Introduction
Cardiovascular diseases (CVD) are a significant
cause of mortality worldwide and the main cause of CVD is acute
myocardial infarction (AMI). In the United States, acute coronary
syndrome (ACS) affects 1.3 million individuals (1). Clinically, early detection is
essential for the treatment and prognosis of myocardial infarction.
At present, the serum levels of cardiac troponins cTnI and cTnT,
are used to diagnose early myocardial infarction (2). In addition, the uptake of radioactive
tracers, such as exogenous glucose and acetate, is applied
clinically to predict cardiac functional improvement (3).
Ischemia and myocardial infarction are different
stages in the progression of heart failure. Identifying the early
changes of various metabolic parameters in myocardial ischemia is
of clinical importance (4). The
myocardium benefits from reperfusion-based therapeutic approaches
if ischemia is detected in the initial period. Therefore, detecting
early metabolic parameter changes in myocardial ischemia may have
diagnostic value (5).
Magnetic resonance spectroscopy (MRS) is a unique
non-invasive method for detecting cardiac metabolic changes, which
usually occur significantly earlier than irreversible organic
lesions in myocardial infarction (6). This method detects biochemical
processes and evaluates metabolism without blood sampling or the
use of radionuclides (5). To date,
cardiovascular magnetic resonance studies using 1H MRS
have focused on its sensitivity and specificity in the study of
myocardial ischemia (7,8).
31P-MRS is commonly used to study
myocardial energy metabolism (9,10).
In previous studies, 31P-MRS has been employed to
measure indicators, including phosphocreatine (PCr)/ATP and
PCr/inorganic phosphate (Pi) ratios, in humans to guide heart
transplantation and myocardial infarction non-invasively (6,11,12).
It is well recognized that protons have the highest
magnetic moment of all biologically relevant nuclei, with a high
concentration in organic molecules. These features make it possible
to achieve enhanced spatial resolution by 1H MRS.
Compared with 31P MRS, 1H MRS provides higher
sensitivity and detects more metabolites, such as unphosphorylated
creatine (3,13). 1H nuclear magnetic
resonance (NMR) has been used to evaluate heart tissue ex
vivo to produce a high-resolution metabolic profile in
myocardial infarction animal models. However, cardiac motion,
respiratory motion and epicardial fat make it difficult to apply
1H NMR clinically (5).
Metabolomics is a rapidly evolving field that aims
to identify and quantify the concentration changes of all
metabolites in a model system. This approach involves large
metabolite datasets and high-throughput techniques, including NMR
spectroscopy or mass spectroscopy. High-resolution magic angle
spinning (HRMAS) 1H NMR spectroscopy has been used to
investigate cardiac metabolites in rodent models (14–17).
Multivariate pattern recognition analysis coupled with the use of
HRMAS was able to identify metabolic biomarkers of disease in
intact tissue. The metabolic information obtained from HRMAS
spectra may be transferred to the clinical environment (18,19).
Taurine (Tau), a sulfur-containing β-amino acid, is
the most abundant free amino acid in the myocardium (∼60%)
(4). The Tau content is high in
mammalian hearts, ranging between 5 and 40 μmol/g wet weight
(20). It has been demonstrated
that Tau has a number of functions, including the regulation of
intracellular calcium balance, antioxidant and anti-inflammatory
actions, immune regulation and cardiovascular protection (4,20–22).
Cardiac muscle lacks the ability to synthesize Tau (23). This suggests that Tau originates
from a transport process. It has been recognized that myocardial
Tau synthesis is limited while the majority of the Tau in cardiac
tissue is accumulated by uptake from the blood (20). As such, myocardial Tau levels may
change when ischemia occurs. The Tau transporter, located on the
cell membrane, is important in Tau metabolism in the myocardium. In
Tau transporter knockout mice, the level of Tau was decreased by
98% in the myocardium, which indicates that the Tau uptake process
is solely Tau transporter dependent without compensation from other
transport systems (24,25). We hypothesize that changes in the
Tau level in myocardial tissue may be a potential indicator of
myocardial ischemia and early infarction.
In the present study, a myocardial ischemia model
was created in rats. Metabolic markers, including Tau, creatine
(Cre), choline (Cho) and lactate (Lac), were analyzed at various
time points ex vivo using HRMAS 1H NMR. The
results were further confirmed by high performance liquid
chromatography (HPLC).
Materials and methods
Animal model and cell culture
The animals were provided by the Animal Center of
Fudan University (Shanghai, China). All procedures were performed
with approval from the Animal Care and Use Committee of Shanghai,
China. Adult Sprague-Dawley (SD) rats (male, body weight 200–250 g)
were anesthetized with pentobarbital sodium (30 mg/kg I.P.; Sigma,
St. Louis, MO, USA; Lot No. P3761). The left anterior descending
coronary artery (LAD) was ligated with a 6-0 suture (Jinhuan
Medical, Shanghai, China; Lot No. 20F101) as previously reported
(26). Sham-operated animals
underwent the same procedure but the LAD was left untied. The
animals were divided into seven groups according to the time points
following ligation: 0 min (control, sham-operated), 5 min, 20 min,
30 min, 45 min, 1 h and 6 h. The heart was then excised. The
infarction zone was isolated and then frozen in liquid nitrogen for
further analysis. For three rats from the 6 h group, the rat LAD
ligation model was verified by 1% triphenyltetrazolium chloride
(TTC) and Evans Blue double staining, as previously described
(27). The H9c2 rat cardiomyoblast
cell line was obtained from the Cell Bank of the Chinese Academy of
Sciences (Beijing, China). For the HPLC and apoptosis assay, the
cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM,
HyClone, Waltham, MA, USA) supplemented with 10% FBS, 100 U/ml
penicillin G and 100 mg/ml streptomycin. The cells were cultured in
standardized cell culture incubator conditions at 37°C in a
humidified atmosphere containing 5% CO2.
HRMAS 1H NMR
For the HRMAS 1H NMR analysis, the tissue
samples (from the control and myocardial ischemia groups, including
the 5 min, 20 min, 30 min, 45 min, 1 h and 6 h groups, n≥3),
weighing 25±2 mg each, were placed into a 25 μl zirconium
oxide rotor with drops of D2O (deuterium lock
reference). NMR tests were performed on a Bruker DRX-500 (Bruker
BioSpin GmbH, Rheinstetten, Germany) spectrometer (1H
frequency at 500.13 MHz) at 300.0 K, with a rotor spin rate of 5
kHz. Carr Purcell Meiboom Gill (CPMG) pulse sequences were used
with solvent presaturation during the relaxation delay of 2 sec.
The NMR spectra were acquired with 256 scans collected into 64,000
data points with a spectral width of 15 kHz. The CPMG pulse
sequence was applied to suppress signals from the molecules with
short T2 values, such as macromolecules and lipids, using a total
echo time (TE) of 320 msec. The stability of tissue samples was
evaluated by repeating a one-dimensional NMR experiment following
overall acquisition. No biochemical degradation was observed in any
of the tissue samples. Spectral assignments were further confirmed
by two-dimensional 1H-1H total correlation
spectroscopy (TOCSY)and 1H-1H correlation
spectroscopy (COSY; data not shown) with values obtained from the
literature (14,28).
Principal component analysis (PCA)
Spectral data were phased and baseline-corrected
using XWINNMR (Bruker Biospin GmbH). All free induction decays
(FIDs) were multiplied by an exponential function equivalent to a
0.3-Hz line-broadening factor prior to Fourier transformation. Each
HRMAS 1H NMR spectrum was segmented into 236 regions of
equal width (0.04 ppm) over the region δ0.00–10.00 and the signal
intensity in each region was integrated by AMIX (version 3.6,
Bruker Biospin GmbH). The region δ4.40–5.00 was removed to
eliminate the baseline effects of imperfect water saturation. Prior
to PCA, each integral region was normalized by dividing by the sum
of all integral regions for each spectrum (29,30).
PCA was used to calculate a new, smaller set of orthogonal
variables from linear combinations of the intensity variables while
retaining the maximum variability present within the data. These
new variables were the derived principal components and the
distribution of their values (scores) permitted the simple
visualization of separation or clustering between samples. The
weightings (loadings) applied to each integral region in
calculating the principal components allowed for the identification
of those spectral regions having the greatest effects on separation
and clustering and, hence, the deduction of the characteristic
metabolites of myocardial ischemia.
HPLC
The ischemic myocardial tissue (250 mg) was
homogenized by ultrasound (BILON96-II; Bilon Instruments, Shanghai,
China) and mixed with 18% sulfosalicylic acid (SCRC, 250
μl/100 mg). The mixture was centrifuged at 13,000 rpm for 5
min. The supernatant was then taken and filtered through a 0.22
μm membrane. O-phthalaldehyde (OPA) precolumn derivatization
was performed as previously described (31). After OPA derivatization, the sample
extract was immediately detected by HPLC (Agilent 1100; Agilent,
Santa Clara, CA, USA) with the parameters as follows: column,
ZORBAX Eclipse XDB-C18 4.6x150 mm, 5 μm (Agilent); mobile
phase A, methanol:acetonitrile:H2O = 45:45:10 (v/v/v);
mobile phase B1, methanol (0.05 mol/l):sodium acetate buffer (pH
5.3):tetrahydrofuran = 42:57:1; mobile phase B2, 40 mM phosphate
buffer (Na2HPO4, pH 7.8). Fluorescence
detection was performed at 450 nm. L-norvaline (Agilent, Lot
No.1103756) was added as the internal standard.
Hypoxia treatment and apoptosis
assay
A cardiac myoblast cell line hypoxia model was
established as described previously (32). Briefly, after washing with PBS, the
cells were placed in serum- and glucose-free DMEM and incubated in
a sealed, hypoxic anaerobic rectangular jar fitted with a catalyst
(BioMérieux, Marcy l’Etoile, France) to scavenge free oxygen. For
the Tau treated group, Tau was added to these cultures (final
concentration 40 nM) and allowed to incubate for 3 h before hypoxia
treatment was commenced. The myocardial cells were digested and
collected by centrifuging. The myocardial cell single cell
suspension was stained with an apoptosis assay kit (Annexin V-FITC
and propidium iodide, KeyGene Biotech, Wageningen, the Netherlands)
at room temperature for 15 min. The sample was then evaluated by
flow cytometry (BD FACS Calibur) and analyzed using CellQuest (BD,
version 5.1).
Statistical analysis
Statistical analysis was performed using the SPSS
statistical program (version 11.0, SPSS Inc., Chicago, IL, USA).
All values were expressed as the mean ± standard deviation (SD).
Differences between groups were evaluated using a one-factor ANOVA
test and P<0.05 was considered to indicate a statistically
significant difference.
Results
LAD ligation model and HRMAS
1H NMR spectroscopy
TTC and Evans Blue double staining were performed on
excised hearts obtained from the 6 h group. The infarct area (TTC
unstained), risk area (Evans Blue unstained) and non-ischemic area
(Evans Blue stained) are shown in Fig.
1A. The result indicated that the LAD ligation model
effectively mimicked myocardial infarction.
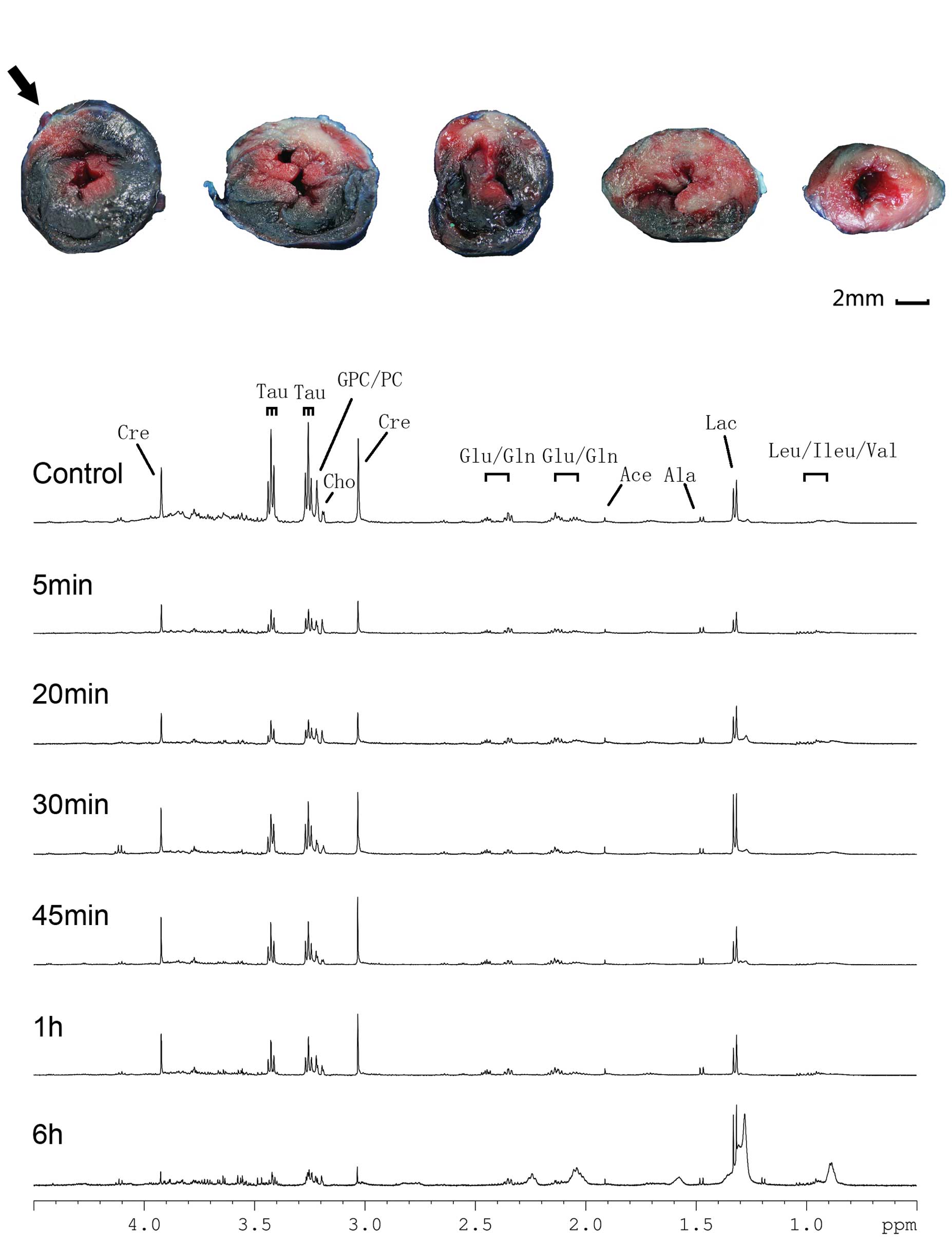 | Figure 1.TTC/Evans Blue staining and HRMAS
1H CPMG NMR spectra of the infarcted myocardium at
various time points. (A) Five rat heart slices from the anatomical
top to bottom are shown from left to right; infarct area (white),
risk area (unstained) and non-ischemic area (blue) are shown and
the ligation site is indicated by an arrow (bar = 2 mm). (B) Peak
assignments are as follows: Cre, creatine; Tau, taurine; GPC/PC,
glyceryl phosphorylcholine/phosphorylcholine; Cho, choline;
Glu/Gln, glutamate/glutamine; Ace, acetone; Ala, alanine; Lac,
lactate; and Leu/Ileu/Val, leucine/isoleucine/valine. TTC,
triphenyltetrazolium chloride; HRMAS, high-resolution magic angle
spinning; CPMG, Carr Purcell Meiboom Gill; NMR, nuclear magnetic
resonance. |
Representative 1H CPMG NMR spectra of the
control, 5 min, 20 min, 30 min, 45 min, 1 h and 6 h groups
following LAD ligation are shown in Fig. 1B. The main metabolites, including
leucine (Leu), isoleucine (Ileu), valine (Val), Lac, alanine (Ala),
glutamate (Glu), glutamine (Gln), Cre, Cho, phosphorylcholine (PC),
glyceryl phosphorylcholine (GPC) and Tau were assigned. The
Leu/Ileu/Val and Lac levels increased following LAD ligation,
whereas the Tau, Cho and PC/GPC levels decreased in a time
dependent manner compared with those in the control group,.
Tau decreased most notably among the
potential markers ex vivo
The data acquired from the 1H CPMG NMR
spectra were analyzed and metabolites were assigned. The levels of
potential markers, including Tau, Cre and Cho, are shown in
Fig. 2A. The Tau, Cre and Cho
levels declined significantly in the 5 min ischemia group compared
with the controls (P<0.05). In the 20 min to 6 h groups, Tau,
Cre and Cho remained at low levels, while Lac accumulated from 1 to
6 h. At 5 min after ischemia, the relative Tau level decreased from
20.27±6.48 to 8.81±0.04 (56.5%); this reduction was greater than
that of Cre (48.5%) or Cho (47.7%).
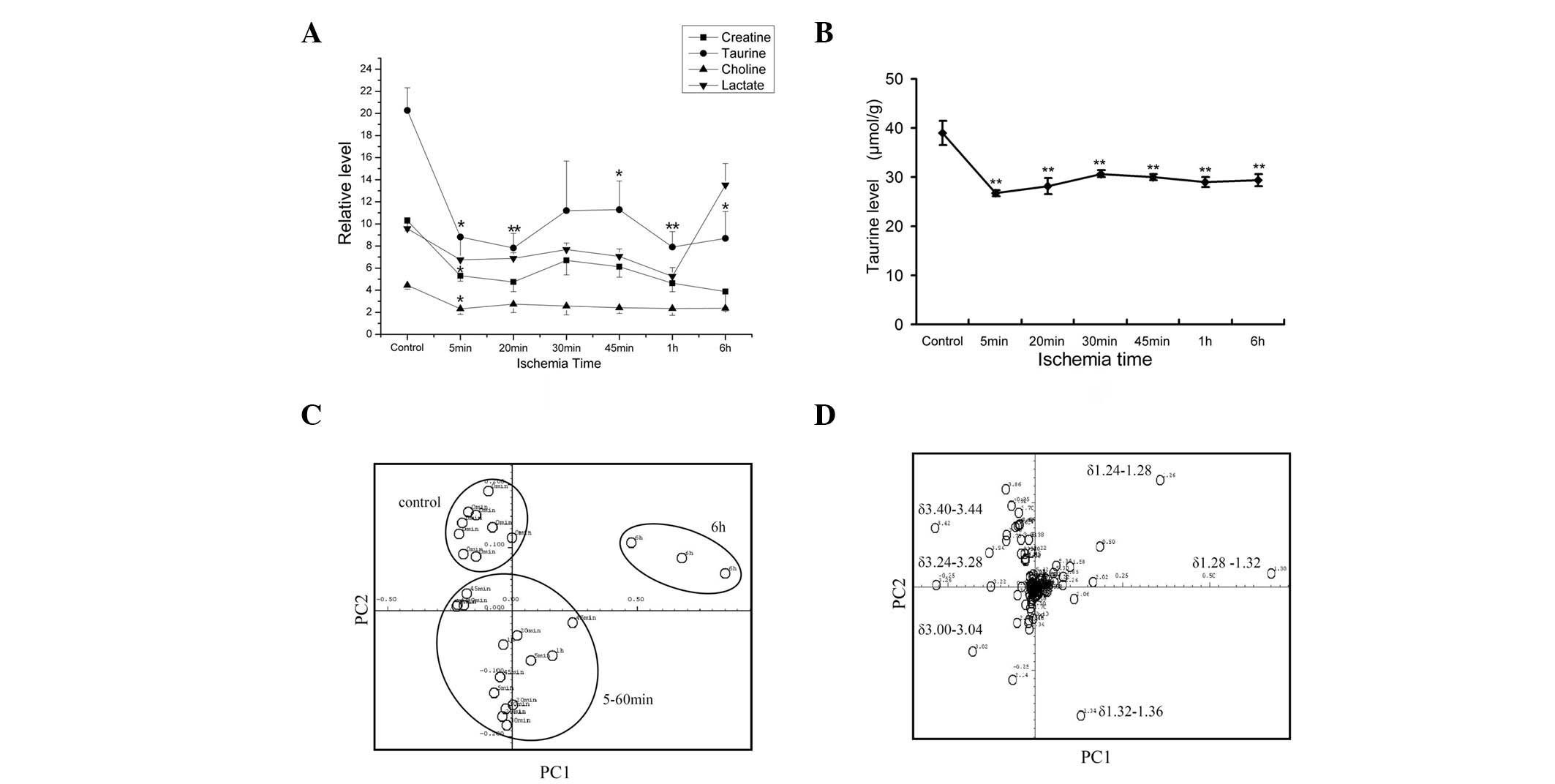 | Figure 2.Taurine decreased most notably of the
potential markers ex vivo. (A) Levels of creatine, taurine,
choline and lactate over ischemia time; the y-axis shows the
relative level adjusted by alanine level; all statistical
significances were tested in comparison with the control.
*P<0.05, **P<0.01 vs. 0 min, n≥3. (B)
Taurine was derivatized by OPA and detected by HPLC; L-norvaline
was used as the inner control; conditions as described in Materials
and methods; *P<0.05, **P< 0.01 vs. 0
min, n≥3 (C) Score plot of the first (PC1) and second (PC2)
principal components (horizontal and vertical axis, respectively)
of the 1H NMR spectra. Samples were divided into three
groups: a control group, a 5–60 min group and a 6 h group. (D) The
corresponding loading plot of the PC1 and second PC2 principal
components (horizontal and vertical axis, respectively) of the
1H NMR spectra. Bands contributing the most to the
distinction between the groups are shown. OPA, O-phthalaldehyde;
HPLC, high-performance liquid chromatography; NMR, nuclear magnetic
resonance. |
PCA was applied to all the CPMG 1H NMR
spectra results (Fig. 2C and D).
In the score plot (Fig. 2C), the
control group showed intra-group similarity. Samples from the 5 min
to 1 h groups clustered together, while the 6 h group formed
another cluster. The three clusters were separated from each
other.
The corresponding loading plot (Fig. 2D) revealed that lipids (δ1.24–1.28
and δ1.28–1.32), Lac (δ1.32–1.36), Cr (δ3.00–3.04), PC/GPC
(δ3.24–3.28) and Tau (δ3.24–3.28 and δ3.40–3.44) contributed the
most to the separation. The lipids mainly contributed to the
identification of the 6 h group. In the area of small molecule
metabolites (δ3.00–3.44), Tau contributed the most to
distinguishing the ischemia group from the control group. The PCA
result was in accordance with the CPMG 1H NMR spectral
results.
The myocardial tissue Tau level was confirmed by
HPLC (Fig. 2B). Consistent with
the results of the spectroscopy, the Tau level decreased
significantly within 5 min when compared with the control group
(P<0.01). No further changes were observed after 5 min.
These results revealed that, within potential
metabolic markers, Tau experienced the largest reduction in its
levels within the first 5 min. Tau contributed the most to
distinguishing the ischemia group from the control group. The NMR
ex vivo detection was confirmed by HPLC.
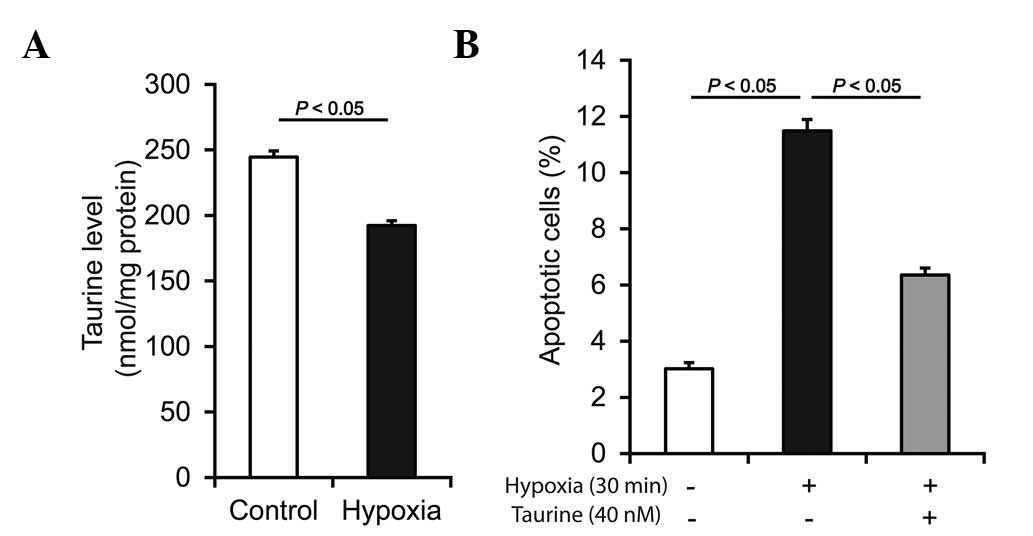
Tau decreases in vitro and shows
protective effects in hypoxia
To confirm the findings, the Tau level was detected
in vitro. Myocardial infarction may be described as oxygen
and glucose deprivation. As shown in Fig. 3A, following hypoxia injury, the Tau
level decreased significantly in the H9c2 rat cardiomyoblast cell
line. In accordance with the ex vivo data, the results
showed that the Tau level also decreased in vitro. Moreover,
hypoxia increased H9c2 cell apoptosis in vitro, while Tau
demonstrated a protective effect against hypoxia-induced myocardial
cell apoptosis (Fig. 3B). This
result suggests that, besides being a potential indicator of
ischemia, Tau may also have therapeutic potential, which is of
greater clinical importance.
Discussion
The HRMAS 1H NMR technique is a
developing technique in NMR spectroscopy. This approach requires
minimal sample preparation and, unlike conventional spectroscopy of
tissue extracts, enables aqueous and lipid-soluble metabolites to
be observed simultaneously in situ. Therefore, HRMAS
1H NMR is an efficient method for studying various
tissue abnormalities.
Tau is a sulfur-containing β-amino acid present at a
high concentration in the myocardium. It has been widely used as a
nutritional supplement and therapeutic tool for cardiovascular
diseases, including congestive heart failure, myocardial infarction
and hypertension (20,21).
In the present study, ischemic myocardial tissue
samples from various time points were used for HRMAS 1H
NMR detection. It has been reported that Cre levels decreased
significantly following LAD ligation in a swine model (5). The present study showed that in the
rat LAD ligation model, Tau and Cre levels decreased significantly
within 5 min compared with those in the control group. This result
indicates that Tau may be an indicator of early heart ischemia as
well as Cre. Cardiac tissue lacks a Tau synthesis mechanism and the
myocardial Tau level is Tau transporter-dependent. Therefore, the
rapid decline of Tau levels may reflect myocardial ischemia.
At present, troponin (troponins T, I and C) is used
as the golden standard in the clinical diagnosis of myocardial
infarction. Troponins T and I have distinct isoforms that exist in
skeletal and cardiac muscle. The release of these proteins from
necrotic cardiomyocytes into the bloodstream accounts for their
utility as biomarkers of acute coronary syndromes (33). However, irreversible changes in the
myocardium occur at 6 h postischemia, while troponin levels
increase at 4–8 h postischemia and reach a peak at 12–48 h
(34). The levels of a novel
diagnostic indicator, heart type fatty acid binding protein
(H-FABP), increase at 1–3 h postischemia (35,36).
However, the change in the Tau level may be detected in the
ischemic zone at 5 min after LAD ligation, which is significantly
earlier compared with the detection of increased troponin or H-FABP
levels. With regard to specificity, troponin exists solely in the
myocardium, while Tau is present at high levels in the myocardium
and skeletal muscle. However, MRS detection would be able to focus
on myocardial tissue in vivo specifically and non-invasively
if improved algorithms were developed to eliminate cardiac and
respiratory motion. In the present ex vivo study, ischemic
tissue was excised and detected by HRMAS 1H NMR. Further
investigation of Tau detection in vivo is expected.
It has previously been shown that Cre levels,
belonging to the myocardial energy index, are related to myocardial
functional performance under ischemic conditions in animal hearts
in vivo(37). In
31P-MRS, PCr and ATP represent the level of myocardial
energy. A reduced PCr/ATP ratio indicates cardiac failure and is a
predictor of mortality. A decrease in total Cre is an early signal
of heart failure (38). Total Cre
may serve as a compensatory mechanism for minimizing the reduction
of the total purine pool in the failing heart and reflects changes
in myocardial function (12). In
acute myocardial ischemia, which may lead to heart failure
(39), energy loss appears
significantly earlier than functional changes. The present data
showed that Tau and Cre levels decreased in the early period of
ischemia. The association of Tau with energy consumption in the
myocardium merits further investigation.
Clinically, myocardial ischemia may also be detected
by MRI (40). However, MRS
provides mostly chemical composite information which is useful for
research and diagnosis. It is possible that MRI and MRS may
interface more often within cardiovascular magnetic resonance
studies. Tau may serve as an indicator of early myocardial
ischemia. This also raises an interesting question as to whether
the Tau metabolism in the heart tissue as well as association of
Tau with energy metabolism should also be studied.
In conclusion, by detecting metabolite changes using
HRMAS 1H NMR, it was observed that Tau levels decreased
significantly within 5 min after ischemia in myocardial tissue.
These results suggest that the Tau level is a potential indicator
of early myocardial ischemia in cardiovascular magnetic resonance
studies.
Acknowledgements
This study was supported by the
Science and Technology Commission of Shanghai Municipality (Grant
No.04JC14027).
References
|
1.
|
Thom T, Haase N, Rosamond W, et al: Heart
disease and stroke statistics - 2006 update: a report from the
American Heart Association Statistics Committee and Stroke
Statistics Subcommittee. Circulation. 113:e85–e151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Ilva T, Lassus J, Siirilä-Waris K, et al:
Clinical significance of cardiac troponins I and T in acute heart
failure. Eur J Heart Fail. 10:772–779. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Bottomley PA and Weiss RG: Non-invasive
magnetic-resonance detection of creatine depletion in non-viable
infarcted myocardium. Lancet. 351:714–718. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Friess U and Stark M: Cardiac markers: a
clear cause for point-of-care testing. Anal Bioanal Chem.
393:1453–1462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Barba I, Jaimez-Auguets E,
Rodriguez-Sinovas A and Garcia-Dorado D: 1H NMR-based
metabolomic identification of at-risk areas after myocardial
infarction in swine. MAGMA. 20:265–271. 2007. View Article : Google Scholar
|
|
6.
|
Beer M, Buchner S, Sandstede J, et al:
(31)P-MR Spectroscopy for the evaluation of energy metabolism in
intact residual myocardium after acute myocardial infarction in
humans. MAGMA. 13:70–75. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Cousins JP: Clinical MR spectroscopy:
fundamentals, current applications, and future potential. AJR Am J
Roentgenol. 164:1337–1347. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Walecki J, Michalak MJ, Michalak E and
Pasowicz M: Use of magnetic resonance spectroscopy in cardiology.
State of the art. Przegl Lek. 59:601–605. 2002.(In Polish).
|
|
9.
|
Horn M: Cardiac magnetic resonance
spectroscopy: a window for studying physiology. Methods Mol Med.
124:225–248. 2006.PubMed/NCBI
|
|
10.
|
Hudsmith LE and Neubauer S: Magnetic
resonance spectroscopy in myocardial disease. JACC Cardiovasc
Imaging. 2:87–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Caus T, Kober F, Mouly-Bandini A, et al:
31P MRS of heart grafts provides metabolic markers of
early dysfunction. Eur J Cardiothorac Surg. 28:576–580. 2005.
View Article : Google Scholar
|
|
12.
|
Beer M, Seyfarth T, Sandstede J, et al:
Absolute concentrations of high-energy phosphate metabolites in
normal, hypertrophied, and failing human myocardium measured
noninvasively with 31P-SLOOP magnetic resonance spectroscopy. J Am
Coll Cardiol. 40:1267–1274. 2002. View Article : Google Scholar
|
|
13.
|
Holloway C, ten Hove M, Clarke K and
Neubauer S: MR spectroscopy in heart failure. Front Biosci (Schol
Ed). 3:331–340. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Garrod S, Humpfer E, Spraul M, et al:
High-resolution magic angle spinning 1H NMR
spectroscopic studies on intact rat renal cortex and medulla. Magn
Reson Med. 41:1108–1118. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Perrine SA, Michaels MS, Ghoddoussi F,
Hyde EM, Tancer ME and Galloway MP: Cardiac effects of MDMA on the
metabolic profile, determined with 1H-magnetic resonance
spectroscopy in the rat. NMR Biomed. 22:419–425. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Bollard ME, Murray AJ, Clarke K, Nicholson
JK and Griffin JL: A study of metabolic compartmentation in the rat
heart and cardiac mitochondria using high-resolution magic angle
spinning 1H NMR spectroscopy. FEBS Lett. 553:73–78.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Griffin JL, Sang E, Evens T, Davies K and
Clarke K: Metabolic profiles of dystrophin and utrophin expression
in mouse models of Duchenne muscular dystrophy. FEBS Lett.
530:109–116. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Jones GL, Sang E, Goddard C, et al: A
functional analysis of mouse models of cardiac disease through
metabolic profiling. J Biol Chem. 280:7530–7539. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Barba I, de León G, Martin E, et al:
Nuclear magnetic resonance-based metabolomics predicts
exercise-induced ischemia in patients with suspected coronary
artery disease. Magn Reson Med. 60:27–32. 2008. View Article : Google Scholar
|
|
20.
|
Gupta RC, Win T and Bittner S: Taurine
analogues; a new class of therapeutics: retrospect and prospects.
Curr Med Chem. 12:2021–2039. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Ueno T, Iguro Y, Yotsumoto G, et al:
Taurine at early reperfusion significantly reduces myocardial
damage and preserves cardiac function in the isolated rat heart.
Resuscitation. 73:287–295. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Oriyanhan W, Yamazaki K, Miwa S, Takaba K,
Ikeda T and Komeda M: Taurine prevents myocardial
ischemia/reperfusion-induced oxidative stress and apoptosis in
prolonged hypothermic rat heart preservation. Heart Vessels.
20:278–285. 2005. View Article : Google Scholar
|
|
23.
|
Hayes KC and Sturman JA: Taurine in
metabolism. Annu Rev Nutr. 1:401–425. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Warskulat U, Flögel U, Jacoby C, et al:
Taurine transporter knockout depletes muscle taurine levels and
results in severe skeletal muscle impairment but leaves cardiac
function uncompromised. FASEB J. 18:577–579. 2004.
|
|
25.
|
Heller-Stilb B, van Roeyen C, Rascher K,
et al: Disruption of the taurine transporter gene (taut) leads to
retinal degeneration in mice. FASEB J. 16:231–233. 2002.PubMed/NCBI
|
|
26.
|
Liu X, Gu X, Li Z, et al:
Neuregulin-1/erbB-activation improves cardiac function and survival
in models of ischemic, dilated, and viral cardiomyopathy. J Am Coll
Cardiol. 48:1438–1447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Garcia-Dorado D, González MA, Barrabés JA,
et al: Prevention of ischemic rigor contracture during coronary
occlusion by inhibition of Na+-H+ exchange.
Cardiovasc Res. 35:80–89. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Griffin JL, Mann CJ, Scott J, Shoulders CC
and Nicholson JK: Choline containing metabolites during cell
transfection: an insight into magnetic resonance spectroscopy
detectable changes. FEBS Lett. 509:263–266. 2001. View Article : Google Scholar
|
|
29.
|
Holmes E, Nicholls AW, Lindon JC, et al:
Chemometric models for toxicity classification based on NMR spectra
of biofluids. Chem Res Toxicol. 13:471–478. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Lindon JC, Holmes E and Nicholson JK:
Pattern recognition methods and applications in biomedical magnetic
resonance. Prog Nucl Magn Reson Spectrosc. 39:1–40. 2001.
View Article : Google Scholar
|
|
31.
|
Rowley HL, Martin KF and Marsden CA:
Determination of in vivo amino acid neurotransmitters by
high-performance liquid chromatography with
o-phthalaldehyde-sulphite derivatisation. J Neurosci Methods.
57:93–99. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Zhu W, Chen J, Cong X, Hu S and Chen X:
Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem
cells. Stem Cells. 24:416–425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Wang TJ: Significance of circulating
troponins in heart failure: if these walls could talk. Circulation.
116:1217–1220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Collinson PO, Boa FG and Gaze DC:
Measurement of cardiac troponins. Ann Clin Biochem. 38:423–449.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Chan CP, Sanderson JE, Glatz JF, Cheng WS,
Hempel A and Renneberg R: A superior early myocardial infarction
marker. Human heart-type fatty acid-binding protein. Z Kardiol.
93:388–397. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Seino Y, Tomita Y, Takano T and Ohbayashi
K; Tokyo Rapid-Test Office Cardiologists (Tokyo-ROC) Study: Office
cardiologists cooperative study on whole blood rapid panel tests in
patients with suspicious acute myocardial infarction: comparison
between heart-type fatty acid-binding protein and troponin T tests.
Circ J. 68:144–148. 2004. View Article : Google Scholar
|
|
37.
|
Schaefer S, Schwartz GG, Gober JR, et al:
Relationship between myocardial metabolites and contractile
abnormalities during graded regional ischemia. Phosphorus-31
nuclear magnetic resonance studies of porcine myocardium in vivo. J
Clin Invest. 85:706–713. 1990. View Article : Google Scholar
|
|
38.
|
Shen W, Asai K, Uechi M, et al:
Progressive loss of myocardial ATP due to a loss of total purines
during the development of heart failure in dogs: a compensatory
role for the parallel loss of creatine. Circulation. 100:2113–2118.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Van de Werf F, Bax J, Betriu A, et al:
Management of acute myocardial infarction in patients presenting
with persistent ST-segment elevation: the Task Force on the
Management of ST-Segment Elevation Acute Myocardial Infarction of
the European Society of Cardiology. Eur Heart J. 29:2909–2945.
2008.
|
|
40.
|
Mahrholdt H, Klem I and Sechtem U:
Cardiovascular MRI for detection of myocardial viability and
ischaemia. Heart. 93:122–129. 2007. View Article : Google Scholar : PubMed/NCBI
|