Introduction
A large number of factors, receptors and downstream
elements in cell signaling cascades regulate the proliferation and
apoptosis of hepatic carcinoma. Dysregulation of the balance
between these processes represents a pro-tumorigenic principle in
human hepatic carcinogenesis, where there is usually activation of
proliferative signals and an inhibition of death processes, leading
to the survival and subsequent proliferation of carcinoma cells.
Apoptosis represents a physiological method for eliminating excess
cells during liver development and regeneration (1). Insufficient apoptosis has been
associated with the development and progression of tumors in the
liver and biliary tree (1).
Hepatocellular carcinoma (HCC) is the fifth most frequent neoplasm
worldwide and the third leading cause of cancer-related mortality.
To date, systemic chemotherapeutic treatment is ineffective against
HCC (2), in part due to the
resistance to apoptosis that is observed in HCC cells.
Apoptotic signaling within the cell is mainly
transduced via two molecular pathways: the death receptor pathway
(also called the extrinsic pathway) and the mitochondrial pathway
(also called the intrinsic pathway) (1). However, molecular alterations have
been reported for HCC that alter its apoptotic response, including
the p53 and transforming growth factor (TGF)-β pathways. Therefore,
researchers have been committed to finding ways to promote
apoptosis, independent of the altered molecules in HCC. The
intrinsic pathway is triggered by various extra or intracellular
signals that induce mitochondrial dysfunction, resulting in altered
membrane permeability and the release of mitochondrial proteins
into the cytosol, including the pro-apoptogenic factors cytochrome
c and HtrA serine peptidase 2 (HtrA2/Omi) (3). Once this intrinsic pathway is
activated, hepatic carcinogenesis may be restricted; therefore,
these proteins are also targets in cancer research. Hu Qisan (HQS),
a Chinese traditional medicine, has notable therapeutic
effectiveness against hepatocirrhosis as well as the ability to
block and reverse hepatocarcinogenesis. Our previous study
confirmed that HQS has a clear effect on hepatocarcinogenesis
(4). In the present study, we used
HQS to activate mitochondrial-controlled apoptosis in HCC. In
addition, the components of HQS that contribute to the proapoptotic
process are discussed.
Materials and methods
Preparation of Hu Qisan
HQS was created from 8 medicinal herbs containing
glycoprival granules. The herbal drugs, including Ramulus
Visci, Radix Astragali seu Hedysari, Radix
Curcumae and Radix Salviae Miltiorrhizae, were gently
boiled in distilled water for 60 min to reduce the volume. The
herbal mixture was soaked for 1 h before boiling. The decoction was
filtered through delipidated gauze and then concentrated to an
ointment through decompression treatment. The ointment was dried in
a vacuum to form extractum, which was ground. The powdered
preparation was stored in a refrigerator until subsequent use.
Preparation of components of Hu
Qisan
Mistletoe alkali
Mistletoe was crushed to small particles, 1 kg of
which was immersed into various acidic aqueous solutions (1.5, 1.0
and 0.5% HCl) for 48 h. The ratio of particles to HCl was 20:1.
Then, the solutions were distilled 3 times for 2 h at 50°C. The
solutions were filtered and centrifuged for extraction of the
supernatant. The concentrated supernatants (extractum) were
dissolved in 2% HCl, which was centrifuged twice to extract the
supernatant. Chloroform was used to remove the lipid from the
supernatant solution and obtain the total alkali A. Normal butyl
alcohol was used to extract the total alkali B from the supernatant
solution. Total alkali A was mixed with total alkali B to produce
the total alkali used in the following experiments.
Mistletoe polysaccharide
Powder crushed from dried mistletoe (1 kg) was
dissolved in water at a ratio of 1:28, powder to water (weight/v).
After 3 h in a 95°C water bath, the polysaccharide was dissolved in
water. Then, the solvent was filtered and centrifuged at 5,867 × g
to remove the dregs. In order to condense the polysaccharide
solvent, 95% ethanol was added to the solvent to yield 80% ethanol
dissolved in polysaccharide solvent. The solvent was incubated at
4°C overnight and the precipitate at the bottom was dissolved in
water. In order to remove the protein from the solvent, 50%
trichloroacetic acid was added to yield a final concentration of
10% trichloroacetic acid and was centrifuged at 1,467 × g to remove
the precipitate. The pH was adjusted to 7.0. To obtain the
polysaccharide powder, 95% ethanol was then added, followed by
centrifugation at 1,467 × g. The precipitate was recovered and
acetone was added, followed by evaporation to remove the water and
ethanol. We used ion-exchange chromatography to purify the
polysaccharide at a neutral location. After qualitative and
quantitative analysis, the purified polysaccharide included 12%
neutral arabinogalactan and a small amount of xylose glycan. The
extraction efficiency was 3.98%. The polysaccharide powder was
dissolved in saline.
Animals and treatment
Male Wistar rats (6 weeks old) weighing 135–149 g
were purchased from the Animal Department of Capital Medical
University. The experiment was performed as previously described
(5), using the Solt-Farber
two-step test model of precancerous liver lesions.
Diethylnitrosamine (DEN; 200 mg/kg) was injected into the abdominal
cavity of experimental rats as an initiating agent. After 2 weeks,
we performed a selective promoting procedure [feeding with a diet
containing 0.015% 2-acetyl aminofluorene (2-AAF)], which lasted for
6 weeks. The majority of the liver was removed from the rats at the
end of the third week. Sixty rats were allowed free access to a
pellet diet and water and were divided into four groups: model
group, high-dose therapeutic group (8 g/kg/day HQS), low-dose
therapeutic group (4 g/kg/day HQS) and a 5-fluorouracil (5-FU)
group (250 mg/day on days 1–5 of each week for 4 weeks). Rats in
the therapeutic groups began the treatment with HQS 1 week after
the majority of the liver was removed, for 4 weeks. Rats in the
normal group were fed normally. After 8 weeks, all rats were
sacrificed under anesthesia with pentobarbital after 24 h fasting.
Hepatic tissue was taken from the right anterior, right back and
caudal lobe.
Histochemical staining for γ-
GTase-positive foci
γ-Glutamyl-transpeptidase isoenzyme
(γ-GTase)-positive foci were determined as previously described
(6). Fresh liver tissue was cut
into 8-μm-thick sections, which were mounted on slices and
air-dried. Fresh solution containing
γ-glutamyl-4-methoxy-2-naphthylamide (GMNA), γ-GTase, azo-coupling
dye, Fast blue BBN (diazotized 4′-amino-2′,5′-diethoxybenzanilide),
0.1 mol/l Tris buffer (pH 7.4) and saline was prepared and pipetted
onto the sections which were then incubated in the dark for 30–45
min. Following incubation, the slices were rinsed in saline for 2
min and transferred to 0.1 mol/l cupric sulphate solution for 2
min. The slices were rinsed in saline followed by distilled water.
The sections were air-dried, placed into 10% glycerol and observed
under a light microscope. Four sections from each liver were
examined under 5 fields. The γ-GTase-positive foci counted were
divided according to various size ranges and the results were
expressed as the percentage of foci area in sections. Sections of
kidney tissue served as positive controls for γ-GTase staining.
Isolation of mitochondrial and
cytosolic fractions from HepG2 cells
HepG2 cells were cultured with 10% fetal bovine
serum (FBS) in Dulbecco’s modified Eagle’s medium (DMEM). HQS at a
high concentration (3 mg/ml) and low concentration (1.5 mg/ml),
mistletoe polysaccharide (25 mg/ml), mistletoe alkali (20 mg/ml)
and 5-FU (1 μg/ml) were administered to promote apoptosis of
the cells for 12 h to detect HtrA2/Omi release from the
mitochondria and for 24 h to detect caspase-3 activation.
Mitochondrial and cytosolic fractions were isolated
to detect HtrA2/Omi distribution using the mitochondria isolation
kit for cultured cells (89874; Pierce, Rockford, IL, USA). Briefly,
reagents were added in order and centrifuged, the cytosolic
fraction was removed and the pellet containing the isolated
mitochondria was washed and centrifuged again. Lysis buffer was
added and centrifuged to obtain the mitochondrial protein.
Western blotting of protein
expression
Following treatment, 40 μg cell lysate was
subjected to sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE). The gel was transferred to a
polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA,
USA), after which the membrane was blocked with 5% BSA/0.05%
Tween-20/phosphate-buffered saline (PBS), followed by overnight
incubation at 4°C with primary antibody diluted in 5% BSA/0.05%
Tween-20/PBS. The primary antibodies used were as follows: rabbit
anti-HtrA2/Omi, goat anti-cleaved caspase-3 (1:750 dilution; Cell
Signaling Technology, Danvers, MA, USA), mouse anti-voltage
dependent anion channel (VDAC; Cell Signaling Technology) and mouse
anti-β-actin (Sigma, Munich, Germany). The membrane was washed 3
times with 0.05% Tween-20/PBS and incubated with alkaline
phosphataseconjugated secondary antibody (Cell Signaling
Technology) at a 1:2,000 dilution (5% BSA/0.05% Tween-20/PBS; 1 h
at room temperature). The membrane was then rewashed 3 times and
developed by the addition of SuperSignal West Pico chemiluminescent
substrate (Cat: 34077; Thermo Scientific, Rockford, IL, USA). The
membrane was scanned and analyzed by densitometry using Adobe
Photoshop (Adobe Systems Inc., San Jose, CA, USA) and Image J (NIH,
Bethesda, MD, USA). Signal intensities of the activated proteins
were normalized to β-actin or VDAC.
Reverse transcription-polymerase chain
reaction (RT-PCR) protocol
The reverse transcription system and PCR Master mix
were purchased from Promega (Madison, WI, USA). The primers for
HtrA2/Omi (F, 5′-TGTGTTCTTCAGAGCCCAG GACTGC-3′; R, 5′-CTACAGCT
CCGAGAGCCAAGTTTCC-3′) and β-actin (F,
5′-TGGAATCCTGTGGCATCCATGAAAC-3′; R,
5′-TAAAACGCAGCTCAGTAACAGTCCG-3′) were synthesized by Genecore
(Shanghai, China). RNA was extracted from the tissues using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA). After denaturing at 94°C
for 1 min, the PCR reaction consisted of 30 cycles (HtrA2/Omi and
β-actin) of denaturation at 94°C for 30 sec, annealing at 60°C for
40 sec and elongation at 72°C for 45 sec, followed by one cycle of
final extension at 72°C for 5 min. A negative control without
template cDNA was always included. The expected sizes of the PCR
products were 402 bp for HtrA2/Omi and 320 bp for β-actin and all
PCR products were analyzed by 2% agarose gel electrophoresis with
ethidium bromide staining. The optical density (OD) of the expected
bands was measured and the OD ratio of HtrA2/Omi mRNA to β-actin
mRNA was used to determine the relative amount of
HtrA2/Omi/β-actin.
Statistical analyses
Comparisons were made using one-way analysis of
variance (ANOVA). All experiments were repeated at least 3 times.
P<0.05 was considered to indicate a statistically significant
difference. Data are presented as the means ± standard error of the
mean.
Results
DEN and liver resection promoted
carcinogenesis in vivo
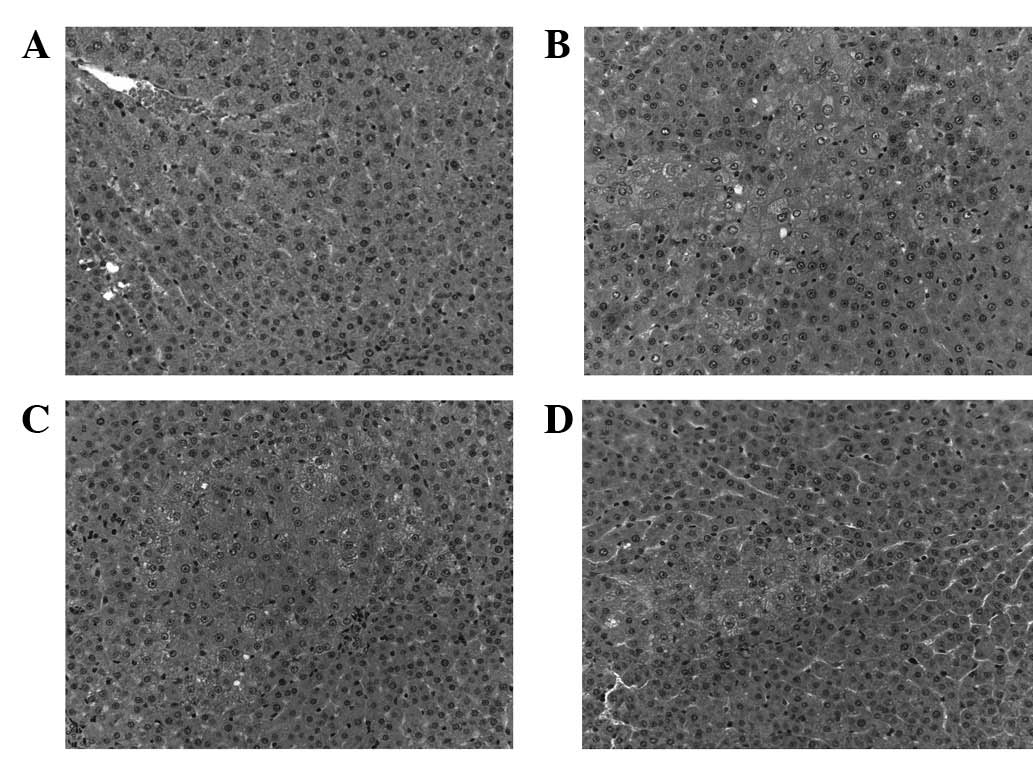
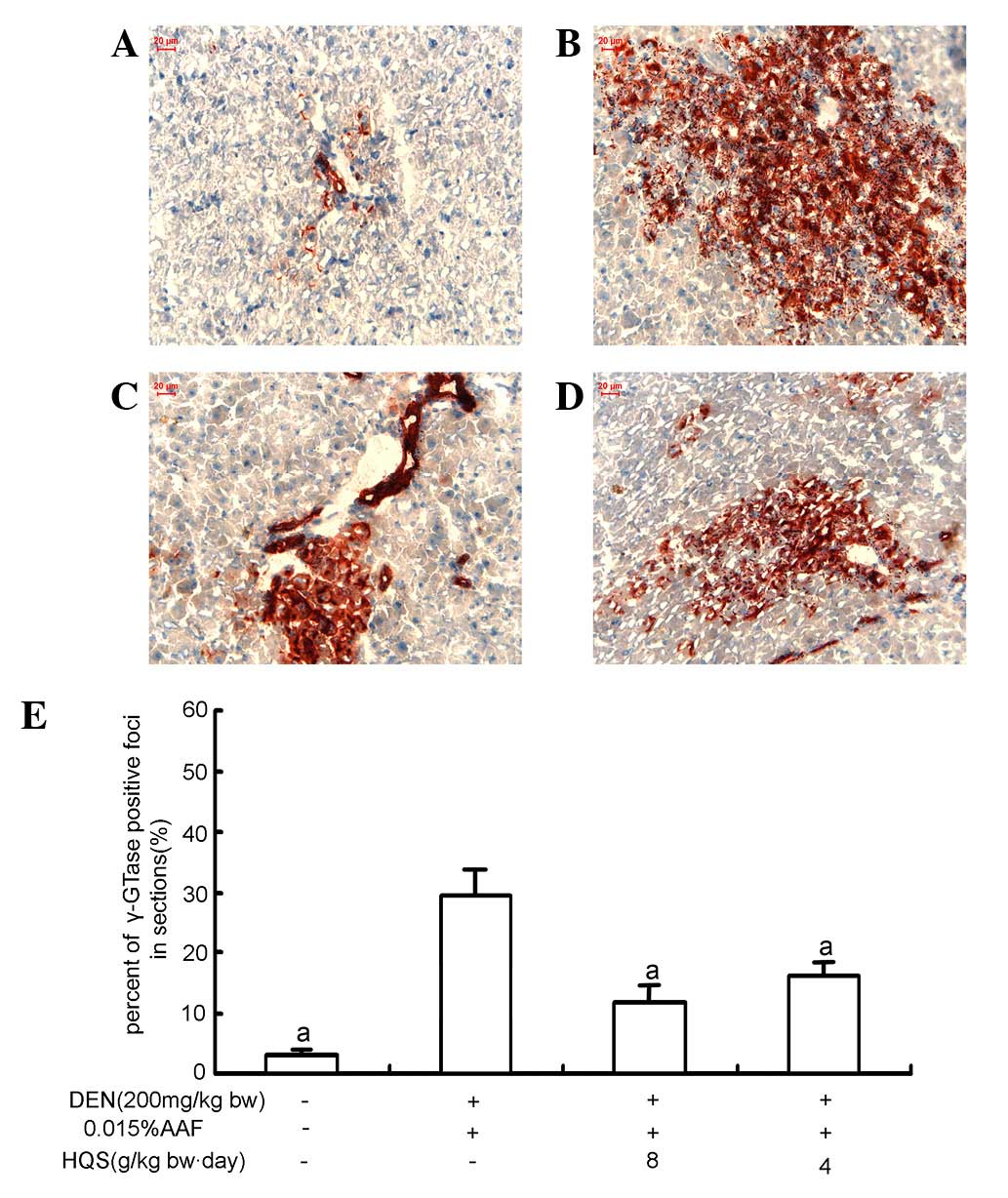
H&E and γ-GTase staining were used to
demonstrate that DEN and liver resection promotes carcinogenesis in
rats. In the control group, the hepatic lobules were clear and
intact; and the liver cells were polygonal and radially arranged
around the central vein (Fig. 1A).
γ-GTase staining demonstrated little γ-GTase expression in the
portal areas of normal rat liver (Fig.
2A). In the DEN and liver resection group, hepatic cells
demonstrated early HCC transformation. The normal hepatic cord
structure and sinusoids were damaged. Cells proliferated to form
foci containing basophilic or acidophilic cells. A number of these
cells invaded surrounding tissues and some cloudy cells were
slightly larger with prominent nucleoli. γ-GTase staining showed
that γ-GTase-positive foci were brown/red and scattered in the
hepatic tissue. At the edge of γ-GTase-positive foci, hepatic cells
were suppressed by proliferating cells (Fig. 2B).
HQS treatment attenuated precancerous
lesions in rat liver
Following treatment with HQS, carcinogenesis in
hepatic tissues triggered by DEN and liver resection was partly
reversed or blocked. H&E staining revealed that the number of
proliferating foci significantly decreased and the number of
enlarged nuclei with prominent nucleoli also decreased (Fig. 1C and D). γ-GTase staining showed
that the size of γ-GTase positive foci was smaller and the number
of suppressed hepatic cells was attenuated (Fig. 2C–E).
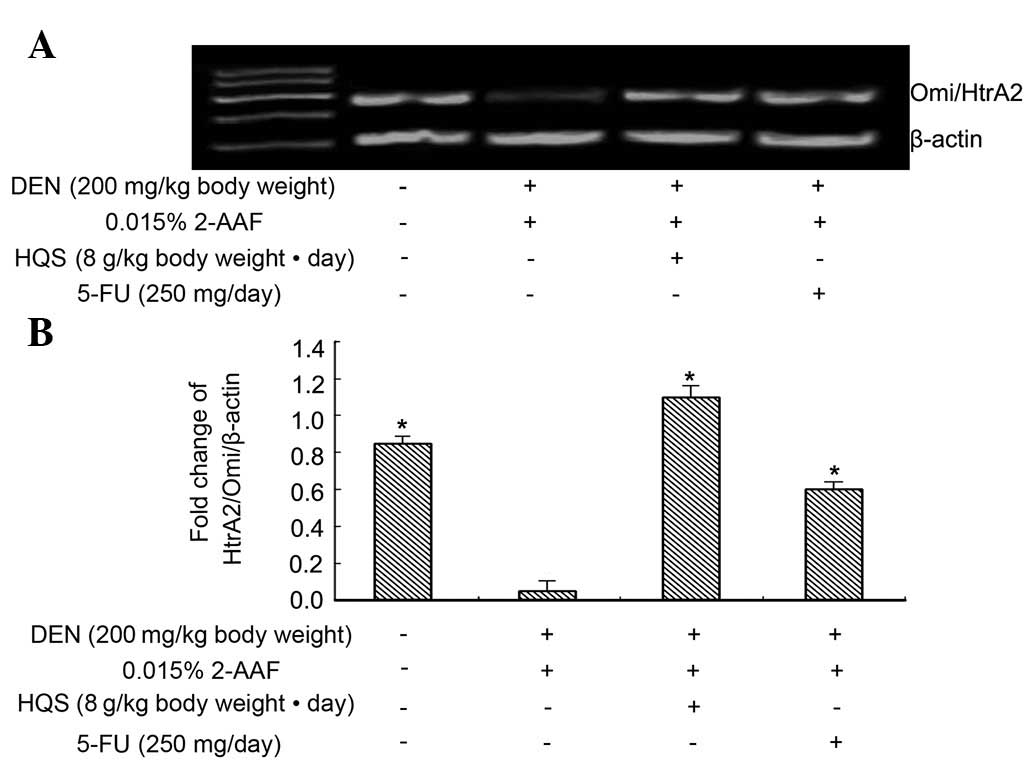
HQS upregulated HtrA2/Omi expression
in precancerous lesions in rat liver
RT-PCR results revealed that HtrA2/Omi is expressed
in normal and carcinogen-treated hepatic tissues and its expression
in carcinogen-treated hepatic tissues is much lower than in normal
tissues. Used as a control to prevent carcinogenesis, 5-FU did not
change the expression of HtrA2/Omi in the tissues exposed to
carcinogen, while HQS significantly increased its expression
(Fig. 3).
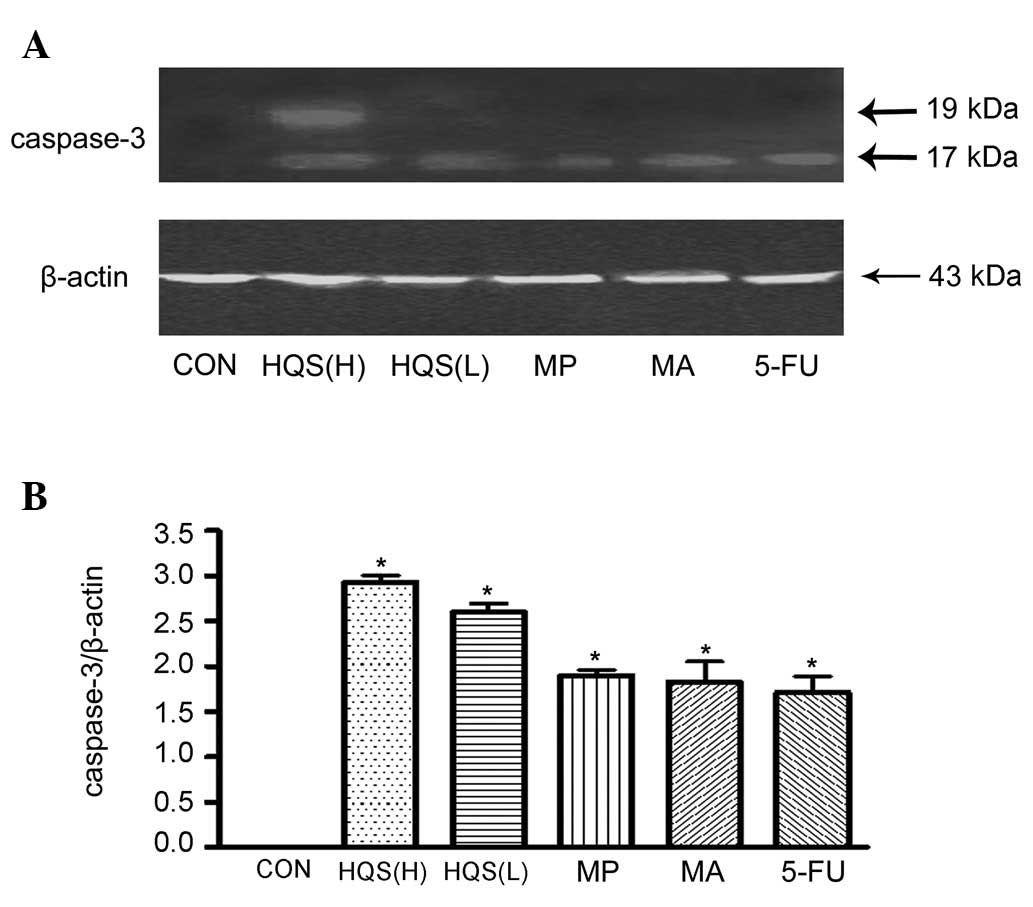
HQS stimulated HtrA2/Omi release from mitochondria
and caspase-3 activation. Consistent with the apoptotic nature of
DEN-induced neuronal carcinogenesis, there was little HtrA2/Omi
release from the mitochondria into the cytosol in the control HepG2
cells. HQS in high doses promoted HtrA2/Omi release from the
mitochondria to the cytosol, as measured by western blot analysis
(Fig. 4A and C). The HtrA2/Omi
levels in the mitochondria were higher than in the control HepG2
cells (Fig. 4A and B) following
HQS treatment, which confirms the HtrA2/Omi mRNA upregulation by
HQS in the tissues exposed to carcinogen. HtrA2/Omi also
substantially promoted caspase-3 activation following the onset of
HQS treatment (Fig. 5).
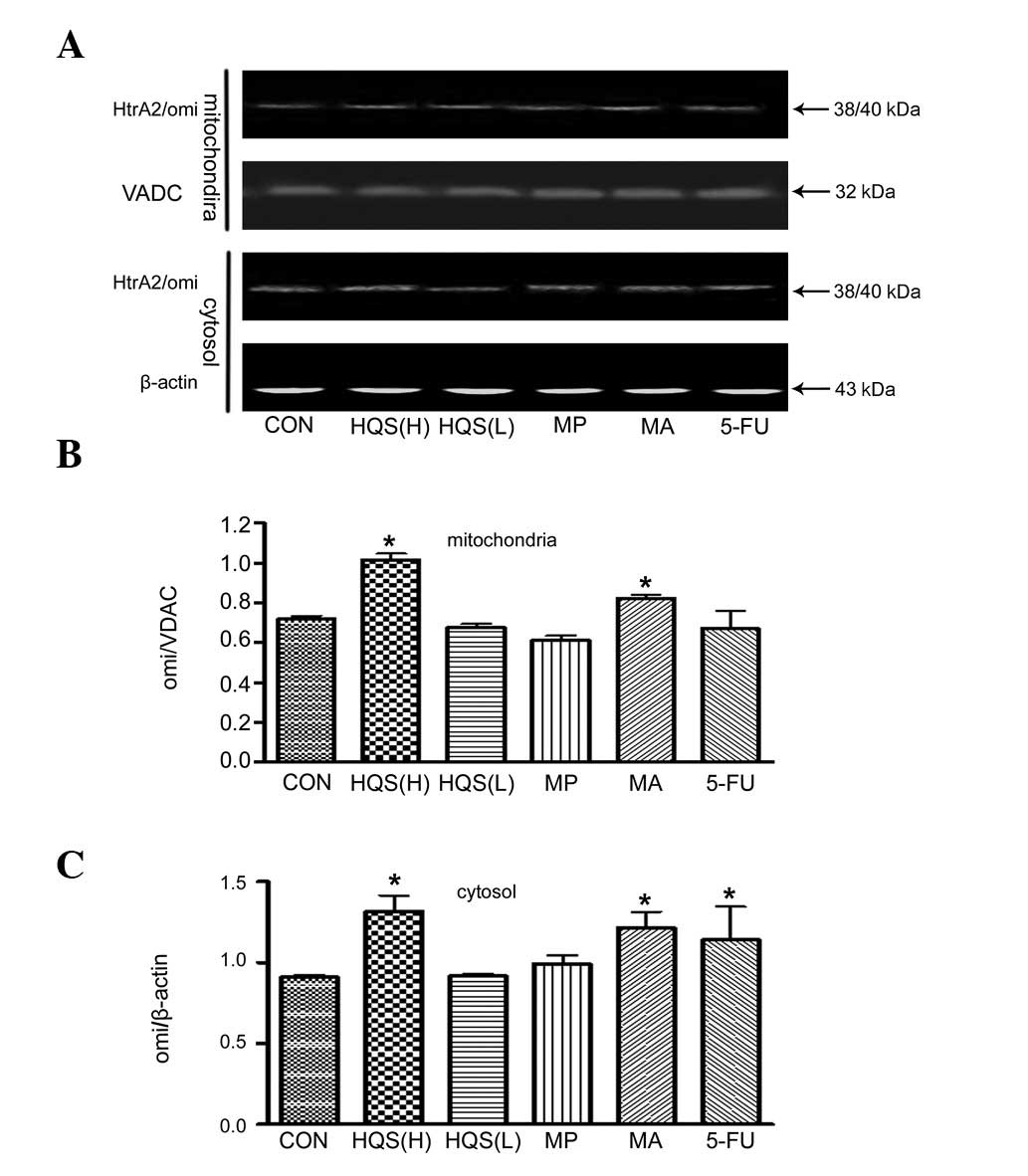 | Figure 4.Effect of HQS on HtrA2/Omi release in
HepG2 cells. HtrA2/Omi content in mitochondrial and cytoplasmic
compartments was evaluated using western blotting. Protein
expression was corrected according to the loading control of VDAC
and β-actin in mitochondria and cytosolic fractions, respectively.
(A) Western blotting gel shows the HtrA2/Omi contents of
mitochondrial and cytoplasmic compartments with different
treatments, as indicated. (B) Summarized data from experiments in
(A). High-dose HQS significantly increased the HtrA2/Omi content of
the mitochondria, as did its component mistletoe alkali. 5-FU, as a
control to prevent carcinogenesis, diminished the HtrA2/Omi content
in the mitochondria. (C) Summarized data from experiments in (A).
High-dose HQS significantly increased HtrA2/Omi release from the
mitochondria and its component mistletoe alkali increased its
release to the cytoplasm effectively. As a control to prevent
carcinogenesis, 5-FU promoted HtrA2/Omi release into the cytoplasm.
n=5; *P<0.05 compared with untreated HepG2 cells.
HQS, Hu Qisan; HQS(H), HQS at 8 g/kg body weight/day; HQS(L), HQS
at 4 g/kg body weight/day; MP, mistletoe polysaccharide; MA,
mistletoe alkali; VADC, voltage-dependent anion channel; 5-FU,
5-fluorouracil. |
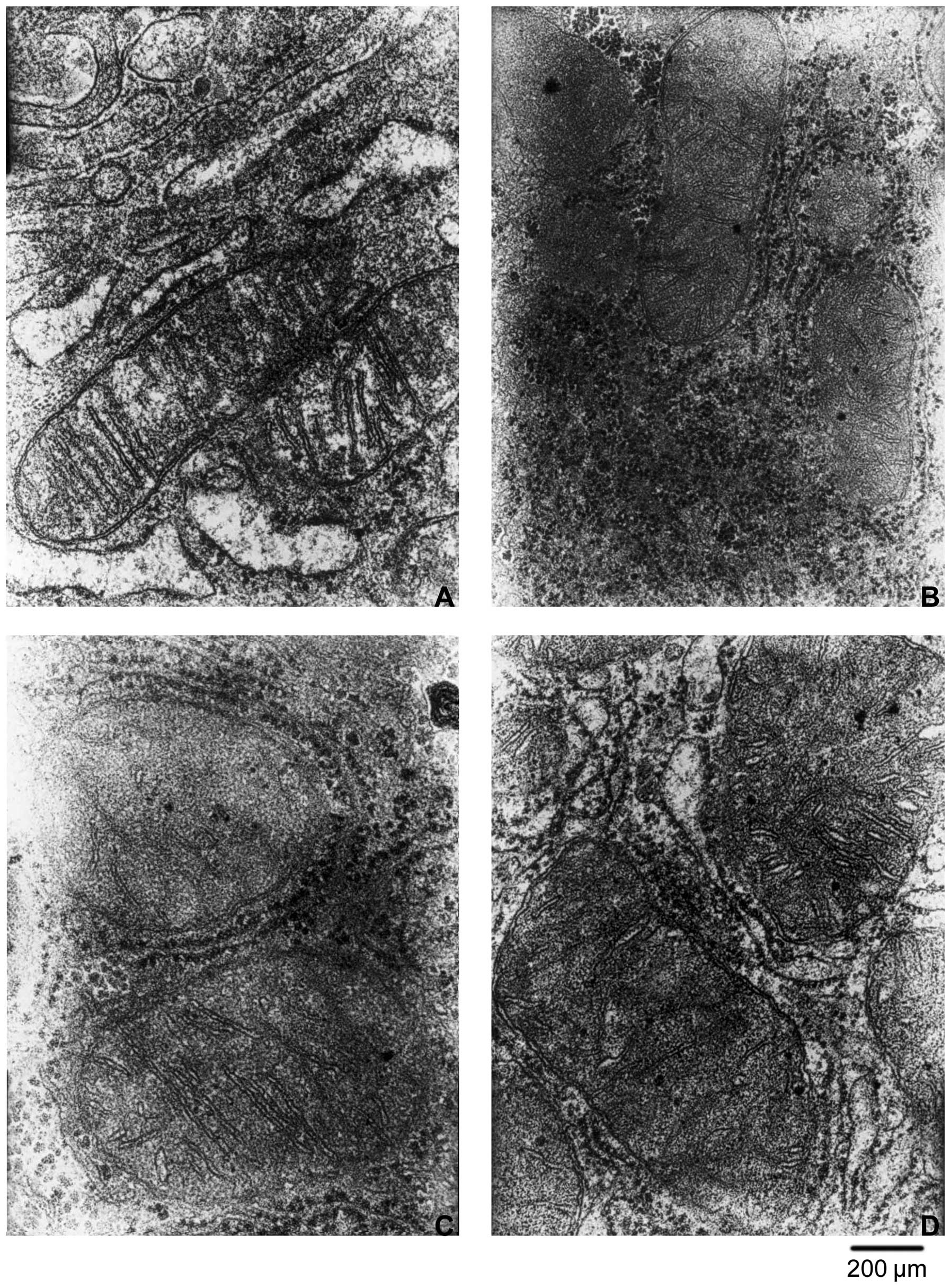
Consistent with the above observations, HQS induced
a change in the number and morphology of mitochondria 4 weeks after
treatment. At 8 g/kg body weight, HQS treatment increased the
number and size of mitochondria in HCC, decreased the number of
crista in mitochondria and the mitochondrial membrane was not
intact. At 4 g/kg body weight, HQS showed no significant change in
the number and morphology of mitochondria (Fig. 6).
Discussion
Cancer occurs or progresses because malignant cells
fail to undergo apoptosis, either spontaneously or in response to
chemotherapy. Failure to activate caspases may account for this
resistance to apoptosis. Many strategies for restoring apoptosis
sensitivity in refractory cancers have been tested (7–9) and
some are undergoing clinical testing in humans. A potential
advantage of inhibitors of apoptosis proteins (IAPs) as drug
targets is that they operate at distal points in apoptotic
pathways, potentially bypassing many upstream defects in
apoptosis-regulatory mechanisms in tumors (10). However, this strategy is only valid
if tumor and normal cells demonstrate differential sensitivities to
IAP suppression.
In this study, we describe HQS, a traditional
Chinese medicine and inhibitor of pre-hepatocarcinoma, as was
determined from studies using the classical preneoplastic marker
enzyme for hepatic chemical carcinogenesis, γ-GTase. γ-GTase is a
cell surface enzyme that initiates the cleavage of extracellular
glutathione and glutathione conjugates. Hydrolysis of glutathione
in glomerular filtrate and fluids in other ducts and glands
throughout the body provides a mechanism for the body to retain
amino acids containing glutathione. In the absence of
γ-GTase-initiated cleavage, glutathione is excreted from the body,
resulting in fatal cysteine deficiency. In rodents, γ-GTase is a
single copy gene whose expression is regulated in a developmental
and tissue specific manner. Several γ-GTase genes or pseudogenes
are present in humans. γ-GTase activity is elevated in
carcinogen-induced tumors of animals. Common human epithelial
tumors, including breast, ovarian and prostate tumors, are
γ-GTase-positive. Synthesis of γ-glutamyl pro-drugs is used as a
new approach in chemotherapeutic drugs to treat γ-GTase-positive
tumors. The placenta form of γ-GTase is hardly detectable in normal
rat liver and is markedly induced in liver-bearing foci and nodules
(11). Our histochemical data
demonstrated that DEN exposure formed γ-GTase foci which are
correlated with the development of HCC. However, HQS at different
doses prevented the formation of γ-GTase foci (Fig. 1). Since the synthesis of γ-glutamyl
prodrugs is used as a new approach for targeting γ-GTase-positive
tumors, the effect of HQS on preventing the formation of γ-GTase
foci in liver indicates that it is capable of protecting against
DEN-induced hepatocarcinogenesis.
X-linked inhibitor of apoptosis protein (XIAP) is
the best studied protein in the human IAP family of proteins from
the standpoint of biochemical mechanism (12) and its overexpression in several
types of human cancers has been documented (13–16).
XIAP suppresses the downstream proteases, including caspase-3 and
-7, via its BIR2 region (17–19).
The objective of this study was to demonstrate that HQS overcomes
the inhibitory effects of XIAP on proteases in hepatocarcinoma
cells and tissues. Our study indicates that HQS inhibits XIAP by
promoting HtrA2/Omi expression and release from mitochondria in
hepatocarcinoma. Two HQS components were responsible for inducing
HtrA2/Omi release from the mitochondria. Moreover, a perfect
correlation was observed between HtrA2/Omi release and induction of
caspase-3 activation in HepG2 cells. Cancer cells have an intrinsic
drive to activate caspases while upregulated IAP expression
counteracts it (20). Therefore,
functional removal of IAPs permits apoptosis to occur in tumor
cells but not in normal cells (20). This is consistent with evidence of
processed caspase-3 in tumor cell lines and tumor tissues being
offset by the overexpression of XIAP or other IAP family members
(20). In this regard, many causes
of caspase activation in tumor cells may be envisioned, including
protooncogene activation, disobeyance of cell cycle checkpoints
associated with defective DNA replication and chromosome
segregation and loss of cell attachment (anchorage-independent
growth), all of which are known to drive apoptosis unless countered
by anti-apoptotic proteins (21).
By contrast, normal cells are expected to have less drive to
caspase activation, thus rendering them less dependent on IAPs; a
theory supported by gene ablation studies in mice, which have
revealed little or no adverse phenotypes in animals lacking XIAP,
cIAP1, cIAP2, or neuronal apoptosis inhibitory protein (NAIP)
(22,23)
HtrA2/Omi has a dual role as a caspase activator via
inhibition of IAP proteins and as an effector of necrosis-like
programmed cell death through its serine protease activity
(24,25). In our study, cytosolic release of
HtrA2/Omi was enhanced by HQS (Fig.
4C) in HepG2 cells. Moreover, levels of HtrA2/Omi mRNA and
HtrA2/Omi in the mitochondria were elevated by HQS (Figs. 3 and 4B) in HepG2 cells. These results suggest
that HQS targets XIAP to activate caspases, which then induce
apoptosis in hepatic cancer cells.
XIAP is the most studied of the human IAP family
members from the standpoint of biochemical mechanisms and its
overexpression in several types of human cancers has been
documented. XIAP suppresses the downstream effector protease,
caspase-3. Our results demonstrate the inhibitory effect of HQS on
XIAP and its downstream proteins (Fig.
5). In addition, HQS is able to suppress the proliferation of
γ-GTase-positive (Fig. 2) cells in
hepatic tissues. Therefore, HQS may be a potential
anti-carcinogenic agent through its inhibitory action on the XIAP
pathway.
In conclusion, results from this study clearly
revealed that HQS at high and low doses is able to promote
HtrA2/Omi expression and release from mitochondria in HCC. The
components responsible for this effect are identified as mistletoe
alkali and mistletoe polysaccharide. Our findings support the use
of HQS as antitumor medicine to promote apoptosis, which was
inhibited by XIAP in cancer cells.
Acknowledgements
The authors wish to acknowledge the
support of a grant from Beijing Natural Science Foundation
(7112010) and a grant from the National Natural Science Foundation
of China (81272757).
References
|
1.
|
Guicciardi ME and Gores GJ: Apoptosis: a
mechanism of acute and chronic liver injury. Gut. 54:1024–1033.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Bruix J, Hessheimer AJ, Forner A, Boix L,
Vilana R and Llovet JM: New aspects of diagnosis and therapy of
hepatocellular carcinoma. Oncogene. 25:3848–3856. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Bulteau AL and Bayot A: Mitochondrial
proteases and cancer. Biochim Biophys Acta. 1807:595–601. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Li X, Shi ZM, Feng P, Wen ZY and Wang XL:
Effect of Qi-protecting powder (Huqi San) on expression of c-jun,
c-fos and c-myc in diethylnitrosamine-mediated
hepatocarcinogenesis. World J Gastroenterol. 13:4192–4198.
2007.PubMed/NCBI
|
|
5.
|
Craddock VM: Effect of a single treatment
with the alkylating carcinogens dimethylnitrosamine,
diethylnitrosamine and methyl methanesulphonate, on liver
regenerating after partial hepatectomy. I Test for induction of
liver carcinomas. Chem Biol Interact. 10:313–321. 1975. View Article : Google Scholar
|
|
6.
|
McNeil CM, Sergio CM, Anderson LR, et al:
c-Myc overexpression and endocrine resistance in breast cancer. J
Steroid Biochem Mol Biol. 102:147–155. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Chao MP, Majeti R, Weissman IL, Kesari S,
Antony ML and Singh SV: Programmed cell removal: a new obstacle in
the road to developing cancer. Nat Rev Cancer. 12:58–67.
2011.PubMed/NCBI
|
|
8.
|
Kesari S, Antony ML and Singh SV:
Understanding glioblastoma tumor biology: the potential to improve
current diagnosis and treatments. Semin Oncol. 38(Suppl 4): S2–S10.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Antony ML and Singh SV: Molecular
mechanisms and targets of cancer chemoprevention by garlic-derived
bioactive compound diallyl trisulfide. Indian J Exp Biol.
49:805–816. 2011.PubMed/NCBI
|
|
10.
|
Zurawa-Janicka D, Skorko-Glonek J and
Lipinska B: HtrA proteins as targets in therapy of cancer and other
diseases. Expert Opin Ther Targets. 14:665–679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Liu J, Yang CF, Wasser S, Shen HM, Tan CE
and Ong CN: Protection of salvia miltiorrhiza against
aflatoxin-B1-induced hepatocarcinogenesis in Fischer 344 rats dual
mechanisms involved. Life Sci. 69:309–326. 2001.
|
|
12.
|
Stennicke HR, Ryan CA and Salvesen GS:
Reprieval from execution: the molecular basis of caspase
inhibition. Trends Biochem Sci. 27:94–101. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ferreira CG, van der Valk P, Span SW, et
al: Assessment of IAP (inhibitor of apoptosis) proteins as
predictors of response to chemotherapy in advanced non-small-cell
lung cancer patients. Ann Oncol. 12:799–805. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Hofmann HS, Simm A, Hammer A, Silber RE
and Bartling B: Expression of inhibitors of apoptosis (IAP)
proteins in non-small cell human lung cancer. J Cancer Res Clin
Oncol. 128:554–560. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Krajewska M, Krajewski S, Banares S, et
al: Elevated expression of inhibitor of apoptosis proteins in
prostate cancer. Clin Cancer Res. 9:4914–4925. 2003.PubMed/NCBI
|
|
16.
|
Tamm I, Kornblau SM, Segall H, et al:
Expression and prognostic significance of IAP-family genes in human
cancers and myeloid leukemias. Clin Cancer Res. 6:1796–1803.
2000.PubMed/NCBI
|
|
17.
|
Deveraux QL, Takahashi R, Salvesen GS and
Reed JC: X-linked IAP is a direct inhibitor of cell-death
proteases. Nature. 388:300–304. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Deveraux QL, Leo E, Stennicke HR, Welsh K,
Salvesen GS and Reed JC: Cleavage of human inhibitor of apoptosis
protein XIAP results in fragments with distinct specificities for
caspases. EMBO J. 18:5242–5251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Takahashi R, Deveraux Q, Tamm I, et al: A
single BIR domain of XIAP sufficient for inhibiting caspases. J
Biol Chem. 273:7787–7790. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Yang L, Cao Z, Yan H and Wood WC:
Coexistence of high levels of apoptotic signaling and inhibitor of
apoptosis proteins in human tumor cells: implication for cancer
specific therapy. Cancer Res. 63:6815–6824. 2003.PubMed/NCBI
|
|
21.
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Holcik M, Thompson CS, Yaraghi Z, Lefebvre
CA, MacKenzie AE and Korneluk RG: The hippocampal neurons of
neuronal apoptosis inhibitory protein 1 (NAIP1)-deleted mice
display increased vulnerability to kainic acid-induced injury. Proc
Natl Acad Sci USA. 97:2286–2290. 2000. View Article : Google Scholar
|
|
23.
|
Harlin H, Reffey SB, Duckett CS, Lindsten
T and Thompson CB: Characterization of XIAP-deficient mice. Mol
Cell Biol. 21:3604–3608. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Okada M, Adachi S, Imai T, et al: A novel
mechanism for imatinib mesylate-induced cell death of
BCR-ABL-positive human leukemic cells: caspase-independent,
necrosis-like programmed cell death mediated by serine protease
activity. Blood. 103:2299–2307. 2004. View Article : Google Scholar
|
|
25.
|
Reed JC: The Survivin saga goes in vivo. J
Clin Invest. 108:965–969. 2001. View
Article : Google Scholar : PubMed/NCBI
|