Introduction
It is currently unknown whether the distance of
mesorectal extension (DME) is applicable as a parameter for
adjuvant treatment and is associated with the prognosis of rectal
cancer. In 1990, the clinical importance of subdividing the
mesorectal extension at a cut-off point of 4 mm was advocated
(1). In 1993, the International
Union Against Cancer (UICC) proposed optional subdivisions for pT3
and pT4 tumors (2). Thereafter,
several studies have shown the prognostic heterogeneity of pT3
rectal cancers (3–12). However, appropriate treatment
strategy for T3/pT3 rectal cancer based on the DME remains unclear.
In European countries, the standard strategy for T3 rectal cancer
is preoperative chemoradiotherapy (CRT) (13,14).
However, not all T3 rectal cancers are necessarily suitable for
CRT. Moreover, it is considerably difficult to evaluate not only
DME but also conventional prognostic factors such as lymphatic,
venous and perineural invasion in pathological specimens following
preoperative CRT. When preoperative CRT is not administered to
certain patients with T3 rectal cancers, it appears to be vital to
accurately assess the DME and to evaluate the prognosis following
surgery. In the current study, we analyzed a large collection of
data obtained from a multi-institutional study promoted by the
Japanese Society for Cancer of the Colon and Rectum (JSCCR). This
study confirms the benefit of the measurement of mesorectal
extension and selection of patients for postoperative adjuvant
treatment strategy in pT3N1-2 rectal cancers based on the TNM
classification (6th edition) (15,16).
Patients and methods
Patients
Approval from the ethics committees of both the
JSCCR and the local Institutional Review Board were obtained in
order to review medical records and to permit follow-up patient
contact. However, informed consent could not be obtained from all
patients, since this study was retrospective and some patients may
be deceased. Data were obtained from 1009 patients with pT3 rectal
cancer from 28 institutes that are members of the Study Group of
the JSCCR on Extramural Mesorectal Extension of Rectal Cancer. All
patients had a primary rectal adenocarcinoma located in the middle
or lower rectum. Patients with rectosigmoid colon cancer were not
included in this study. Histologically defined curative surgery was
performed between 1995 and 1999. Patients undergoing non-curative
surgery (R2 operation) were excluded from this study. Patients were
staged according to the pathological TNM classification (6th
edition) (15,16). The present study focused on
postoperative treatment strategy in patients with Stage III
(pT3N1-2) disease. After staging, 512 patients with Stage III
disease remained enrolled in this study, including 321 with Stage
IIIB and 191 with Stage IIIC diseases. Clinicopathological
information was available and eligible for analysis. Neither
radiotherapy nor neoadjuvant chemotherapy was performed prior to
surgery in these enrolled patients. All 512 patients underwent
total mesorectal excision. According to the postoperative adjuvant
treatment protocol of each institute, peroral 5-fluouracil
(5-Fu)-based chemotherapy, such as 5′-DFUR (doxifluridine), HCFU
(1-hexylcarbamoyl-5-fluorouracil), or UFT (tegafur-uracil) were
most frequently administrated. Clinicopathological data and
follow-up system were based on the Japanese rules defined by the
JSCCR (17). The follow-up system
consisted of the measurement of serum tumor markers, chest X-ray
and abdominal ultrasound examination every three months for the
first three years, and then every six months for the next two
years. When the development of recurrence was suspected by serum
tumor markers, digital examination and/or ultrasonography, the
final diagnosis was carried out using rectoscopy, computed
tomography (CT) and/or magnetic resonance imaging (MRI) and other
diagnostic tools. Local recurrence was defined as the presence of a
radiologically confirmed or histologically proven tumor
non-hematogenously occurring in the pelvis within the field of the
initial surgery. Distant metastasis included hematogenous
metastases to the liver, lung, bone, brain, kidney or other organs.
The other-organ recurrences were defined as recurrence other than
local recurrence or distant metastasis, i.e., peritoneal
dissemination, intra-abdominal, para-aortic, subclavicular,
mediastinal and inguinal lymph node metastases. The outcomes of all
patients were investigated in detail. From January 1995, eligible
surviving patients were followed for a median of 86 months (range,
1–166).
Measurement of mesorectal extension
All surgically resected specimens were opened along
the anti-tumor or anti-mesenteric side. Specimens were fixed in 20%
formalin for at least 48 h after being pinned to a wood or cork
board. One or more longitudinal sections of the tumor were sliced
at the point of maximum extramural invasion and were embedded in
paraffin after being divided into blocks of suitable size. These
tissue blocks were then routinely processed for hematoxylin and
eosin and Elastica Von Gieson staining. Tumor category pT3 sections
were subdivided based on the histological measurement of the
maximum depth of invasion beyond the outer border of the muscular
layer (in mm). Without any knowledge of clinical information,
histological measurement was performed according to our previous
methods (18). When the outer
border of the muscular layer was completely identifiable (sometimes
identifiable as fragments of muscle), the distance from the outer
border of the muscular layer to the deepest part of the invasion
was measured. When the outer border of the muscular layer was not
entirely identifiable, due to destruction by the invasion or
excessive inflammatory reaction, an estimate of the outer border
was obtained by drawing a straight solid line between both break
points in the muscular layer.
Statistical analysis
Statistical analysis was performed using StatView
5.0 and JMP 8.0 (SAS Institute, Inc., Cary, NC, USA) software for
Windows. All clinicopathological independent variables (13 items)
were coded for analysis. Overall recurrence, distant metastasis,
local recurrence and survival were coded as dependent variables.
Cox regression analyses were used to determine the optimal cut-off
point of the mesorectal extension for postoperative recurrence. The
Cox regression analysis was also used to estimate the independent
risk factors for either distant metastasis or local recurrence. The
Kaplan-Meier method and the log-rank test were used for calculating
survival rates. Statistical significance was determined at
p<0.05 and the confidence interval (CI) was determined at
95%.
Results
Distance of ME
The DME in these 512 cases (pT3N1-2 tumor) was
measured histologically. The mean DME was 5.4±4.4 mm, and the
median DME was 4.3 mm (range, 0.1–28.4).
Postoperative recurrence pattern
Postoperative overall recurrence occurred in 247
(48.2%) of the 521 patients. A total of 55 patients (10.7%) had
local recurrence only, and 124 (24.2%) had distant metastasis only.
Furthermore, 30 patients (5.9%) had both local and distant
recurrences. The remaining 38 patients exhibited other recurrences,
that is, peritoneal dissemination, intra-abdominal, para-aortic,
subclavicular, mediastinal and inguinal lymph node metastases.
Cut-off point for subdividing mesorectal
extension
The multivariate Cox regression analyses for
recurrence-free survival are shown in Table I. A cut-off value of 4 mm showed
the highest Chi-square (17.463), lowest p-value (p=0.00003), and
high hazard ratio (HR, 1.72). The L/U ratio (lower/upper limits of
CI) showed higher reliability (0.5950) among other cut-off points.
A cut-off value of 4 mm was found to be the best predictor of
recurrence-free survival. Overall, the best cut-off point was
determined to be 4 mm, therefore, the patients were divided into
two groups according to mesorectal extension: ≤4 mm and >4
mm.
 | Table I.Cut-off points of distance of
mesorectal extension for recurrence-free survival using
multivariate Cox regression analysis. |
Table I.
Cut-off points of distance of
mesorectal extension for recurrence-free survival using
multivariate Cox regression analysis.
| DME (mm) | No. of patients | RF survival at 5
years (%) | Chi-square | HR (95% CI, L-U) | L/U ratio | p-value |
|---|
| >1 vs. ≤1 | 445 vs. 67 | 52 vs. 64 | 4.174 | 1.53
(1.012–2.317) | 0.4368 | 0.0411 |
| >2 vs. ≤2 | 391 vs. 121 | 51 vs. 65 | 10.366 | 1.70
(1.224–2.370) | 0.5165 | 0.0013 |
| >3 vs. ≤3 | 330 vs. 182 | 48 vs. 65 | 14.423 | 1.71
(1.290–2.270) | 0.5683 | 0.0001 |
| >4 vs. ≤4 | 267 vs. 245 | 46 vs. 63 | 17.463 | 1.72
(1.328–2.232) | 0.5950 | 0.00003 |
| >5 vs. ≤5 | 204 vs. 308 | 44 vs. 60 | 16.331 | 1.67
(1.297–2.155) | 0.6019 | 0.00005 |
| >6 vs. ≤6 | 167 vs. 345 | 46 vs. 58 | 11.059 | 155
(1.191–2.006) | 0.5937 | 0.0009 |
| >7 vs. ≤7 | 135 vs. 377 | 43 vs. 58 | 13.061 | 1.63
(1.246–2.140) | 0.5822 | 0.0003 |
| >8 vs. ≤8 | 98 vs. 414 | 39 vs. 58 | 16.071 | 1.80
(1.341–2.407) | 0.5572 | 0.00006 |
| >9 vs. ≤9 | 79 vs. 433 | 39 vs. 57 | 12.495 | 1.74
(1.273–2.386) | 0.5335 | 0.0004 |
| >10 vs. ≤10 | 59 vs. 453 | 39 vs. 56 | 11.980 | 1.82
(1.287–2.575) | 0.4998 | 0.0005 |
Independent risk factors for distant
metastasis and local recurrence
Distant and/or local recurrence-related independent
variables used for analyses are listed in Table II. Multivariate Cox regression
analysis showed that the DME was a powerful independent risk factor
for distant metastasis (HR, 1.82; 95% CI, 1.300–2.538; p= 0.0005)
and for local recurrence (HR, 1.74; 95% CI, 1.107–2.744;
p=0.0164).
 | Table II.Independent risk factors for distant
metastasis and local recurrence using multivariate Cox regression
analysis. |
Table II.
Independent risk factors for distant
metastasis and local recurrence using multivariate Cox regression
analysis.
| Variable | Distant metastasis
| Local recurrence
|
|---|
| Rate of DM (%) | HR (95% CI) | p-value | Rate of LR | HR (95% CI) | p-value |
|---|
| Gender | 28 vs. 31 | n.a. | | 15 vs. 16 | n.a. | |
| Male vs.
female | | | | | | |
| Size of tumor | 26 vs. 31 | n.a. | | 15 vs. 16 | n.a. | |
| >5 vs. ≤5
cm | | | | | | |
| Location of
tumor | 31 vs. 24 | 1.28
(0.845–1.947) | 0.2425 | 11 vs. 18 | 1.44
(0.784–2.629) | 0.2411 |
| Lower vs.
middle | | | | | | |
| Gross type | 27 vs. 29 | n.a. | | 20 vs. 15 | n.a. | |
| Infiltrative vs.
expansive | | | | | | |
| Histology | 30 vs. 27 | n.a. | | 15 vs. 16 | n.a. | |
| Others vs.
well | | | | | | |
| Lymphatic
invasion | 30 vs. 28 | n.a. | | 17 vs. 14 | n.a. | |
| ly2–3 vs.
ly0–1 | | | | | | |
| Venous
invasion | 29 vs. 29 | n.a. | | 14 vs. 17 | n.a. | |
| v2–3 vs.
v0–1 | | | | | | |
| DME | 34 vs. 24 | 1.82
(1.300–2.538) | 0.0005 | 18 vs. 13 | 1.74
(1.107–2.744) | 0.0164 |
| >4 vs. ≤4
mm | | | | | | |
| CRM | 28 vs. 29 | n.a. | | 14 vs. 16 | n.a. | |
| ≤1 vs. >1
mm | | | | | | |
| Number of retrieved
LN | 25 vs. 29 | n.a. | | 14 vs. 15 | n.a. | |
| <12 vs.
≥12 | | | | | | |
| Operative
methods | 34 vs. 25 | 1.50
(1.025–2.197) | 0.0370 | 11 vs. 20 | 1.97
(1.160–3.339) | 0.0121 |
| APR vs. SSO | | | | | | |
| Autonomic
nerve-saving | 29 vs. 26 | n.a. | | 16 vs. 13 | n.a. | |
| Yes vs. no | | | | | | |
| Chemotherapy | 27 vs. 31 | n.a. | | 17 vs. 14 | n.a. | |
| Yes vs. no | | | | | | |
Distant metastasis and local recurrence
based on the cut-off value
Stage-specific distant metastasis and local
recurrence occurred in 86 (26.8%) and 40 patients (12.5%),
respectively, at Stage IIIB, and 68 (35.6%) and 45 patients
(23.6%), respectively, at Stage IIIC (Table III). Taking into account the
cut-off value of 4 mm, the rates of distant metastasis at IIIB and
IIIC were significantly higher (32.1 and 41.9%, respectively) in
patients with DME >4 mm compared to patients with DME ≤4 mm
(21.4 and 27.9%, respectively). Local recurrence showed a trend
toward a higher rate at the cut-off value at any Stage.
 | Table III.Distant metastasis and local
recurrence at the cut-off value of 4 mm using Cox regression
analysis. |
Table III.
Distant metastasis and local
recurrence at the cut-off value of 4 mm using Cox regression
analysis.
| Distant metastasis
| Local recurrence
|
|---|
| TNM Stage (6th
edition) | No. of DM patients
(%) | HR (95% CI) | p-value | No. of LR patients
(%) | HR (95% CI) | p-value |
|---|
| Stage IIIB
(n=321) | 86 (26.8) | | | 40 (12.5) | | |
| ≤4 mm
(n=159) | 34 (21.4) | 1 | | 16 (10.1) | 1 | |
| >4 mm
(n=162) | 52 (32.1) | 1.79
(1.154–2.773) | 0.0094 | 24 (14.8) | 1.66
(0.878–3.151) | 0.1186 |
| Stage IIIC
(n=191) | 68 (35.6) | | | 45 (23.6) | | |
| ≤4 mm (n=86) | 24 (27.9) | 1 | | 16 (18.6) | 1 | |
| >4 mm
(n=105) | 44 (41.9) | 1.82
(1.106–3.008) | 0.0186 | 29 (27.6) | 1.79
(0.964–3.331) | 0.0652 |
| Overall
(n=512) | 154 (30.0) | | | 85 (16.6) | | |
| ≤4 mm
(n=245) | 58 (23.7) | 1 | | 32 (13.1) | 1 | |
| >4 mm
(n=267) | 96 (36.0) | 1.83
(1.314–2.541) | 0.0003 | 53 (19.9) | 1.75
(1.125–2.736) | 0.0132 |
Recurrence-free and cancer-specific
survival rates
The recurrence-free 5-year survival rates of the DME
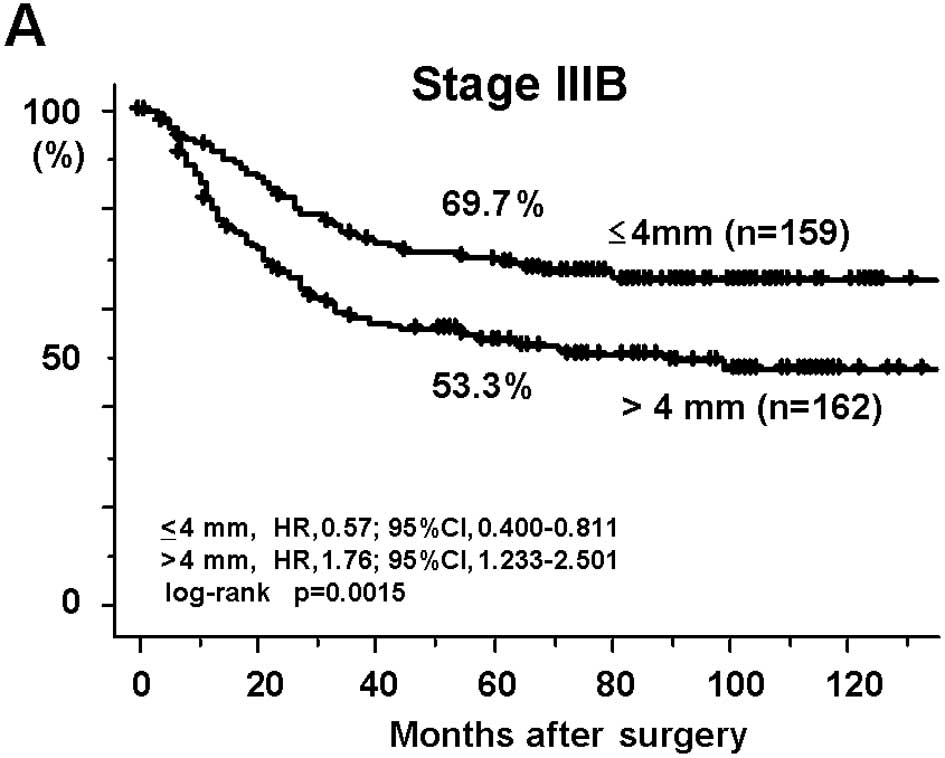
>4 mm group were significantly worse [53.3% at Stage IIIB (HR,
1.76; 95% CI, 1.233–2.501; p=0.0015) and 32.9% at Stage IIIC (HR,
1.64; 95% CI, 1.119–2.407; p=0.0095)] than those of the patients
with a DME ≤4 mm (69.7 and 50.4%, respectively; Fig. 1A and B). The cancer-specific 5-year
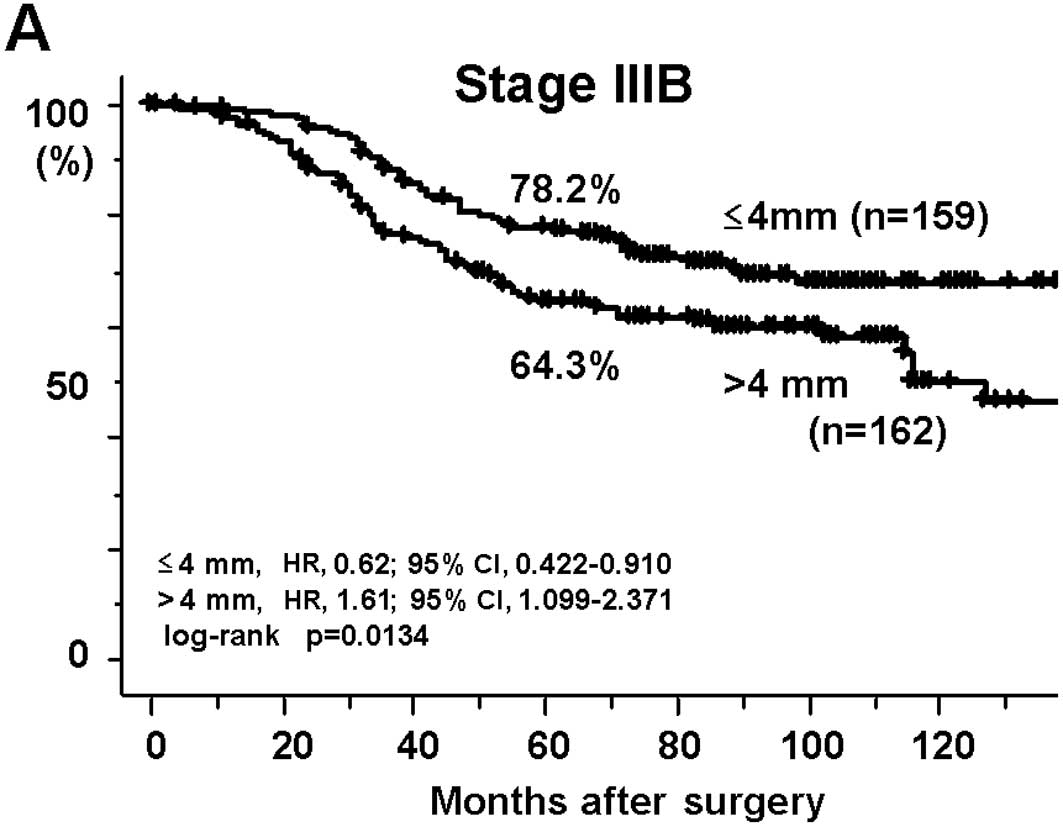
survival rates of the DME >4 mm group were also significantly
worse at Stage IIIB (64.3%; HR, 1.61; 95% CI, 1.099–2.371; p=
0.0134) and at Stage IIIC (42.6%; HR, 1.93; 95% CI, 1.288–2.901;
p=0.0011) than those of patients with a DME ≤4 mm (78.2 and 65.9%,
respectively; Fig. 2A and B).
Discussion
In the early 1990s, the clinical importance of
subdividing the mesorectal extension for pT3 and pT4 tumors was
advocated (1,2). Thereafter, the importance was
reported by several authors, who showed the prognostic
heterogeneity of pT3 rectal cancers (1,3,5–11).
At a cut-off point of 4 mm, the DME >4 mm was confirmed as an
independent adverse prognostic factor for survival using
multivariate analysis (1,7,8).
Other authors found prognostic heterogeneity of N1-2 tumors between
pT3a (≤5 mm) and pT3b (>5 mm) groups (4) and reported prognostic heterogeneity
of pT3N1-2 tumors at the cut-off point of 6 mm from two different
patient databases (6). Thus, the
majority of studies found prognostic heterogeneity of mesorectal
extension in pT3 rectal cancers at various cut-off points. However,
the clinical significance and statistical appropriateness of these
cut-off points remain controversial, partly because these studies
had small sample sizes with underpowered statistical analyses and
included cohorts from only a single institution. Based on the
statistical analyses in the present study, the appropriate
prognostic cut-off point was theoretically set at a value of 4 mm,
and the patients were divided into two groups based on mesorectal
extension: DME ≤4 mm and DME >4 mm. A recent multi-institutional
study by our group demonstrated that a cut-off point of 4 mm
independently delineated adverse prognosis among pT3N0 rectal
cancers based on TNM classification (6th edition) (18). However, the appropriate
postoperative treatment strategy for pT3N1-2 rectal cancers based
on the DME remains unclear.
Dutch and Swedish trials have reported that
preoperative CRT has decreased local recurrence rate to 15% in
Stage III rectal cancers (13,14),
which is similar to our data without using preoperative CRT.
However, there have been only a few reports on the correlation of
DME and local recurrence. Merkel et al(4) reported that the local recurrence rate
was significantly higher in pT3b tumors with DME >5 mm (N1–2;
34.0%) compared with pT3a tumors ≤5 mm (N1–2; 17.1%). Another study
did not find any correlation between local recurrence and DME
(6). Our data showed no
significant difference with regard to stage-specific local
recurrence at the cut-off point in any stage due to the small
number of patients. Overall, our study indicates that local
recurrence occurs at a high rate in Stage III patients with a DME
of >4 mm (p=0.0132, Table
III).
Multivariate Cox regression analysis showed that the
DME was an important parameter to predict distant and local
recurrences, and was more effective than conventional prognostic
parameters such as lymphatic invasion, venous invasion,
circumferential resection margin, and total number of retrieved
lymph nodes (Table II). As the DME
becomes deeper, it is considered that undetectable lymphovascular
invasions or micro-tumor deposits increase in the mesorectal
adipose tissues. These isolated tumor cells may cause local
recurrence and/or distant metastases. In European countries,
preoperative CRT is the standard strategy for selected patients
with T3 rectal cancer to eradicate those isolated tumor cells and
to control local recurrence. However, it is considerably difficult
to evaluate not only DME but also those pathological parameters
following preoperative CRT. The current study also determined that
the DME was a useful predictor to estimate survival rates (Figs. 1 and 2), which was similar to results reported
by other authors (4,6). When preoperative CRT is not applied
for some patients with pT3 rectal cancer, it appears to be vital to
accurately assess the DME and evaluate the prognosis following
surgery (3). In addition, the
present study supported the reproducibility of a cut-off point of 4
mm even in pT3N1–2 disease as in pT3N0 disease (TNM 6th edition)
(18).
Diagnostic techniques using MRI enable accurate
measurement of the mesorectal extension that strongly correlates
with the pathological measurement (19,20).
If the cut-off value can be applied to the preoperative MRI-based
diagnosis, then this would be more efficient for stratifying the
appropriate patients for preoperative CRT. In the present series
between 1995 and 1999, postoperative adjuvant chemotherapy was
administered orally under the criteria for each institute. More
intensive adjuvant treatments are required for patients with a DME
of >4 mm to eradicate isolated tumor cells, prevent
postoperative recurrence and improve survival.
In conclusion, a value of 4 mm provides the best
cut-off point for subdividing the mesorectal extension to predict
oncologic outcomes. The current study suggests that DME is a highly
beneficial parameter with which to stratify patients for
postoperative adjuvant treatments. However, further prospective
studies are required to assure the reproducibility and validity of
this cut-off point.
Acknowledgements
We are grateful to Kenta Murotani from
the Department of Biostatistics, Kurume University Graduate School
of Medicine, for the help with the statistical analyses. We also
thank the following surgeons and pathologists: Koya Hida, Division
of Gastrointestinal Surgery, Department of Surgery, Graduate School
of Medicine, Kyoto University; Toru Inoue, Department of Surgical
Oncology, Osaka City University Graduate School of Medicine;
Tomohisa Furuhata, First Department of Surgery, Sapporo Medical
University; Tatsuro Yamaguchi, Tokyo Metropolitan Cancer and
Infectious Diseases Center, Komagome Hospital; Tetsuro Higuchi,
Department of Surgical Oncology, Graduate School, Tokyo Medical and
Dental University; Tomoaki Mizobe, Department of Surgery, Kurume
University School of Medicine; Yutaka Ogata, Department of Surgery,
Kurume University School of Medicine; Yutaka Kawamura, Department
of Surgery, Saitama Medical Center, Jichi Medical University;
Toshimasa Yatsuoka, Division of Gastroenterological Surgery,
Saitama Cancer Center; Seiichi Shinji, Department of Surgery,
Chiba-Hokusoh Hospital, Nippon Medical School; Kimihiko Funahashi,
Division of General and Gastroenterological Surgery, Department of
Surgery (Omori), Faculty of Medicine, Toho University; Kazuhiko
Yoshimatsu, Department of Surgery, Tokyo Women’s Medical
University, Medical Center East; Fumikazu Koyama, Department of
Surgery, Nara Medical University; Takanori Goi, First Department of
Surgery, Faculty of Medical Sciences, University of Fukui; Shingo
Kameoka, Department of Surgery II, Tokyo Women’s Medical
University; Wataru Onozato, Kitasato University School of Medicine;
Keiichiro Ishibashi, Department of Digestive Tract and General
Surgery, Saitama Medical Center, Saitama Medical University;
Yoshihiro Kakeji, Department of Surgery and Science, Graduate
School of Medical Sciences, Kyushu University; Akihiko Kataoka,
Department of General Surgery, Hokkaido University Graduate School
of Medicine; Yoshiro Kubo, Department of Surgery, National Hospital
Organization, Shikoku Cancer Center; Shunji Ogata, Coloproctology
Center, Takano Hospital; Mitsugu Sekimoto, Department of
Gastroenterological Surgery, Osaka University, Graduate School of
Medicine; Masaki Kitazono, Department of Surgical Oncology and
Digestive Surgery, Kagoshima University Graduate School of Medical
and Dental Sciences; Shinichiro Yoshitani, Department of Surgical
Oncology, Kanazawa Medical University; Takashi Yao, Department of
Human Pathology, Juntendo University School of Medicine; Michiyo
Higashi, Department of Human Pathology, Field of Oncology,
Kagoshima University Graduate School of Medical and Dental
Sciences; Hirokazu Fukui, Department of Surgical and Molecular
Pathology, Dokkyo Medical University; Yoichi Ajioka, Division of
Molecular and Diagnostic Pathology, Niigata University Graduate
School of Medicine and Dental Sciences; Tadakazu Shimoda, Center
for Cancer Control and Information Services, National Cancer Center
Hospital; Atsushi Ochiai, Pathology Division, Research Center for
Innovative Oncology, National Cancer Center Hospital East.
References
|
1.
|
Cawthorn SJ, Parums DV, Gibbs NM, et al:
Extent of mesorectal spread and involvement of lateral resection
margin as prognostic factors after surgery for rectal cancer.
Lancet. 1:1055–1059. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Hermanek P, Henson DE, Hutter RVP, et al:
TNM Supplement. A Commentary on Uniform Use. Springer; New York,
NY: 1993
|
|
3.
|
Willett CG, Badizadegan K, Ancukiewicz M
and Shellito PC: Prognostic factors in stage T3N0 rectal cancer: Do
all patients require postoperative pelvic irradiation and
chemotherapy? Dis Colon Rectum. 42:167–173. 1990. View Article : Google Scholar
|
|
4.
|
Merkel S, Mansmann U, Siassi M,
Papadopoulos T, Hohenberger W and Hermanek P: The prognostic
inhomogeneity in pT3 rectal carcinomas. Int J Colorectal Dis.
16:298–304. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Steel MC, Woods R, Mackay JM and Chen F:
Extent of mesorectal invasion is a prognostic indicator in T3
rectal carcinoma. ANZ J Surg. 72:483–487. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Miyoshi M, Ueno H, Hashiguchi Y, Mochizuki
H and Talbot IC: Extent of mesorectal tumor invasion as a
prognostic factor after curative surgery for T3 rectal cancer
patients. Ann Surg. 243:492–497. 2006. View Article : Google Scholar
|
|
7.
|
Katsumata D, Fukui H, Ono Y, et al: Depth
of tumor invasion in locally advanced rectal cancer correlates with
patients’ prognosis: the usefulness of elastic stain for its
measurement. Surg Today. 38:115–122. 2008.PubMed/NCBI
|
|
8.
|
Yoshida K, Yoshimatsu K, Otani T, Yokomizo
H and Ogawa K: The depth of invasion beyond the outer border of the
muscularis propria as a prognostic factor for T3
rectal/rectosigmoid cancer. Anticancer Res. 28:1773–1778.
2008.PubMed/NCBI
|
|
9.
|
Tokoro T, Okuno K, Hida J, Ishimaru E,
Ueda K and Yoshifuji T: Depth of mesorectal invasion has
prognositic significance in T3N0 low rectal cancer.
Hepatogastroenterology. 56:124–127. 2009.PubMed/NCBI
|
|
10.
|
Bori R, Sejben I, Svebis M, et al:
Heterogeneity of pT3 colorectal carcinomas according to the depth
of invasion. Pathol Oncol. 15:527–532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Pollheimer MJ, Kornprat P, Pollheimer VS,
et al: Clinical significance of pT sub-classification in surgical
pathology of colorectal cancer. Int J Colorectal Dis. 25:187–196.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Picon AI, Moore HG, Sternberg SS, et al:
Prognostic significance of depth of gross or microscopic perirectal
fat invasion in T3 N0 M0 rectal cancers following sharp mesorectal
excision and no adjuvant therapy. Int J Colorectal Dis. 18:487–492.
2003. View Article : Google Scholar
|
|
13.
|
Swedish Rectal Cancer Trial: Improved
survival with preoperative radiotherapy in resectable rectal
cancer. N Engl J Med. 336:980–987. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kapiteijn E, Marijnen CA, Nagtegaal ID,
Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B,
van Krieken JH, Leer JW and van de Velde CJ; Dutch Colorectal
Cancer Group: Preoperative radiotherapy combined with total
mesorectal excision for resectable rectal cancer. N Engl J Med.
345:638–646. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Sobin LH and Wittekind C: UICC TNM
Classification of Malignant Tumours. 6th edition. Wiley-Liss;
Hoboken, NJ: 2002
|
|
16.
|
Greene FL, Page DL, Fleming ID, et al:
AJCC Cancer Staging Manual. 6th Edition. Springer; New York, NY:
2002, View Article : Google Scholar
|
|
17.
|
Japanese Society for Cancer of the Colon
and Rectum (JSCCR): Japanese Classification of Colorectal
Carcinoma. 2nd English edition. Kanehara & Co., Ltd; Tokyo:
2009
|
|
18.
|
Shirouzu K, Akagi Y, Fujita S, et al:
Clinical significance of mesorectal extension of rectal cancer. A
Japanese multi-institutional study. Ann Surg. 253:704–710. 2011.
View Article : Google Scholar
|
|
19.
|
Brown G, Radcliffe AG, Newcombe RG,
Dallimore NS, Bourne MW and Williams GT: Preoperative assessment of
prognostic factors in rectal cancer using high-resolution magnetic
resonance imaging. Br J Surg. 90:355–364. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
MERCURY Study Group: Extramural depth of
tumor invasion at thin-section MR in patients with rectal cancer.
Results of the MERCURY study. Radiology. 243:132–139. 2007.
View Article : Google Scholar : PubMed/NCBI
|