Introduction
Naturally occurring compounds have attracted
considerable attention as cancer chemopreventive agents due to
their beneficial effects on human health and their potent
anticancer effects (1). Certain
naturally occurring compounds have been reported to effectively
suppress cell proliferation and tumor progression in in vivo
and in vitro experimental models of cancer by inducing
apoptosis (2). In particular,
naturally occurring compounds derived from plant sources, including
curcumin, polyphenols, betulinic acid and ellagic acid, have been
studied in various models as modulators of proliferation,
angiogenesis, apoptosis and inflammation (3). It has also been demonstrated that the
antitumor activities of naturally occurring compounds are
associated with the regulation of numerous molecular targets,
including p53, VEGF, STAT3, MAPK and PI3K/AKT signaling pathways
(1,3). Therefore, it is important to
understand the antitumor effects and molecular mechanisms of
naturally occurring compounds for chemoprevention and
chemotherapy.
Specificity protein 1 (Sp1) is a transcription
factor which binds GC/GT-rich promoter elements via three
Cys2His2-type zinc fingers and plays key
roles in tumorigenesis (4,5). Sp1 regulates several cancer
associated genes associated with the cell cycle, proliferation,
differentiation and apoptosis (6).
In addition, Sp1 is overexpressed in several cancers and is closely
correlated with the prognosis of patients (7–9).
Notably, naturally occurring compounds, such as curcumin and
betulinic acid, have been reported to suppress tumor growth via the
downregulation of Sp1 expression in prostate and bladder cancer
cells (10,11). Isorhapontigenin has also
demonstrated an anticancer effect by inducing apoptosis through the
down-regulation of the Sp1/XIAP pathway (12). Therefore, the downregulation of Sp1
by naturally occurring compounds may be a potential chemopreventive
and chemotherapeutic strategy for cancer.
The present study demonstrates that methanol
extracts of C. officinale Makino (MECO) and C.
bursa-pastoris (MECB) decrease cell growth and induce apoptosis
via the downregulation of Sp1 in HSC-2 human oral cancer cells.
Materials and methods
Chemicals and antibodies
MECO and MECB were provided by Professor Ki-Han Kwon
(Kwangju University, Kwangju, Korea). The DC Protein Assay kit was
acquired from Bio-Rad Laboratories Inc., (Madison, WI, USA). Sp1
and actin antibodies were obtained from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). Poly (ADP-ribose) polymerase (PARP) antibody
was provided by BD Pharmingen™ (San Jose, CA, USA). Antibodies
against Bak, Bax, Bcl-xL and Mcl-1 were supplied by Cell Signaling
Technology (Charlottesville, VA, USA).
Cell culture and chemical treatment
HSC-2 human oral cancer cells were provided by
Hokkaido University (Hokkaido, Japan). Cells were maintained in
DMEM supplemented with 10% fetal bovine serum and 1%
penicillin/streptomycin at 37°C in a 5% CO2 incubator.
The cells were treated with DMSO or various concentrations (300,
600 and 900 μg/ml) of MECO or MECB for 24 or 48 h.
MTS assay
The effects of MECO and MECB on cell growth were
investigated using the CellTiter 96® Aqueous One Solution Cell
Proliferation Assay kit (Promega, Madison, WI, USA). Cells were
seeded in 96-well plates and treated with MECO or MECB for 24 or 48
h. MTS solution was added to each well and incubated for 2 h at
37°C. The absorbance was measured using an ELISA microplate reader
(Bio-Tek Instruments, Inc., Madison, WI, USA) at 490 and 690 nm (as
a blank control).
Detection of nuclear morphological
changes
The effects of MECO and MECB on nuclear
morphological change were confirmed using the fluorescent nuclear
dye, DAPI (Sigma, St. Louis, MO, USA). HSC-2 cells were seeded and
treated with MECO or MECB for 48 h. The cells were harvested by
trypsinization, resuspended in PBS and then fixed in 100% methanol
at room temperature for 10 min. The cells were deposited on slides
and stained with DAPI solution (2 μg/ml). The cell
morphological change was observed using a fluorescence microscope
equipped with a suitable filter for the DAPI fluorescent dye.
Western blot analysis
The protein concentration of the supernatant was
determined using the DC Protein Assay kit. The samples containing
equal amounts of protein were resolved by SDS-PAGE and transferred
to Immun-Blot PVDF membranes (Bio-Rad Laboratories, Hercules, CA,
USA). The membranes were blocked with 5% skimmed milk in TBST at
room temperature for 2 h and probed overnight at 4°C with various
primary antibodies. They were then incubated with HRP-conjugated
secondary antibodies. After 2 h, the membranes were washed and
detected using the ECL Western Blotting Luminol reagent (Santa Cruz
Biotechnology).
RNA interference
ON-TARGETplus SMARTpool siRNA sequences targeting
Sp1 and non-targeting control were supplied by Dharmacon Research
(Lafayette, CO, USA). The HSC-2 cells were seeded in 6-well plates
and transiently transfected with 25 nM siRNA using a DharmaFECT2
transfection reagent (Thermo Scientific, Lafayette, CO, USA). After
48 h, transfected cells were harvested and examined by trypan blue
exclusion assay and western blot analysis.
Trypan blue exclusion assay
The HSC-2 cells were transfected with 25 nM siRNA
for 48 h and the number of viable cells was counted using a
hemocytometer with trypan blue (0.4%). The result was expressed as
the mean ± standard deviation.
Statistical analysis
A Student’s t-test was used to determine the
significance of differences between the control and treatment
groups. P<0.05 was considered to indicate a statistically
significant result.
Results
MECO and MECB inhibit cell growth and
induce apoptosis in HSC-2 human oral cancer cells
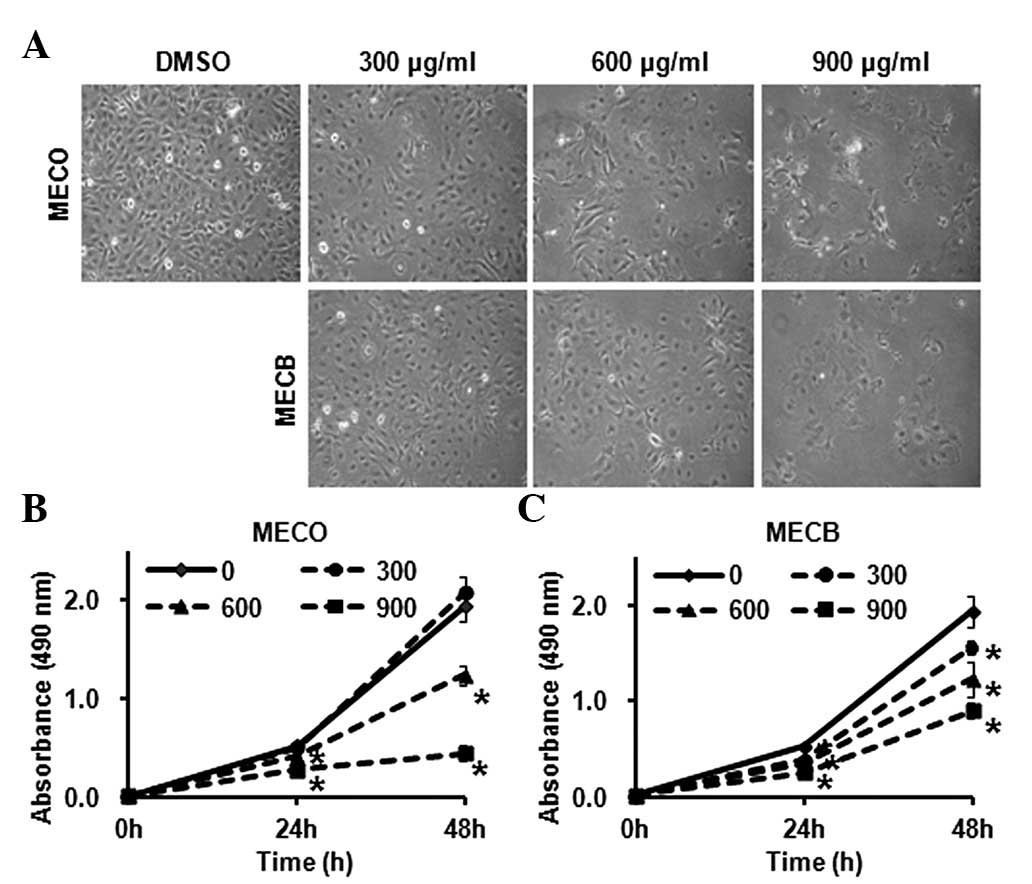
To investigate the anticancer effects of MECO and
MECB on HSC-2 human oral cancer cells, we assessed the growth
inhibitory effects of MECO and MECB. Cells were treated with DMSO
or various concentrations (300, 600 and 900 μg/ml) of MECO
or MECB for 24 or 48 h. Morphological changes of the MECO- and
MECB-treated cells were observed under an optical microscope after
48 h. As shown in Fig. 1A, a
number of MECO- and MECB-treated cells were detached in the medium
in a concentration-dependent manner. The effects of MECO and MECB
on cell viability were examined using an MTS assay. The results
showed that MECO and MECB significantly decreased cell viability in
HSC-2 cells (Fig. 1B and C). We
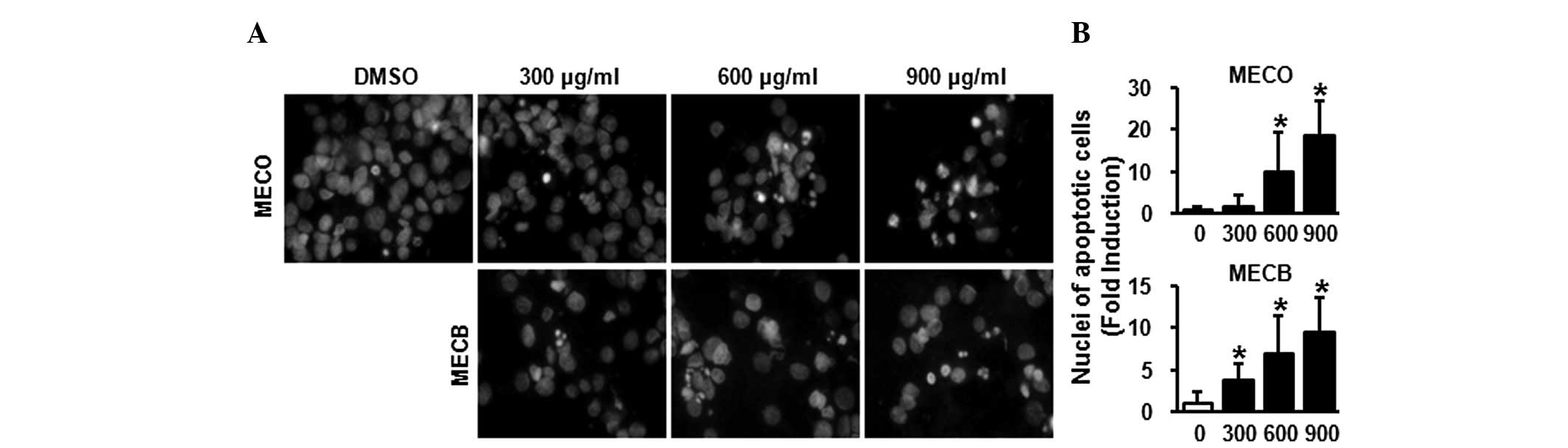
then evaluated whether the growth inhibitory effects of MECO and
MECB were associated with apoptotic effects using DAPI staining. As
shown in Fig. 2A and B, cells
treated with MECO or MECB for 48 h exhibited nuclear fragmentation
and chromatin condensation in a concentration-dependent manner.
These results demonstrate that MECO and MECB inhibited cell growth
and induced apoptosis in HSC-2 human oral cancer cells.
Downregulation of Sp1 by MECO and MECB
correlates with the regulation of several Bcl-2 family
proteins
A previous study has suggested that the
downregulation of Sp1 inhibits malignant transformation via the
induction of apoptosis (13).
Since Sp1 is highly expressed in oral tumor tissues compared with
normal oral tissues (14), we
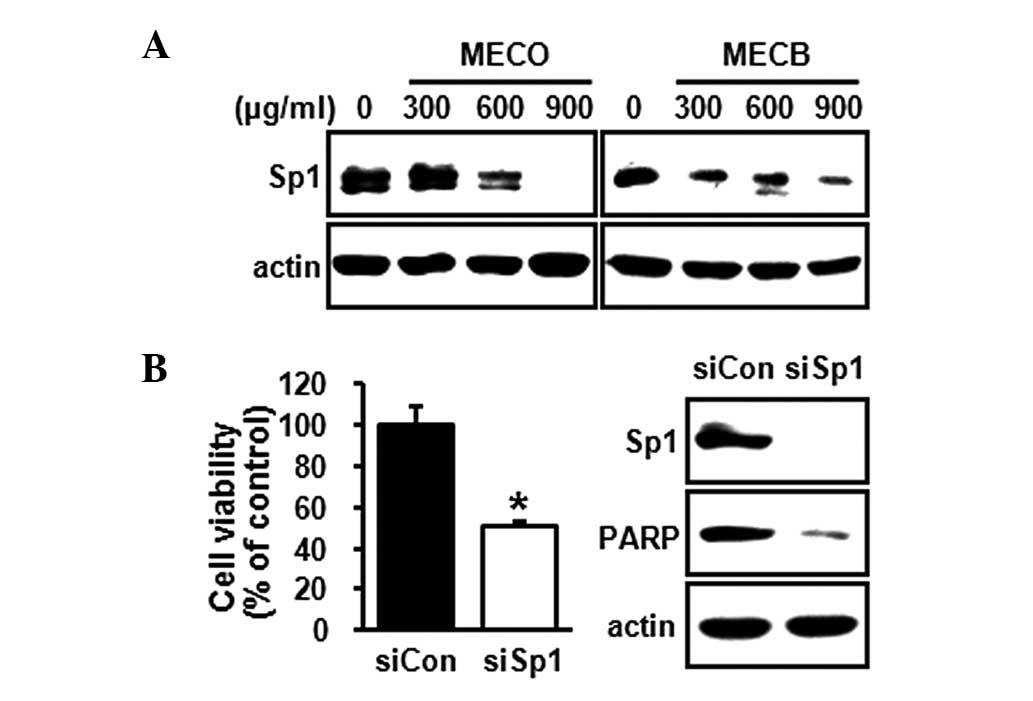
investigated whether MECO and MECB affect Sp1 expression in HSC-2
cells. The results showed that MECO and MECB decreased Sp1
expression (Fig. 3A). To confirm
that the downregulation of Sp1 was associated with the induction of
apoptosis, we knocked down the expression of Sp1 in the HSC-2 cells
using siRNA technology. As shown in Fig. 3B, the knockdown of Sp1 markedly
decreased cell growth and total PARP expression, indicating that
downregulation of Sp1 was sufficient to inhibit cell growth and
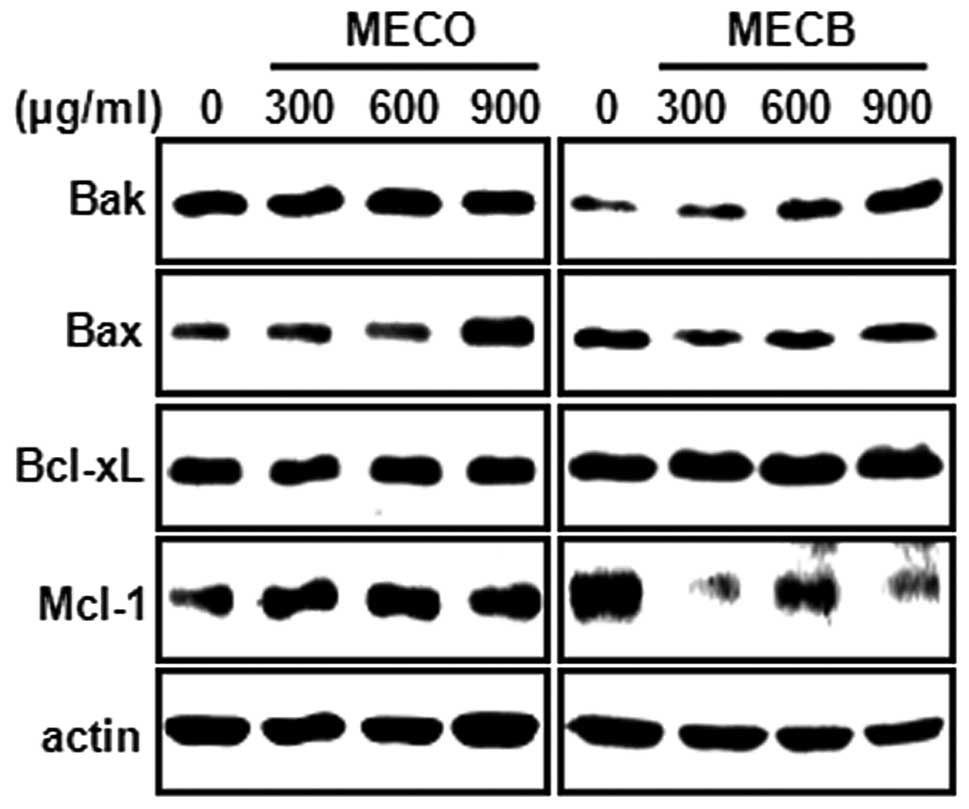
induce apoptosis. To further investigate the molecular mechanism of
MECO- and MECB-induced apoptosis, we examined whether MECO and MECB
regulated the expression of Bcl-2 family proteins. Fig. 4 shows that MECO caused a marked
increase in Bax expression levels. MECB increased Bak expression
and decreased Mcl-1 expression. These results indicate that MECO
and MECB may induce apoptosis through the regulation of several
Bcl-2 family proteins.
Discussion
Since Sp1 is a critical transcription factor which
regulates several cancer associated genes associated with cell
survival, proliferation and angiogenesis, the abnormal expression
or increased binding activity of Sp1 may contribute to tumor
development and progression. In a previous study, the abnormally
activated Sp1 expression was shown to represent a potential risk
for poor prognosis and directly caused gastric cancer progression
(15). The expression of Sp1 in
breast cancer tissues is positively associated with TNM stage,
tumor invasion and lymph node metastasis (16). In addition, the overexpression of
Sp1 is involved in the malignant transformation of human
fibroblasts (13). Previous
studies have also evaluated different approaches for targeting Sp1,
including Sp1 ribozyme, siRNA and dominant negative mutant, in
experimental models. The downregulation of Sp1 by Sp1 ribozyme
correlated with increased apoptosis (13), and the silencing of Sp1 by siRNA
suppressed invasion in human glioma cells (17). The dominant negative mutant of Sp1
demonstrated a growth inhibitory effect in cervical cancer cell
lines (18). Therefore, it is
likely that Sp1 is an important target for cancer therapy.
A recent study in our laboratory has shown that Sp1
is over-expressed in oral cancer tissues compared with normal oral
tissues (14). Notably, several
naturally occurring compounds decreased the cell growth of oral
cancer cells, and exhibited apoptotic activity through the
decreased expression of Sp1 and regulation of its downstream target
proteins (14,19,20).
These findings demonstrate that certain naturally occurring
compounds regulate Sp1 expression to inhibit cell growth and induce
apoptosis in oral cancer.
In the present study, we examined whether MECO and
MECB inhibit cell growth and induce apoptosis through the
downregulation of Sp1 in oral cancer cells. We observed that MECO
and MECB significantly decreased cell growth and induced apoptosis,
which was caused by Sp1 downregulation.
The Bcl-2 family of proteins have emerged as
important regulators in mitochondria-mediated apoptosis due to
protein-protein interactions between pro- and anti-apoptotic
proteins (21). In particular, it
is noteworthy that promoters of Bcl-2 family genes such as Mcl-1
and Bax contain Sp1 binding sites (14,22).
Therefore, we hypothesized that the down-regulation of Sp1 by MECO
and MECB may affect pro- or anti-apoptotic proteins. We observed
that MECO significantly increased Bax expression, but did not
change other Bcl-2 family proteins. MECB markedly increased Bak
expression and decreased Mcl-1 expression. Although the effects of
MECO and MECB on several Bcl-2 family proteins due to the
downregulation of Sp1 are unclear, the regulation of Bcl-2 family
proteins by MECO and MECB may be due, in part, to the effect of
MECO and MECB on Sp1 expression.
In conclusion, the results of the present study
indicate that MECO and MECB treatment inhibited cell growth and
induced apoptosis via the downregulation of Sp1 in HSC-2 human oral
cancer cells. These effects involved the regulation of Bcl-2 family
proteins, including Bax, Bak and Mcl-1. Therefore, we provide
experimental evidence to indicate that MECO and MECB may be
attractive anticancer drug candidates targeting Sp1 in oral
cancers.
Acknowledgements
This study was supported by the Basic
Science Research Program through the National Research Foundation
of Korea (NRF) funded by the Ministry of Education, Science and
Technology (2012001497 and 2012003731).
References
|
1.
|
Fulda S: Modulation of apoptosis by
natural products for cancer therapy. Planta Med. 76:1075–1079.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Nobili S, Lippi D, Witort E, et al:
Natural compounds for cancer treatment and prevention. Pharmacol
Res. 59:365–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Amin AR, Kucuk O, Khuri FR and Shin DM:
Perspectives for cancer prevention with natural compounds. J Clin
Oncol. 27:2712–2725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Safe S and Abdelrahim M: Sp transcription
factor family and its role in cancer. Eur J Cancer. 41:2438–2448.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Black AR, Black JD and Azizkhan-Clifford
J: Sp1 and krüppel-like factor family of transcription factors in
cell growth regulation and cancer. J Cell Physiol. 188:143–160.
2001.
|
|
6.
|
Sankpal UT, Goodison S, Abdelrahim M and
Basha R: Targeting Sp1 transcription factors in prostate cancer
therapy. Med Chem. 7:518–525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Zhang J, Zhu ZG, Ji J, et al:
Transcription factor Sp1 expression in gastric cancer and its
relationship to long-term prognosis. World J Gastroenterol.
11:2213–2217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Wang LW, Li Q, Hua ZL, et al: Expression
of transcription factor Sp1 in human gastric cancer tissue and its
correlation with prognosis. Zhonghua Zhong Liu Za Zhi. 29:107–111.
2007.(In Chinese).
|
|
9.
|
Jia Z, Gao Y, Wang L, Li Q, et al:
Combined treatment of pancreatic cancer with mithramycin A and
tolfenamic acid promotes Sp1 degradation and synergistic antitumor
activity. Cancer Res. 70:1111–1119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Chintharlapalli S, Papineni S, Ramaiah SK
and Safe S: Betulinic acid inhibits prostate cancer growth through
inhibition of specificity protein transcription factors. Cancer
Res. 67:2816–2823. 2007. View Article : Google Scholar
|
|
11.
|
Chadalapaka G, Jutooru I, Chintharlapalli
S, et al: Curcumin decreases specificity protein expression in
bladder cancer cells. Cancer Res. 68:5345–5354. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Fang Y, Yu Y, Hou Q, et al: The Chinese
herb isolate isorhapontigenin induces apoptosis in human cancer
cells by down-regulating overexpression of antiapoptotic protein
XIAP. J Biol Chem. 287:35234–35243. 2012. View Article : Google Scholar
|
|
13.
|
Lou Z, O’Reilly S, Liang H, et al:
Down-regulation of overexpressed sp1 protein in human fibrosarcoma
cell lines inhibits tumor formation. Cancer Res. 65:1007–1017.
2005.PubMed/NCBI
|
|
14.
|
Shin JA, Kim JJ, Choi ES, et al: In vitro
apoptotic effects of methanol extracts of Dianthus chinensis
and Acalypha australis L. targeting specificity protein 1 in
human oral cancer cells. Head Neck. June 25–2012.(Epub ahead of
print).
|
|
15.
|
Wang L, Wei D, Huang S, et al:
Transcription factor Sp1 expression is a significant predictor of
survival in human gastric cancer. Clin Cancer Res. 9:6371–6380.
2003.PubMed/NCBI
|
|
16.
|
Wang XB, Peng WQ, Yi ZJ, Zhu SL and Gan
QH: Expression and prognostic value of transcriptional factor sp1
in breast cancer. Ai Zheng. 26:996–1000. 2007.(In Chinese).
|
|
17.
|
Guan H, Cai J, Zhang N, et al: Sp1 is
upregulated in human glioma, promotes MMP-2-mediated cell invasion
and predicts poor clinical outcome. Int J Cancer. 130:593–601.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Chen F, Zhang F, Rao J and Studzinski GP:
Ectopic expression of truncated Sp1 transcription factor prolongs
the S phase and reduces the growth rate. Anticancer Res.
20:661–667. 2000.PubMed/NCBI
|
|
19.
|
Shin JA, Shim JH, Choi ES, et al:
Chemopreventive effects of synthetic C-substituted
diindolylmethanes originating from cruciferous vegetables in human
oral cancer cells. Eur J Cancer Prev. 20:417–425. 2011. View Article : Google Scholar
|
|
20.
|
Shin JA, Shim JH, Jeon JG, et al:
Apoptotic effect of Polygonum Cuspidatum in oral cancer
cells through the regulation of specificity protein 1. Oral Dis.
17:162–170. 2011.
|
|
21.
|
van Delft MF and Huang DC: How the Bcl-2
family of proteins interact to regulate apoptosis. Cell Res.
16:203–213. 2006.PubMed/NCBI
|
|
22.
|
Schmidt T, Korner K, Karsunky H, et al:
The activity of the murine Bax promoter is regulated by Sp1/3 and
E-box binding proteins but not by p53. Cell Death Differ.
6:873–882. 1999. View Article : Google Scholar : PubMed/NCBI
|