
ventilatory response to hyperoxic hypercapnia. Am J Respir Crit
Care Med. 175:62–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Fullerton DA, Mitchell MB, McIntyre RC Jr,
Banerjee A, Campbell DN, Harken AH and Grover FL: Cold ischemia and
reperfusion each produce pulmonary vasomotor dysfunction in the
transplanted lung. J Thorac Cardiovasc Surg. 106:1213–1217.
1993.PubMed/NCBI
|
|
17.
|
Dignan RJ, Dyke CM, Abd-Elfattah AS, et
al: Coronary artery endothelial cell and smooth muscle dysfunction
after global myocardial ischemia. Ann Thorac Surg. 53:311–317.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Szadujkis-Szadurski R, Tafil-Klawe M,
Szadujkis-Szadurska K, et al: Effect of acetylocholine on reactions
induced by 2 contraction agents - angiotensin II and caffeine. Med
Biol Sci. 24:53–58. 2010.
|
|
19.
|
Szadujkis-Szadurski R, Tafil-Klawe M,
Szadujkis-Szadurska K, et al: Modulation of the contractile effect
of Bay K8644 on human vascular smooth muscle cells by
acetylocholine and calcium ions. Med Biol Sci. 24:59–64. 2010.
|
|
20.
|
Furchgott RF and Zawadzki JW: The
obligatory role of endothelial cells in the relaxation of arterial
smooth muscle by acetylcholine. Nature. 288:373–376. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Szadujkis-Szadurska K, Slupski M,
Szadujkis-Szadurski R, Szadujkis-Szadurski L, Jasiñski M and
Kolodziejska R: The role of the endothelium in the regulation of
vascular smooth muscle cell contractions induced by angiotensin II
after ischemia and reperfusion. Arch Pharm Res. 33:1019–1024. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Zaccolo M and Movsesian MA: cAMP and cGMP
signaling cross-talk: Role of phosphodiesterases and implications
for cardiac pathophysiology. Circ Res. 100:1569–1578. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Bender AT and Beavo JA: Cyclic nucleotide
phosphodiesterases: molecular regulation to clinical use. Pharmacol
Rev. 58:488–520. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Münzel T, Daiber A, Ullrich V and Mülsch
A: Vascular consequences of endothelial nitric oxide synthase
uncoupling for the activity and expression of the soluble guanylyl
cyclase and the cGMP-dependent protein kinase. Arterioscler Thromb
Vasc Biol. 25:1551–1557. 2005.PubMed/NCBI
|
|
25.
|
Yetik-Anacak G, Xia T, Dimitropoulou C,
Venema RC and Catravas JD: Effects of hsp90 binding inhibitors on
sGC-mediated vascular relaxation. Am J Physiol Heart Circ Physiol.
291:H260–H268. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Oberwittler H, Hirschfeld-Warneken A,
Wesch R, et al: Significant pharmacokinetic and pharmacodynamic
interaction of warfarin with the NO-independent sGC activator
HMR1766. J Clin Pharmacol. 47:70–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Yan C, Kim D, Aizawa T and Berk BC:
Functional interplay between angiotensin II and nitric oxide:
cyclic GMP as a key mediator. Arterioscler Thromb Vasc Biol.
23:26–36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Martin E, Sharina I, Kots A and Murad F: A
constitutively activated mutant of human soluble guanylyl cyclase
(sGC): Implication for the mechanism of sGC activation. Proc Natl
Acad Sci USA. 100:9208–9213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Slupski M, Szadujkis-Szadurska K,
Szadujkis-Szadurski R, et al: Nitric oxide and thromboxane A2
modulate pulmonary pressure after ischemia and intestinal
reperfusion. Transplant Proc. 38:334–337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Slupski M, Szadujkis-Szadurski L, Grześk
G, et al: Guanylate cyclase activators influence reactivity of
human mesenteric superior arteries retrieved and preserved in the
same conditions as transplanted kidneys. Transplant Proc.
39:1350–1353. 2007. View Article : Google Scholar
|
|
31.
|
Szadujkis-Szadurska K, Slupski M,
Szadujkis-Szadurski R, et al: Modulation of the reaction of
vascular smooth muscle cells to angiotensin II induced by catalase
and aminotriasol during ischemia-reperfusion. Transplant Proc.
42:1614–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|