Introduction
Renal carcinoma is the most common malignant tumor
of the kidney and accounts for ∼3% of all malignant tumors and 80%
of malignant kidney tumors. Among renal carcinomas, 79% are
classified as clear cell renal cell carcinomas (CCRCC) (1). However, little is known about the
molecular and cellular mechanisms involved in the development of
CCRCC.
Although there are various factors associated with
malignant aggressiveness, destruction of the extracellular matrix
(ECM) is one of the major early steps in a number of malignant
tumors. A major family of ECM-degrading enzymes involved in this
process is the matrix metalloproteinase (MMP) family, which plays
important roles in cancer invasion and metastasis (2,3). In
addition to their invasive function, MMPs are also associated with
cell proliferation and angiogenesis in various cancers (4–6).
Based on these features, a number of researchers are considering
the pathological significance of MMPs in cancer cells and the
potential of MMP inhibitors in the antitumor treatment of various
malignancies. Tissue inhibitors of matrix metalloproteinases
(TIMPs) are known to have the ability to inhibit the catalytic
activity of MMPs. It is thought that the balance between MMPs and
TIMPs determines the proteolytic activity in vivo(7,8). The
ratio of MMPs to TIMPs, which is required to be close to 1 to
neutralize enzymatic activity, means that small changes in MMP and
TIMP levels lead to biologically significant changes in net
proteolytic activity. If MMP expression increases and/or TIMP
expression decreases, the balance is greatly affected (9).
The expression of MMP-1, -2, -3, -9, -10 and -11, as
well as TIMP-1 and -2, has been analyzed in CCRCC (10–17).
However, little or no information concerning the association of MMP
with TIMP in human CCRCC tissues and the clinicopathological
significance of such expression on survival rate has been reported.
Moreover, the correlation between the balance of MMPs and TIMPs in
CCRCC and the clinicopathological characteristics and survival rate
using tissue microarrays have not been reported. In the present
study, we focused on the role of MMP-7 associated with TIMP-2 in
human CCRCC tissues to determine the correlation with
clinicopathological features and survival rate using tissue
microarray, immunohistochemistry and RT-PCR to evaluate the
clinical value of MMP-7 and TIMP-2 proteins in CCRCC.
Materials and methods
Materials and tissue microarray
Subjects diagnosed with CCRCC based on pathological
examination of patient tissues following radical surgery at Taizhou
Central Hospital of Taizhou Enze Medical Group and Taizhou Hospital
of Taizhou Enze Medical Group between January 1997 and December
2006 were selected. The patient population included 63 men and 35
women, with an average age of 55.16±10.40 years (range, 25–83
years). Cases were graded based on the 2004 World Health
Organization (WHO) pathological Fuhrman nuclear grading standards
(18). In all, 47 cases were
classified as grade I, 39 cases as grade II, 8 cases as grade III
and 4 cases as grade IV. According to the 2004 WHO clinical staging
standards, 61 CCRCC patients were stage I, 24 were stage II, 8 were
stage III and 5 were stage IV.
Histopathological examination and
immunohistochemical staining were performed using cancer tissues
from the 98 CCRCC patients enrolled in the study. Paraffin-embedded
CCRCC tissues (98 cases) and normal renal tissues (28 cases) were
retrieved and tissue microarray slides were constructed according
to a previously published method (19). The micro-array contained 126 cases
in total, including CCRCC and control group (CG) specimens. This
study was approved by the Taizhou Enze Medical Group Research
Ethics Committee. All patients provided written informed consent in
order to participate in this study.
Immunohistochemistry
Tissue microarray sections were dewaxed in xylene,
rehydrated in alcohol and immersed in 3% hydrogen peroxide for 10
min to suppress endogenous peroxidase activity. Antigen retrieval
was performed by heating (100°C) each section for 30 min in 0.01
mol/l sodium citrate buffer (pH 6.0). After three rinses (each for
5 min) in phosphate-buffered saline (PBS), sections were incubated
for 2 h at room temperature with a mouse anti-human MMP-7 antibody
(Dako, Carpinteria, CA, USA; 1:100) or mouse anti-human TIMP-2
antibody (Dako; 1:100) diluted in PBS. After three washes (each for
5 min) in PBS, sections were incubated with horseradish
peroxidase-labeled goat anti-mouse immunoglobulin (Dako) for 1 h at
room temperature. After three additional washes, peroxidase
activity was developed with diaminobenzidine (DAB) at room
temperature. EnVision staining was performed. PBS was substituted
for the primary antibody as a negative control and the known
positive slips served as positive controls.
The positive staining of the MMP-7 and TIMP-2
expression were mainly located in the cytoplasm with brown-yellow
granules. In each section, 5 high-power visual fields were randomly
selected and observed. Two hundred cells in each visual field were
counted. The staining was judged according to the percentage of
positive cells: <5% positive cells was negative (-); 5–20%
positive cells was weak positive (+); 20–50% positive cells was
middle positive (++) and >50% positive cells was strong positive
(+++).
RT-PCR
RT-PCR analysis was performed to determine the mRNA
levels for the last 61 CCRCC and 22 CG patients. For these
analyses, fresh renal tumor tissue and normal renal tissue were
harvested during the surgical resection of the tumor and
immediately frozen in liquid nitrogen and stored in a −70°C
ultra-low temperature freezer.
Three pairs of primers for MMP-7, TIMP-2 and GAPDH
(reference gene) were designed using the computer-assisted software
Primer Premier 5.0. Gene sequences from Genbank were used in the
primer design. These primers are summarized in Table I. The specificity of these primers
was confirmed using the BLAST system. The 3 pairs of primers were
synthesized by Shanghai Sangon Bioengineering Co. Ltd. (Shanghai,
China).
 | Table I.MMP-7, TIMP-2 and GAPDH primer
nucleotide sequences. |
Table I.
MMP-7, TIMP-2 and GAPDH primer
nucleotide sequences.
| Gene name | | Primer sequence
(5′-3′) | Product length
(bp) |
|---|
| MMP-7 | Sense |
AGCCATGTATCAGAGTCACCAA | 326 |
| Antisense |
AGTATCAGGAGCAGGAGAG | |
| TIMP-2 | Sense |
GGGGTTTTGGAATGCAGATGTAG | 418 |
| Antisense |
CACAGGAGCCGTCACTTCTCTTG | |
| GAPDH | Sense |
GGATATCGCATCACCATCT | 287 |
| Antisense |
GAGTGCTTTCACGATACCAA | |
Total RNA was isolated using TRIzol and the RNA
purity and concentration were determined based on A260 and A280
absorption. The RT-PCR procedure was performed as follows: i)
reverse transcription was carried out using 2 μg RNA in a
mixture containing 100 ng (2 μl) random primers and 15
μl water. The mixture was heated at 65°C for 5 min and then
cooled to 4°C. This was followed by the addition of 2.0 μl
diethylpyrocarbonate (DEPC) water, 5.0 μl 5X RT-buffer, 1.25
μl 10 mmol/l deoxyribonucleotide triphosphate (dNTP), 0.75
μl 50 U/μl RNAs and 1.0 μl Moloney murine
leukemia virus (MMLV; 10 U/μl). The mixture was heated at
37°C for 60 min and cooled to 4°C for storage. ii) PCR
amplification was performed in a 25-μl PCR reaction
containing 2.5 μl 15 mmol/l MgCl2, 0.5 μl
10 mmol/l dNTP, 1 μl 1.5 units Taq DNA polymerase, 1
μl 3′ and 5′ primers and 2.5 μl template DNA. The
amplification conditions were different for each target gene. MMP-7
and TIMP-2 were amplified for 30 cycles, each including a
predenaturation step at 95°C for 10 min, a denaturation step at
95°C for 60 sec, a renaturation step 52°C for 30 sec and an
extension step at 72°C for 45 sec. During the last cycle, the 72°C
step was held for 5 min. GAPDH was amplified for 30 cycles, each
consisting of predenaturation at 95°C for 10 min, denaturation at
94°C for 60 sec, renaturation at 60°C for 45 sec and extension at
72°C for 60 sec. During the last cycle, the 72°C step was held for
5 min.
For detection of the amplification products, 10
liters of PCR product and 2 liters of loading buffer were separated
on a 2% agarose electrophoresis gel, run at 100 V for 25 min and
the optical density of target bands was analyzed using a gel
analysis system. The ratio of MMP-7 or TIMP-2 band optical density
to GAPDH band optical density was calculated.
Follow-up
The follow-up was carried out by telephone or
letter. The survival time was defined as the time from diagnosis to
mortality or to the final examination. There were 74 cases that
were followed up for >5 years, including 58 cases of survival,
18 cases of mortality due to tumor recurrence and/or metastasis, 2
cases of mortality as a result of another disease and 6 cases which
were lost to follow-up.
Statistical analysis
The SPSS 10.0 (SPSS Inc., Chicago, IL, USA)
statistical software package was used to analyze the data.
Chi-square (χ2) tests were used for results presented as
percentages, t-tests were used for results with means belonging to
a normal distribution and Spearman grade-relevance analysis was
used to determine the correlation between two variables.
Postoperative survival rates were estimated using a lifespan table.
Survival curves were created using the Kaplan-Meier method and
analyzed by log-rank test. In addition, the Kaplan-Meier method and
log-rank test were used for single variant analysis and a Cox model
was used to analyze correlations between survival rates and
multiple variables. All statistical tests were two-sided and
P<0.05 was considered to indicate a statistically significant
difference.
Results
MMP-7 expression in CCRCC and normal
kidney with immunohistochemical staining
Representative sections of CCRCC kidney tissue
stained with hematoxylin and eosin and with immunohistochemical
staining for MMP-7 are shown in Fig.
1A and B, respectively. In the CCRCC kidney tissues, the
cytoplasm of cancerous cells presented positive staining for MMP-7,
with a positive expression rate of 62.2% (61/98). By contrast,
MMP-7 staining in the normal kidney tissue was less dense and was
observed only in the cytoplasm. Additionally, MMP-7 staining was
observed mainly in the kidney tubule epithelium. Compared with
CCRCC tissue, normal tissue demonstrated a significantly lower
percentage of positive staining for MMP-7 (28.6%, 8/28; P<0.01;
Table II). This suggests that
MMP-7 expression is significantly upregulated in CCRCC tissue.
Furthermore, positive associations between MMP-7 expression and
pathological grade (χ2=12.20, P<0.01) and clinical
stage (χ2=8.44, P<0.05) were evident (Fig. 1E and F; Table III).
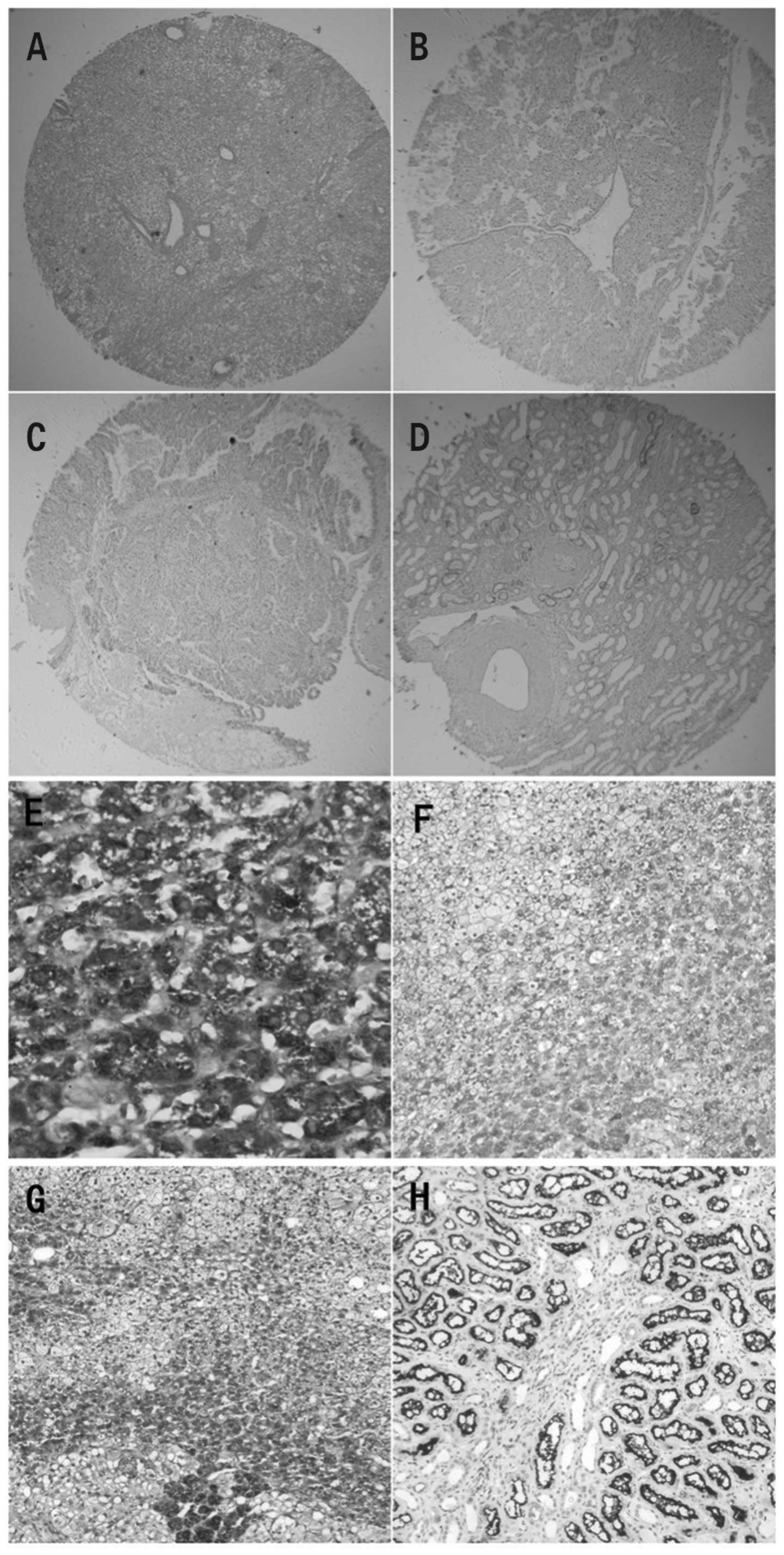 | Figure 1.Expression of MMP-7 and TIMP-2 in
CCRCC and normal tissues in a tissue microarray. (A) H&E
staining of CCRCC (original magnification, ×10). (B) IHC staining
for MMP-7 expression in CCRCC (original magnification, ×10). (C)
IHC staining for TIMP-2 expression in CCRCC (original
magnification, ×10). (D) TIMP-2 expression was restricted to the
tubular structures in normal renal tissues (original magnification,
×10). (E) IHC staining for MMP-7 protein revealed that MMP-7 was
situated mainly in the cytoplasm and on the membrane in stage IV
(original magnification, ×400). (F) MMP-7 was moderately expressed
in grade II (original magnification, 100×). (G) TIMP-2 expression
was strong in grade I (original magnification, ×100). (H) Levels of
TIMP-2 expression in renal tubule epithelial cells (original
magnification, ×100). MMP-7, matrix metalloproteinase 7; TIMP-2
tissue inhibitor of matrix metalloproteinases 2; CCRCC, clear cell
renal cell carcinoma; H&E, hematoxylin and eosin; IHC,
immunohistochemistry. |
 | Table II.Expression of MMP-7 and TIMP-2 in the
CCRCC and control groups. |
Table II.
Expression of MMP-7 and TIMP-2 in the
CCRCC and control groups.
| | MMP-7
| TIMP-2
|
|---|
| Group | n | + - +++ | % | χ2 | P-value | + - +++ | % | χ2 | P-value |
|---|
| CG | 28 | 8 | 28.6 | | | 23 | 82.1 | | |
| CCRCC | 98 | 61 | 62.2 | 9.97 | <0.01 | 44 | 44.9 | 12.13 | 0.01 |
 | Table III.MMP-7 expression, pathological grade
and clinical stage of CCRCC. |
Table III.
MMP-7 expression, pathological grade
and clinical stage of CCRCC.
| MMP-7
|
|---|
| Case | N | - | + | ++ | +++ |
|---|
| Pathological
grade | | | | | |
| I | 47 | 26 | 15 | 6 | 0 |
| II | 39 | 10 | 21 | 7 | 1 |
| III | 8 | 1 | 2 | 3 | 2 |
| IV | 4 | 0 | 0 | 1 | 3 |
| Clinical stage | | | | | |
| I | 61 | 29 | 23 | 9 | 0 |
| II | 24 | 7 | 13 | 3 | 1 |
| III | 8 | 1 | 2 | 3 | 2 |
| IV | 5 | 0 | 0 | 2 | 3 |
TIMP-2 expression in CCRCC and normal
kidney with immunohistochemical staining
A representative kidney section stained
immunohistochemically for TIMP-2 is shown in Fig. 1C. In normal kidney tissue, TIMP-2
staining was observed in the cytoplasm of the kidney tubule
epithelium (Fig. 1D and H), with a
positive expression rate of 82.1% (23/28). By contrast, CCRCC
tissue demonstrated clear TIMP-2 staining only in the cytoplasm of
cancerous cells and this staining was less dense. Normal tissue
underwent significantly more staining than CCRCC tissue (44.9%,
44/98; P<0.01; Table II). This
suggests that in CCRCC tissue TIMP-2 expression is significantly
downregulated. Furthermore, negative associations of TIMP-2
expression with pathological grade (χ2=7.98, P<0.05)
and clinical stage (χ2=7.97, P<0.05) were evident
(Fig. 1G; Table IV).
 | Table IV.TIMP-2 expression, pathological grade
and clinical stage of CCRCC. |
Table IV.
TIMP-2 expression, pathological grade
and clinical stage of CCRCC.
| TIMP-2
|
|---|
| Case | N | - | + | ++ | +++ |
|---|
| Pathological
grade | | | | | |
| I | 47 | 22 | 5 | 9 | 11 |
| II | 39 | 21 | 4 | 6 | 8 |
| III | 8 | 7 | 1 | 0 | 0 |
| IV | 4 | 4 | 0 | 0 | 0 |
| Clinical stage | | | | | |
| I | 1 | 28 | 6 | 12 | 15 |
| II | 24 | 15 | 2 | 3 | 4 |
| III | 8 | 6 | 2 | 0 | 0 |
| IV | 5 | 5 | 0 | 0 | 0 |
Association between MMP-7 and TIMP-2
expression in CCRCC tissues
There was a statistically significant negative
correlation between MMP-7 and TIMP-2 expression (r=-0.416,
P<0.01; Table V).
 | Table V.Expression of MMP-7 associated with
TIMP-2 in CCRCC. |
Table V.
Expression of MMP-7 associated with
TIMP-2 in CCRCC.
| TIMP-2
|
|---|
| MMP-7 | - | + | ++ | +++ | Total |
|---|
| - | 9 | 6 | 8 | 14 | 37 |
| + | 28 | 2 | 5 | 3 | 38 |
| ++ | 12 | 1 | 2 | 2 | 17 |
| +++ | 5 | 1 | 0 | 0 | 6 |
| Total | 54 | 10 | 15 | 19 | 98 |
Association between MMP-7 and TIMP-2
expression in CCRCC tissues and prognosis in CCRCC patients
Seventy-four patients were subjected to a follow-up
and their expected survival curves were calculated using the
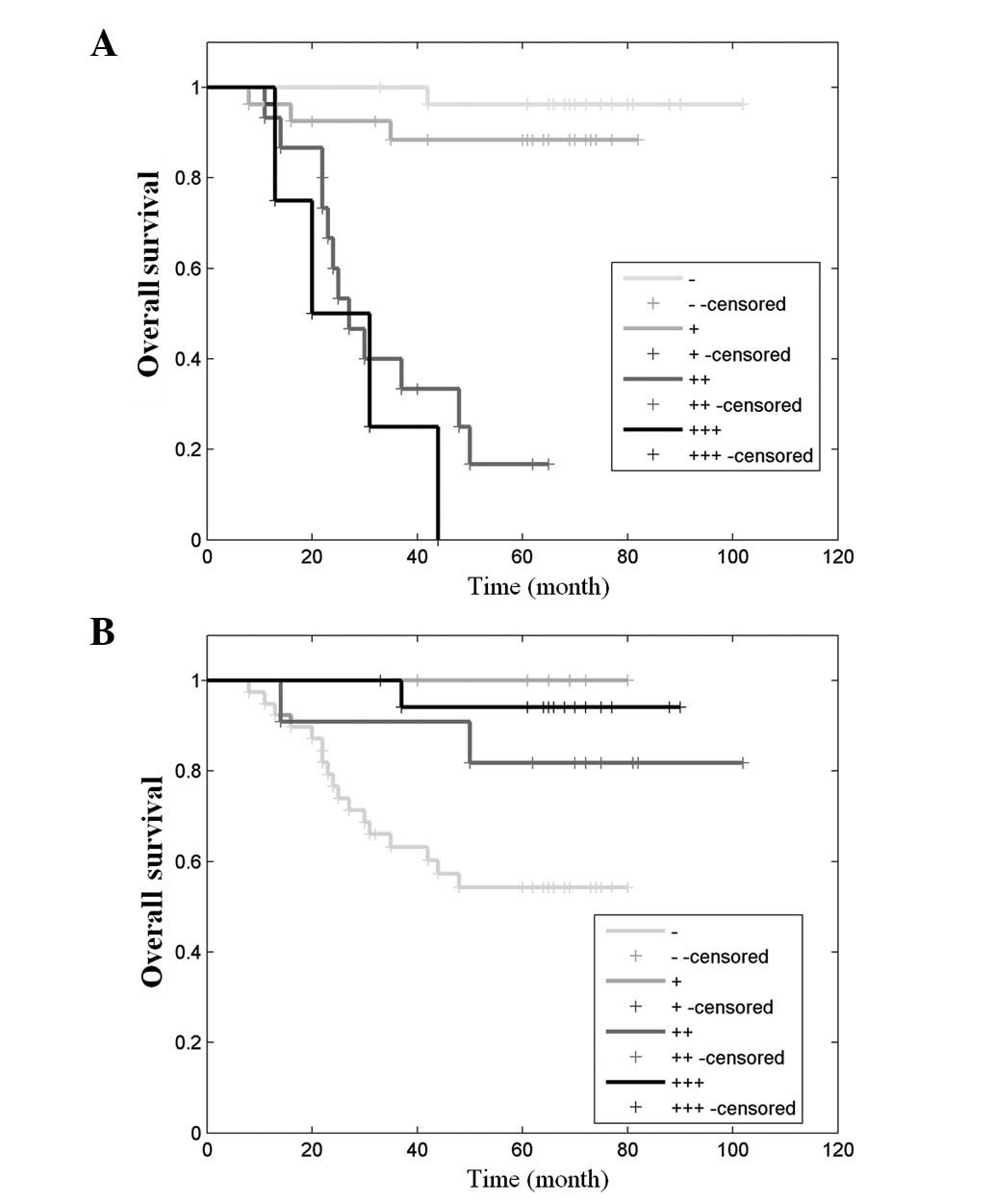
Kaplan-Meier method (Fig. 2).
Survival curves were calculated based on MMP-7 and TIMP-2
expression levels. Significant negative associations between MMP-7
and TIMP-2 expression and survival rates were evident. The survival
rate in the 5 years following tumor resection was estimated to be
95, 85, 20 and 0% for patients with -, +, ++ and +++ MMP-7
expression, respectively, and 50, 80, 93 and 100% for patients with
-, +, ++ and +++ TIMP-2 expression, respectively. Patients with -
and + MMP-7 or ++ and +++ TIMP-2 expression had a significantly
longer expected survival time as compared with those with ++ and
+++ MMP-7 or - and + TIMP-2 expression.
Association between MMP-7 and TIMP-2
expression in CCRCC tissues and pathological grade, clinical stage
and patient prognosis
Univariate survival rate analysis demonstrated a
statistically significant association between patient prognosis and
pathological grade, clinical stage, MMP-7 expression and TIMP-2
expression (P<0.01). However, no significant association was
observed between patient prognosis and gender, age, tumor size or
kidney vein cancer bolt (data not shown). A Cox model was used to
analyze the correlation between survival rate and the four positive
parameters mentioned above. This revealed pathological grade,
clinical stage and MMP-7 expression as three independent factors
negatively correlated with post-CCRCC expected survival rate
(P<0.01, P<0.05 and P<0.05, respectively). Higher
pathological grade, higher clinical stage and increased expression
of MMP-7 correlated with a worse prognosis (Table VI).
 | Table VI.Multiple-factor Cox model regression
variables of CCRCC. |
Table VI.
Multiple-factor Cox model regression
variables of CCRCC.
| 95% CI for HR
|
|---|
| P-value | HR | Lower | Upper |
|---|
| MMP-7 | 0.023 | 1.650 | 0.871 | 3.12 |
| TIMP-2 | 0.320 | 0.741 | 0.410 | 1.338 |
| Pathological
grade | 0.005 | 2.842 | 1.370 | 5.896 |
| Clinical stage | 0.011 | 1.650 | 1.178 | 3.697 |
Expression of MMP-7 and TIMP-2 mRNA in
CCRCC and normal kidney with RT-PCR
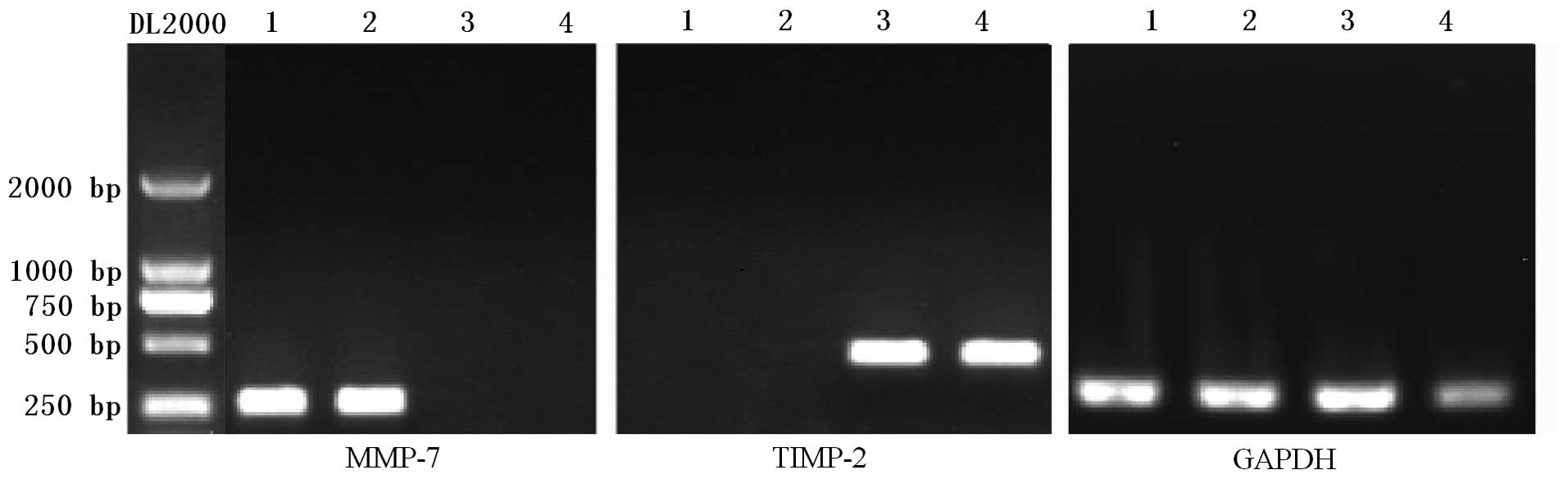
By RT-PCR analysis we observed mRNA expression of
the control gene GAPDH in normal and CCRCC tissue and observed no
significant difference between the GAPDH expression levels in these
two types of tissues (Fig. 3). An
extremely weak expression of MMP-7 mRNA was measured in the CG and
no expression of TIMP-2 mRNA was observed in CCRCC. However, there
was a significant expression of MMP-7 in CCRCC and TIMP-2 mRNA in
the CG.
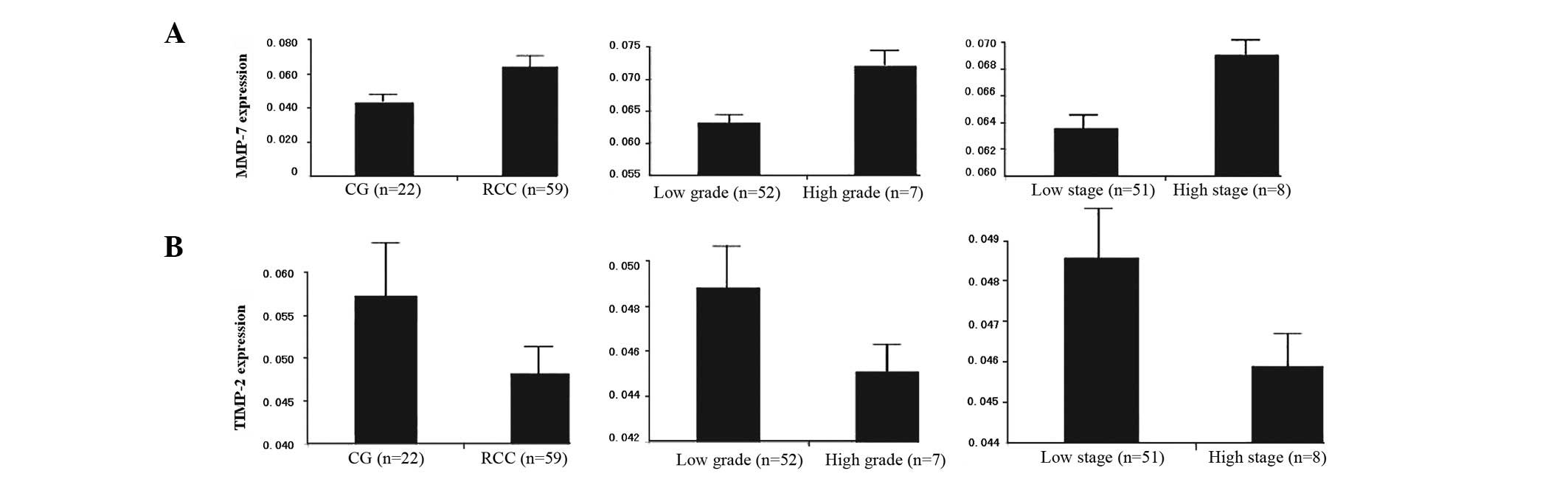
Association between MMP-7 gene expression
in CCRCC tissue and pathological grade and clinical stage
Among the 59 CCRCC cases for which RT-PCR analysis
was performed, there was a significant increase in MMP-7 mRNA
expression as compared with the 22 control samples. Among the CCRCC
patients, as shown in Table VI,
the expression of MMP-7 was significantly upregulated in the high
grade CCRCC (grades III and IV) compared with the low grade CCRCC
(grades I and II; P<0.05). There was also a significant
difference in the positive expression of MMP-7 mRNA between cases
with high clinical stage (stages III and IV) and low clinical stage
(stages I and II; P<0.05; Fig.
4A).
Association between TIMP-2 gene
expression in CCRCC tissues and pathological grade and clinical
stage
Although normal kidney and CCRCC tissues
demonstrated TIMP-2 mRNA expression, quantitative analysis revealed
a significantly lower level of expression of TIMP-2 mRNA in the
CCRCC tissues, as compared with normal kidney tissue. Furthermore,
there were significant associations between TIMP-2 mRNA expression
and pathological grading and clinical staging. The expression of
TIMP-2 mRNA was significantly higher in the low grades than in the
high grades and in the low stages than in the high stages (Fig. 4B).
Discussion
MMPs are enzymes produced by stromal or tumor cells
and are involved in tumor progression. TIMPs are induced in stromal
cells to regulate the proteinase reactions. They are closely
related to a series of pathological processes. The imbalance
between MMP and TIMP plays a critical role in cancer invasion and
metastasis (20–22).
The role of MMPs and TIMPS in RCC growth, metastasis
and angiogenesis has been the focus of intense investigation for a
number of years. Awakura et al demonstrated by univariate
analysis that TIMP-2 is a significant prognostic factor (1). Kawata et al identified that
the expression of TIMP-2 has an essential correlation with the
expression of MMP-2, which may have a correlation with the
prognosis of CCRCC. Moreover, the authors indicated that nuclear
grade and TIMP-2 are significant prognostic factors of CCRCC and
that patients with tumors with a high pathological grade and
strongly expressed MMP-9 and TIMP-2 have a poor outcome (23,24).
Miyata et al reviewed tissue samples of 156 RCC patients and
demonstrated that MMP-7 affects tumor progression by regulating
invasion and angiogenesis and is a predictor of poor prognosis by
multivariate analysis (25).
However, another study reported that MMP-7 expression is not
associated with clinicopathological features, including grade,
invasion and metastasis in a number of cancers (26). Thus, there is a difference of
opinion regarding the clinical significance of MMP-7 in
cancers.
In the current study, we focused on the roles of
MMP-7 and TIMP-2 in the tissue of 98 CCRCC patients in relation to
the clinicopathological characteristics of tumors, including
pathological grade and clinical stage, and the survival rate of the
patients, using tissue microarray, immunohistochemistry and RT-PCR.
The results of immunohistochemical analysis demonstrated that the
expression level of MMP-7 in CCRCC was significantly higher than in
the CG. Moreover, high MMP-7 expression was correlated with the
degree of malignancy, including high grade and high stage. In
addition, univariate survival rate analysis demonstrated that
patients with an elevated expression of MMP-7 in the CCRCC tissue
were predicted to have a poor prognosis. TIMP-2 expression level in
CCRCC was clearly lower than in the CG and the high expression
correlated with low grade and low stage. Univariate survival rate
analysis demonstrated that, contrary to MMP-7 expression, patients
with an elevated expression of TIMP-2 had a good survival rate.
Correlation analysis revealed negative correlations between MMP-7
and TIMP-2 expression levels. Cox multivariate survival rate
analysis demonstrated positive correlations between MMP-7
expression level and a high potential for tumor invasion and
metastasis, and a poor prognosis. Through RT-PCR analysis, we also
confirmed the high expression of MMP-7 and low expression of TIMP-2
in cases with high pathological grade and clinical stage.
These findings revealed that the expression levels
of MMP-7 and TIMP-2 in CCRCC tissues are related to malignant
progression in RCC and also to survival rate following tumor
removal. Therefore, expression of these proteins may be considered
indicators of progression in CCRCC tumors and prognostic predictors
in CCRCC patients. These findings also indicate that MMP-7 is an
independent prognostic factor but TIMP-2 is not, which differs from
the conclusions of other studies (23,24,26).
Thus, MMP-7 and TIMP-2 may be useful molecular markers for
evaluating prognosis in CCRCC patients.
In conclusion, we demonstrated for the first time
that MMP-7 is associated with TIMP-2 expression in CCRCC. The
concentrations of MMP-7 and TIMP-2 in the tissue of 98 CCRCC
patients were assessed in relation to pathological grade, clinical
stage and survival rate. Upregulated expression of MMP-7 and
downregulated expression of TIMP-2 in CCRCC have significant
clinicopathological associations with the aggressiveness observed
for this tumor. In addition, we identified that elevated levels of
MMP-7 in cancer tissues are a strong and independent predictor of
poor prognosis.
Therefore, MMP-7 and TIMP-2 may be useful molecular
markers for evaluating prognosis in CCRCC patients and MMP-7 may be
a new target for the prevention of tumor development and
improvement of survival rate.
References
|
1.
|
Awakura Y, Ito N, Nakamura E, Takahashi T,
Kotani H, Mikami Y, Manabe T, Kamoto T, Habuchi T and Ogawa O:
Matrix metalloproteinase-9 polymorphisms and renal cell carcinoma
in a Japanese population. Cancer Lett. 241:59–63. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Nagase H and Woessner JF: Matrix
metalloproteinases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar
|
|
3.
|
Declerck YA, Mercurio AM, Stack MS,
Chapman HA, Zutter MM and Muschel RJ: Proteases, extracellular
matrix and cancer: a workshop of the path B study section. Am J
Pathol. 164:1131–1139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Di Nezza LA, Misajon A and Zhang J:
Presence of active gelatinases in endometrial carcinoma and
correlation of matrix metalloproteinase expression with increasing
tumor grade and invasion. Cancer. 94:1466–1475. 2002.
|
|
5.
|
Egelblad M and Werb Z: New functions for
the matrix metal-loproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Jiang WG, Davies G, Martin TA, Parr C,
Watkins G, Mason MD and Mansel RE: Expression of membrane type-1
matrix metal-loproteinase, MT1-MMP in human breast cancer and its
impact on invasiveness of breast cancer cells. Int J Mol Med.
17:583–590. 2006.PubMed/NCBI
|
|
7.
|
Ogata Y, Miura K, Ohkita A, Nagase H and
Shirouzu K: Imbalance between matrix metalloproteinase 9 and tissue
inhibitor of metalloproteinases 1 expression by tumor cells
implicated in liver metastasis from colorectal carcinoma. Kurume
Med J. 48:211–218. 2001. View Article : Google Scholar
|
|
8.
|
Moore CS and Crocker SJ: An alternate
perspective on the roles of TIMPs and MMPs in pathology. Am J
Pathol. 180:12–16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Hahne JC, Fuchs T, El Mustapha H, Okuducu
AF, Bories JC and Wernert N: Expression pattern of matrix
metalloproteinase and TIMP genes in fibroblasts derived from Ets-1
knock-out mice compared to wild-type mouse fibroblasts. Int J Mol
Med. 18:153–159. 2006.PubMed/NCBI
|
|
10.
|
Abdel-Wahed MM, Asaad NY and Aleskandarany
M: Expression of matrix metalloproteinase-2 in renal cell
carcinoma. J Egypt Natl Canc Inst. 16:168–177. 2004.PubMed/NCBI
|
|
11.
|
Aoyama T, Yamamoto S, Kanematsu A, Ogawa O
and Tabata Y: Local delivery of matrix metalloproteinase gene
prevents the onset of renal sclerosis in streptozocin-induced
diabetic mice. Tissue Eng. 9:1289–1296. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Hirata H, Naito K, Yoshihiro S, Matsuyama
H, Suehiro Y and Hinoda Y: A single nucleotide polymorphism in the
matrix metalloproteinase-1 promoter is associated with conventional
renal cell carcinoma. Int J Cancer. 106:372–374. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hirata H, Okayama N, Naito K, Inoue R,
Yoshihiro S, Matsuyama H, Suehiro Y, Hamanaka Y and Hinoda Y:
Association of a haplotype of matrix metalloproteinase (MMP)-1 and
MMP-3 polymorphisms with renal cell carcinoma. Carcinogenesis.
25:2379–2384. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Huo N, Ichikawa Y and Kamiyama M: MMP-7
(matrilysin) accelerated growth of human umbilical vein endothelial
cells. Cancer Lett. 177:95–100. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Miyata Y, Iwata T, Maruta S, Kanda S,
Nishikido M, Koga S and Kanetake H: Expression of matrix
metalloproteinase-10 in renal cell carcinoma and its prognostic
role. Eur Urol. 52:791–597. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Struckmann K, Mertz KD, Steu S, Storz M,
Staller P, Krek W, Schraml P and Moch H: pVHL co-ordinately
regulates CXCR4/CXCL12 and MMP2/MMP9 expression in human clear-cell
renal cell carcinoma. J Pathol. 214:464–471. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Yang SD, Sun RC, Mu HJ, Xu ZQ and Zhou ZY:
The expression and clinical significance of TGF-β1 and MMP2 in
human renal clear cell carcinoma. Int J Surg Pathol. 18:85–93.
2010.
|
|
18.
|
Eble JN, Sauter G, Epstein JI and
Sesterhenn IA: World Health Organization Classification of Tumors.
Pathology and Genetics of Tumors of the Urinary System and Male
Genital Organs. IARC Press; Lyon, France: pp. 16–18. 2004
|
|
19.
|
Lu H, Gan M, Zhang G, Zhou T, Yan M and
Wang S: Expression of survivin, caspase-3 and p53 in cervical
cancer assessed by tissue microarray: correlation with
clinicopathology and prognosis. Eur J Gynaecol Oncol. 6:662–666.
2010.PubMed/NCBI
|
|
20.
|
Johansson N, Ahonen M and Kahari VM:
Matrix metalloproteinases in tumor invasion. Cell Mol Life Sci.
57:5–15. 2000. View Article : Google Scholar
|
|
21.
|
Stetler-Stevenson WG, Liotta LA and
Kleiner DE: Extracellular matrix 6: role of matrix
metalloproteinases in tumor invasion and metastasis. Faseb J.
7:1434–1441. 1993.PubMed/NCBI
|
|
22.
|
Stetler-Stevenson WG and Yu AE: Proteases
in invasion: matrix metalloproteinases. Semin Cancer Biol.
11:143–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kawata N, Nagane Y, Hirakata H, Ichinose
T, Okada Y, Yamaguchi K and Takahashi S: Significant relationship
of matrix metalloproteinase 9 with nuclear grade and prognostic
impact of tissue inhibitor of metalloproteinase 2 for incidental
clear cell renal cell carcinoma. Urology. 69:1050–1053. 2007.
View Article : Google Scholar
|
|
24.
|
Kawata N, Nagane Y and Igarashi T: Strong
significant correlation between MMP-9 and systemic symptoms in
patients with localized renal cell carcinoma. Urology. 68:523–527.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Miyata Y, Iwata T, Ohba K, Kanda S,
Nishikido M and Kanetake H: Expression of matrix
metalloproteinase-7 on cancer cells and tissue endothelial cells in
renal cell carcinoma: prognostic implications and clinical
significance for invasion and metastasis. Clin Cancer Res.
12:6998–7003. 2006. View Article : Google Scholar
|
|
26.
|
Kumaki F, Matsui K and Kawai T: Expression
of matrix metal-loproteinases in invasive pulmonary adenocarcinoma
with bronchioloalveolar component and atypical adenomatous
hyperplasia. Am J Pathol. 159:2125–2135. 2001. View Article : Google Scholar
|