Introduction
The renin-angiotensin-aldosterone system (RAAS) is a
key regulator of blood pressure (BP) and body fluid volume, acting
primarily via the effects of angiotensin II (Ang II). The RAAS may
increase the load on the cardiovascular system when activated by
electrolyte abnormalities, wall stress, pressure and volume
(1). Classically, there is an
increased release of renin from the granular cells and in turn, an
increased conversion of angiotensinogen to angiotensin I from the
liver. Angiotensin I is converted to Ang II by the
angiotensin-converting enzyme (ACE). Increased Ang II ultimately
results in an increased adrenal aldosterone release. Ang II and
aldosterone then have various effects on their target organs. In
addition to this traditional angiotensin production method,
RAAS-independent local angiotensin production has also been
described (2), as well as
ACE-independent production pathways for the formation of Ang II
(3,4).
The RALES trial showed that the maximum benefit of
spironolactone was achieved in congestive heart failure patients
with the increased levels of collagen synthesis markers (5). Neurohumoral, genetic and mechanical
para meters affect the operation of this conversion process and in
turn are positively affected by the inhibition of the RAAS-ACE
inhibitors and Ang II type 1 (AT1) receptor antagonists, preventing
the effects of Ang II, but not the negative effects of aldosterone.
Even following the complete inhibition of the RAAS by the ACE
inhibitors (6,7) and the administration of additional
AT1-receptor antagonists (8),
elevated aldosterone levels remain evident in heart failure,
indicating the potential gain that may occur from an additional
inhibitor of aldosterone.
The aim of the present study was to investigate the
protective effects of saponin on a hypertensive target organ (the
kidney) in spontaneously hypertensive rats SHRs and also to explore
the effects of saponin on the RAAS.
Materials and methods
Animals
A total of 24 male or female, 14-week-old SHRs
weighing 200–250 g were used in the present study. The SHRs were
provided by the Beijing Vital River Laboratory Animal Technology
Co., Ltd. (Beijing) with Animal Production License No. SCXK
2007-001. Animals were kept in cages, with controlled light/dark
cycles and temperatures, fed with a normal rat chow and had free
access to tap water. The SHRs were randomly divided into three
groups; the first was adminstered low-dose saponin (27 mg/kg, n=8),
the second with high-dose saponin (108 mg/kg, n=8) and the third
with a placebo as the control group (n=8). Another eight healthy
male Wistar rats were used as the normal group.
BP measurements
The BPs of the rats were determined using an animal
BP-6 non-invasive blood pressure tester after 0, 4, 8, 12 and 10
weeks of drug intervention.
In situ hybridization
Total mRNA was isolated from the kidney cells of
male and female rats at 14 weeks of age. An Ribonuclease Protection
Assay (RPA) was performed according to the manufacturer’s
instructions (Ambion RPA II kit, Foster City, CA, USA). For each
hybridi zation reaction, 40 pg RNA and 50,000 cpm of
32P-labeled transcripts were purified at 42°C overnight.
Correct expression of the transgene was studied by in situ
hybridization, as described above. In short, a
35S-UTP-labeled mRNA probe was built using a fragment of
600 bp.
Quantitative real-time (qRT)-PCR
The purification of total RNA from the cells was
performed using the NucleoSpin RNA kit I (Macherey-Nagel, Düren,
Germany), according to the manufacturer’s instructions. cDNA
corresponding to 50 ng of RNA was added to the SYBR-Green JumpStart
Taq Ready Mix (Sigma-Aldrich, St. Louis, MO, USA). Following this
addition, the cDNA for the 18S rRNA gene was diluted due to the
quantitative superiority of the ribosomal RNA. A duplicate was made
for each sample. qRT-PCR (Mx4000, Stratagene, TX, USA) was
performed using a three-step protocol, followed by a melting curve
analysis to verify the homogeneity of the amplified PCR
products.
Statistical analysis
The data are presented as the mean ± SD. Comparisons
between the groups of data were performed by using a Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference. Data were analyzed with the SPSS 18.0
statistical software package (SPSS Inc., Chicago, IL, USA).
Results
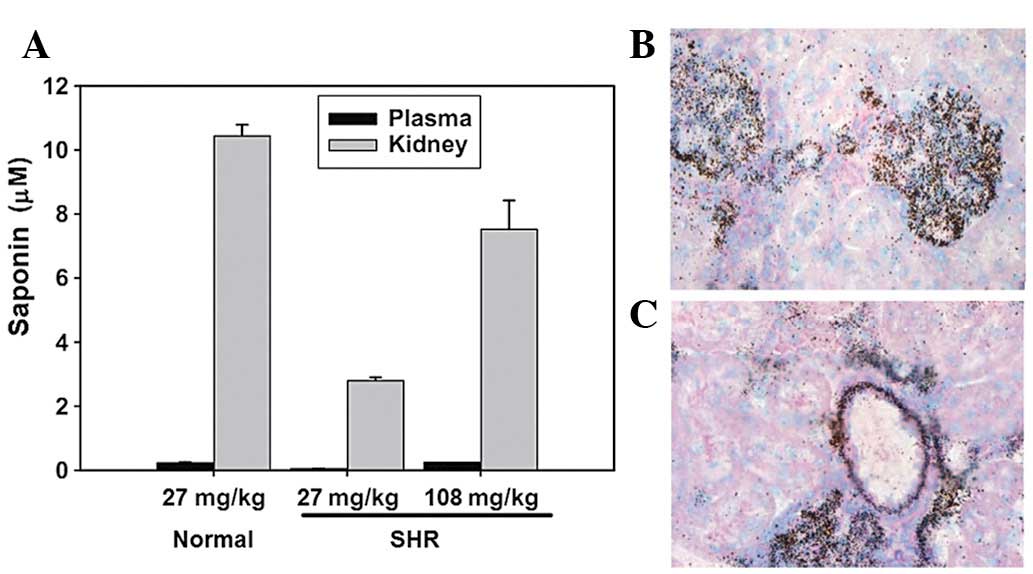
Renal distribution of saponin
Following treatment with the saponin compound for 2
weeks the plasma saponin levels were 127±18 ng/ml in normal rats
(27 mg/kg per day) and 29.7±5.6 and 129±23.7 ng/ml in the SHRs (27
and 108 mg/kg per day, respectively). The mean kidney:plasma
concentration ratio of saponin in rats treated with the compound
for 2 weeks was 45.6 for the normal rats (27 mg/kg per day) and
30.7 and 63.6 for the SHRs (27 and 108 mg/kg per day, respectively;
Fig. 1A), indicating extensive
saponin levels in the kidneys. In the rats treated with 27 mg/kg
saponin per day, the renal and plasma saponin levels were lower in
the SHRs compared with those in the normal rats.
Using light microscopy, autoradiographic grains were
observed in the glomeruli of each renal section and used to
indicate the presence of saponin (Fig.
1B). Extensive saponin levels were also located in the arterial
wall of the small cortical vessels in the kidney (Fig. 1C).
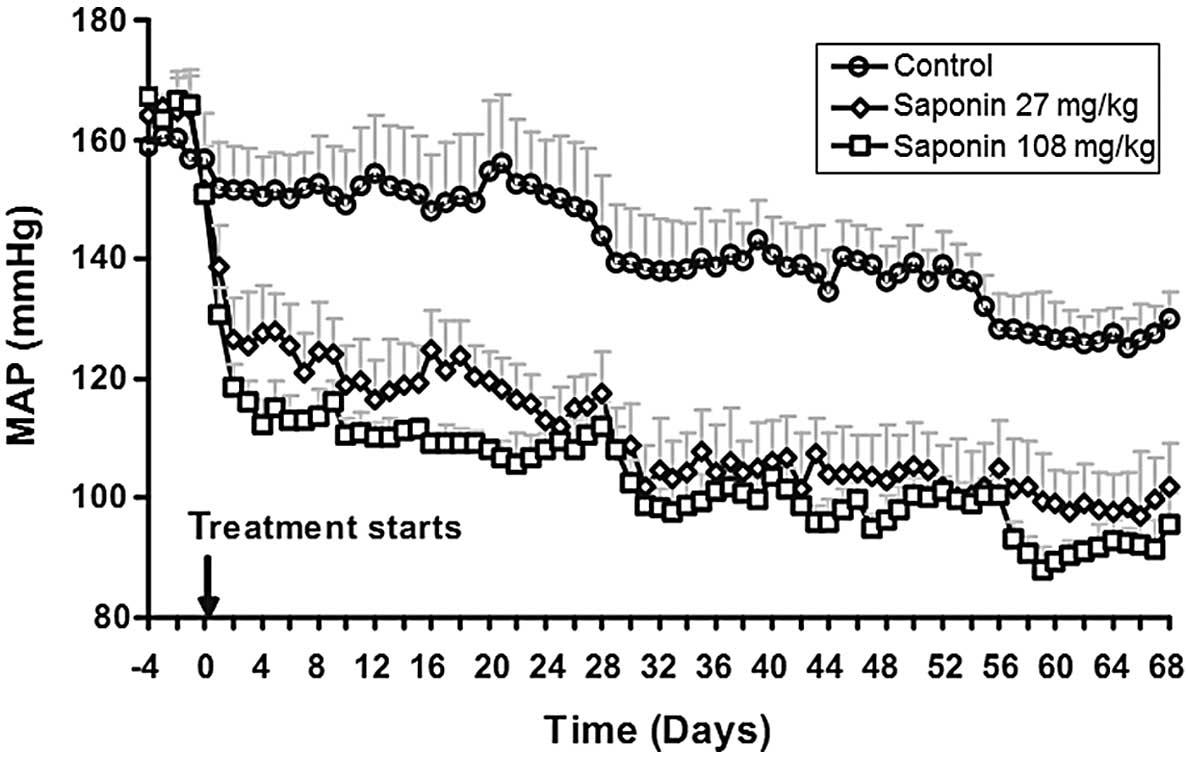
BP declines in saponin-treated SHRs
There was a trend towards a mild and gradual decline
in BP in the saponin-treated SHRs (Fig. 2). By contrast, initiation of the
saponin treatment caused a prompt and sustained reduction in mean
arterial pressure (MAP). MAP was decreased by 36±3 and 51±4 mm Hg
in the low- and high-dose groups, respectively, 5 days after the
saponin treatment. No significant differences were observed in
heart rates following saponin treatment (data not shown).
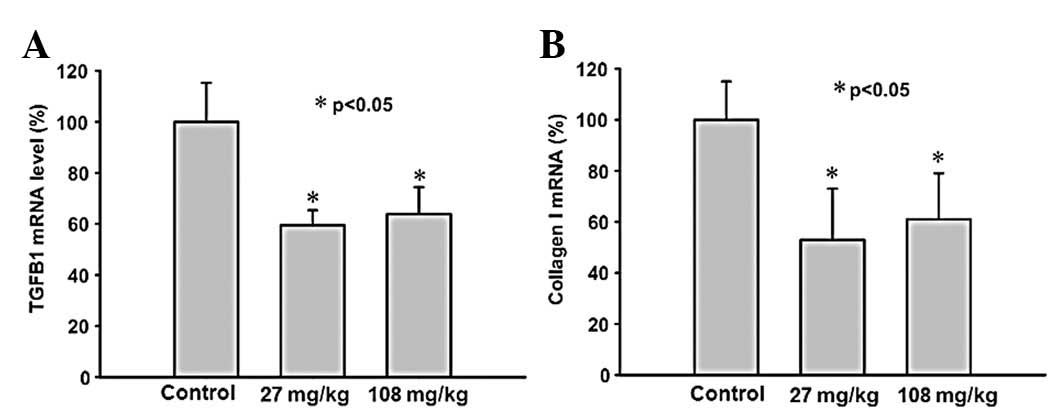
Treatment with saponin suppresses gene
expression of TGFB1 and collagen I
The TGFB1 gene expression in the renal samples was
significantly suppressed in the saponin-treated SHRs (Fig. 3A) compared with the controls, with
the expression levels in the 27 mg/kg per day group tending to be
slightly, but not significantly, more supressed than the 108 mg/kg
per day group. The gene expression of collagen I in the renal
samples was significantly reduced in each of the saponin-treated
SHRs groups (Fig. 3B). No
significant differences were observed in the expression of
collagens III and V between the saponin-treated SHRs and the
controls (data not shown).
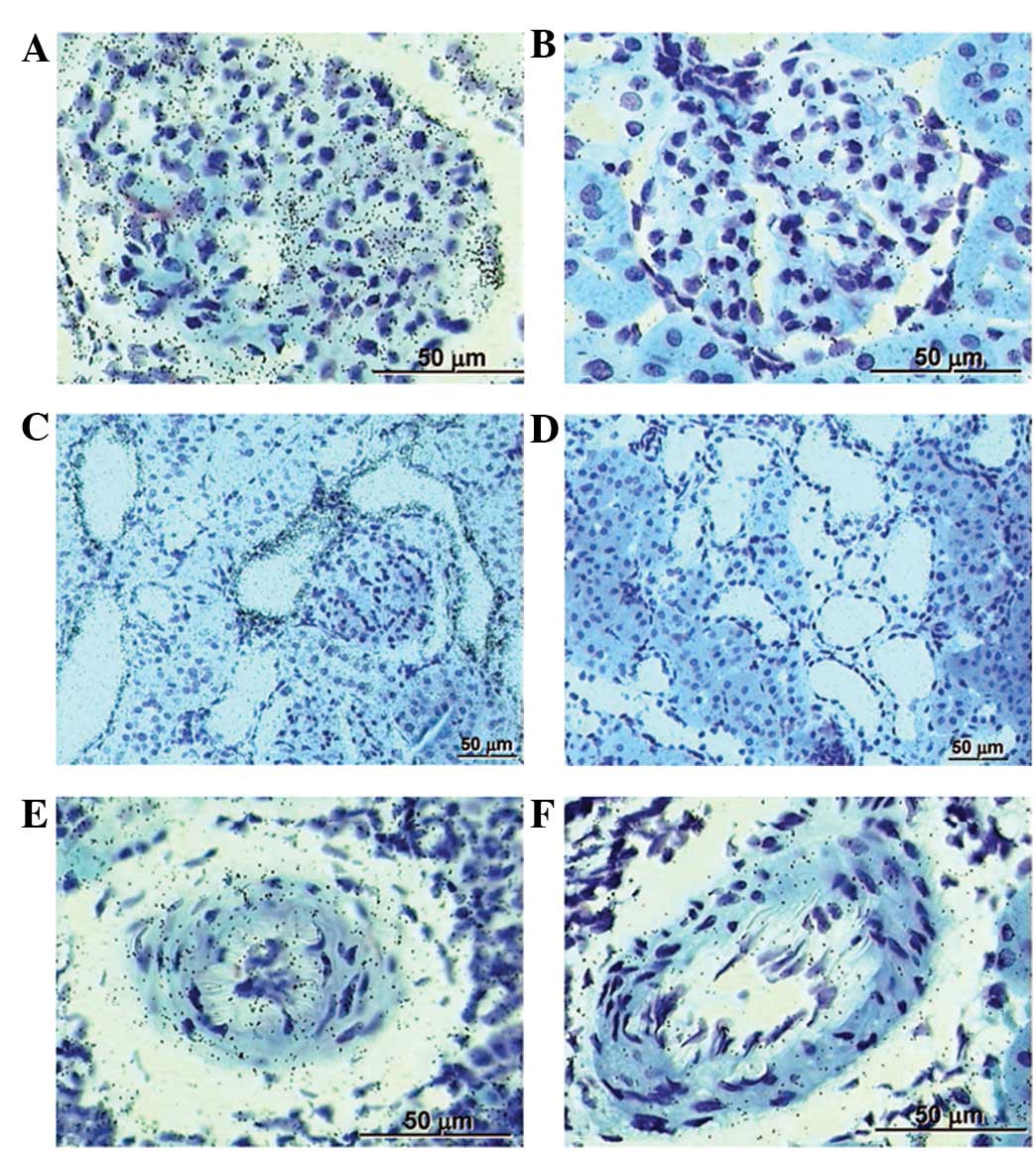
Treatment with saponin suppresses gene
expression of PRR
In situ hybridized renal sections from the
placebo-treated controls showed prominent labeling for PRR in the
glomeruli and tubules, with less labeling evident in the renal
arteries (Fig. 4A, C and E).
However, in the saponin-treated SHRs, the expression of PRR in
these renal sections was significantly suppressed compared with the
placebo-treated controls (Fig. 4B, D
and F).
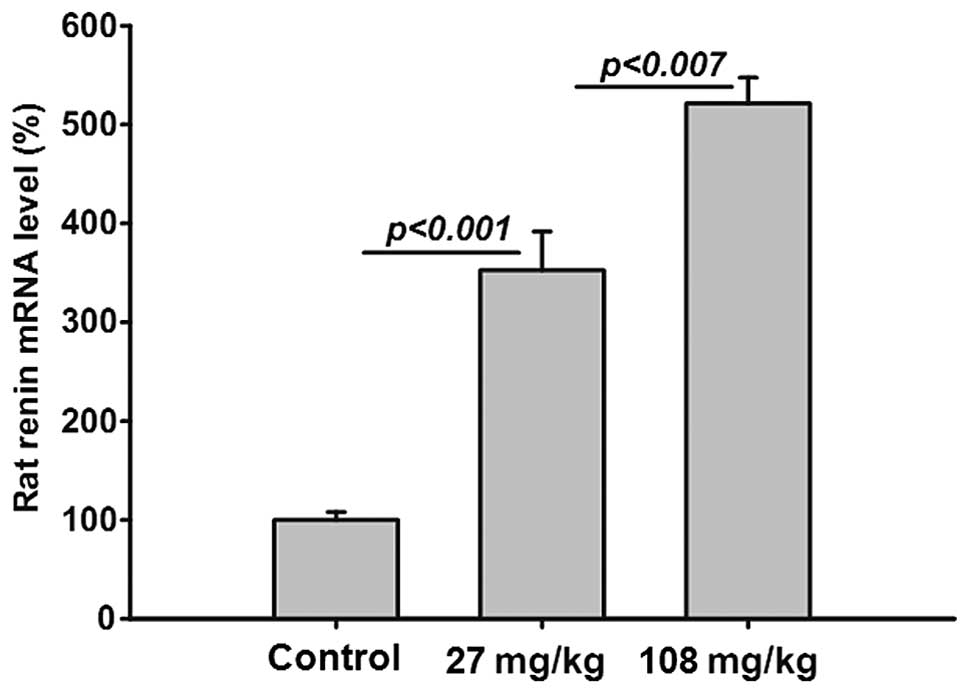
Treatment with saponin increases renal
rat renin gene expression
The gene expression of endogenous rat renin was
significantly and dose-dependently increased in the saponin-treated
SHRs (Fig. 5), indicating a
saponin-induced RAAS blockade.
Discussion
The aim of the present study was to investigate the
protective effects of saponin on a hypertensive target organ (the
kidney) in SHR rats as well as also to explore the effects of
saponin on the RAAS.
Increased renin may lead to an increase in the BP of
SHRs (9,10), thus, the observed inhibitory
potency of saponin against rat renin suggests that saponin lowers
BP in SHRs by inhibiting renin. The increased gene expression of
renin in the renal samples of SHRs in the present study suggested
an RAAS blockade.
The extensive level of saponin in the renal samples
suggested a renoprotective effect via inhibition of the intra-renal
RAAS. Moreover, autoradiographic grains observed in the glomeruli
of each renal sample, which indicated the presence of saponin,
suggested the potential for local renin inhibition in the
glomeruli. Longer exposures to saponin may lead to the accumulation
of saponin in other renal compartments. Notably, the presence of
saponin in the vessel wall suggested that saponin may enter the
granular cells of the afferent arteriole, the renin production
site. Thus, it is possible that saponin may inhibit the production
of renin prior to its release from the granular cells. A previous
study has reported the blockade of intracellular renin by saponin
in cultured myocardial cells (11).
In the present study, the development of albuminuria
was prevented in the SHRs treated with saponin, but was not
prevented in the placebo-treated controls. As albuminuria is
considered a biomarker for the risk of renal decline these findings
are relevant to the overall results (12). The anti-albuminuric effect of
saponin was attributable to its anti-hypertensive effect.
TGFB1 in conjunction with Ang II plays a key role in
renal fibrosis (13). The
observations of the present study indicate that saponin suppressed
the gene expression of TGFB1 in the renal samples of the SHRs
allowing it to inhibit TGFB1-mediated pathways therefore leading
them towards renal fibrosis. Saponin also reduced renal collagen I
gene expression.
A possible correlation between the renoprotective
effects of saponin and PRR has been explored in cardio-renal
disease (14–16). The results of the in situ
hybridization in the present study implicated saponin in the
suppression of PRR gene expression in vivo. Incubation of
saponin did not change the gene expression of PRR in the mesangial
cells. The results showed a different distribution pattern of
saponin in a greater number of renal compartments compared with
that described previously in human kidneys (17).
In conclusion, the present study has demonstrated
that saponin reduces systemic BP and blocks the circulating and
tissue RAAS in SHRs. The administration of saponin was suspected to
have a renoprotective effect via the inhibition of the intrarenal
RAAS.
References
|
1.
|
Delcayre C and Swynghedauw B: Molecular
mechanisms of myocardial remodeling. The role of aldosterone. J Mol
Cell Cardiol. 34:1577–1584. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Weber KT: Extracellular matrix remodeling
in heart failure: a role for de novo angiotensin II generation.
Circulation. 96:4065–4082. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Urata H, Nishimura H, Ganten D and Arakawa
K: Angiotensin-converting enzyme-independent pathways of
angiotensin II formation in human tissues and cardiovascular
diseases. Blood Press Suppl. 2:22–28. 1996.PubMed/NCBI
|
|
4.
|
Olivetti G, Capasso JM, Sonnenblick EH and
Anversa P: Side-to-side slippage of myocytes participates in
ventricular wall remodeling acutely after myocardial infarction in
rats. Circ Res. 67:23–34. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Zannad F, Alla F, Dousset B, et al:
Limitation of excessive extracellular matrix turnover may
contribute to survival benefit of spironolactone therapy in
patients with congestive heart failure: insights from the
randomized aldactone evaluation study (RALES). Rales Investigators
Circulation. 102:2700–2706. 2000. View Article : Google Scholar
|
|
6.
|
Bauersachs J and Fraccarollo D:
Aldosterone antagonism in addition to angiotensin-converting enzyme
inhibitors in heart failure. Minerva Cardioangiol. 51:155–164.
2003.PubMed/NCBI
|
|
7.
|
Jorde UP, Vittorio T, Katz SD, et al:
Elevated plasma aldosterone levels despite complete inhibition of
the vascular angiotensin-converting enzyme in chronic heart
failure. Circulation. 106:1055–1057. 2002. View Article : Google Scholar
|
|
8.
|
McKelvie RS, Yusuf S, Pericak D, et al:
Comparison of candesartan, enalapril, and their combination in
congestive heart failure: randomized evaluation of strategies for
left ventricular dysfunction (RESOLVD) pilot study. The RESOLVD
Pilot Study Investigators. Circulation. 100:1056–1064. 1999.
View Article : Google Scholar
|
|
9.
|
Kodavanti UP, Schladweiler MC, Ledbetter
AD, et al: The spontaneously hypertensive rat as a model of human
cardiovascular disease: evidence of exacerbated cardiopulmonary
injury and oxidative stress from inhaled emission particulate
matter. Toxicol Appl Pharmacol. 164:250–263. 2000. View Article : Google Scholar
|
|
10.
|
Sagvolden T: Behavioral validation of the
spontaneously hypertensive rat (SHR) as an animal model of
attention- deficit/hyperactivity disorder (AD/HD). Neurosci
Biobehav Rev. 24:31–39. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Singh VP, Le B, Bhat VB, et al:
High-glucose-induced regulation of intracellular ANG II synthesis
and nuclear redistribution in cardiac myocytes. Am J Physiol Heart
Circ Physiol. 293:H939–H948. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
de Zeeuw D, Remuzzi G, Parving HH, et al:
Proteinuria, a target for renoprotection in patients with type 2
diabetic nephropathy: lessons from RENAAL. Kidney Int.
65:2309–2320. 2004.PubMed/NCBI
|
|
13.
|
Border WA and Noble NA: Interactions of
transforming growth factor-beta and angiotensin II in renal
fibrosis. Hypertension. 31:181–188. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Ichihara A, Hayashi M, Kaneshiro Y, et al:
Inhibition of diabetic nephropathy by a decoy peptide corresponding
to the ‘handle’ region for nonproteolytic activation of prorenin. J
Clin Invest. 114:1128–1135. 2004.PubMed/NCBI
|
|
15.
|
Ichihara A, Suzuki F, Nakagawa T, et al:
Prorenin receptor blockade inhibits development of
glomerulosclerosis in diabetic angiotensin II type 1a
receptor-deficient mice. J Am Soc Nephrol. 17:1950–1961. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Burcklé CA, Jan Danser AH, Müller DN, et
al: Elevated blood pressure and heart rate in human renin receptor
transgenic rats. Hypertension. 47:552–556. 2006.PubMed/NCBI
|
|
17.
|
Nguyen G, Delarue F, Burcklé C, et al:
Pivotal role of the renin/prorenin receptor in angiotensin II
production and cellular responses to renin. J Clin Invest.
109:1417–1427. 2002. View Article : Google Scholar : PubMed/NCBI
|