Introduction
Transcatheter arterial embolization (TAE) for the
treatment of liver cancers is a minimally invasive procedure which
may improve survival and quality of life (QOL) (1,2).
However, the currently used embolic agents do not congest
microvessels sufficiently and therefore TAE is a palliative
treatment rather than a curative therapy (3). In addition, as a result of cancer
cell ischemia and hypoxia, tumor angiogenesis may be induced by TAE
and may contribute to tumor recurrence and metastasis (4,5).
Therefore, liver tumors almost always require repeated TAE.
Ginseng, a highly valued herb from East Asia has
been used by the Chinese for thousands of years. There is extensive
evidence on the beneficial effects of ginseng (6). The major biologically active
components of ginseng are various types of ginsenosides (7). Of these, ginsenoside Rg3 has been
shown to inhibit cancer cell proliferation (8) and angiogenesis (9). The route by which a drug is delivered
may have a significant effect on its therapeutic effect. To date,
the therapeutic effect of Rg3 on liver tumors when administered via
the hepatic artery has not been investigated. The liver VX2
carcinoma derived from a Shope virus-induced papilloma is an
accepted and suitable model for interventional therapy due to its
continuous tumor growth and rich arterial supply (10). Using rabbit liver VX2 carcinoma
models, we evaluated the therapeutic effect of hepatic artery
administration of Rg3 combined with TAE for the treatment of liver
tumors.
Materials and methods
Sample and randomization
This study was approved by the Institutional Animal
Ethical Committee of Sichuan University (Chengdu, China). A total
of 50 adult male New Zealand white rabbits weighing 2.3–4.2 kg were
acquired from the Experimental Animal Center of West China Medical
Center, Sichuan University. The VX2 allografts were provided by The
Union Hospital, Huazhong University of Science and Technology,
Wuhan, China. The tumors were implanted in lateral muscles of the
hind limbs of donor rabbits. The tumor tissue required for
implantation in liver was obtained 2 weeks later. After ketamine
hydrochloride (40 mg/kg; ShuangHe Pharmaceutical Co., Ltd.,
Beijing, China) and xylazine (5 mg/kg; Yaji Pharmaceutical,
Shanghai, China) anesthesia was intramuscularly injected,
laparotomy was performed. A section of tumor tissue (2×2×2 mm) was
implanted into the left lobe of the recipient liver. The abdominal
wall was closed layer by layer. CT scans of abdomen were performed
on all animals 2 weeks after tumor tissue implantation. Animals
bearing a tumor >10 mm in diameter were included in the
experiment. Rg3 was extracted from a sample of ginseng from
Northeast China provided by Yatai Pharmaceutical Co. (Changchun,
China) and the purity quotient was ≥99.5%.
A total of 48 rabbits were randomly assigned into 4
groups with the aid of a computer-generated table of random
numbers; each group was restricted to 12 rabbits. In Group 1, Rg3
was injected into the tumor via the hepatic artery, in Group 2,
lipiodol was injected, in Group 3, a cocktail of Rg3 mixed with
lipiodol was injected, and in Group 4 saline was injected.
Intervention technique and tissue
preparation
The rabbits were anesthetized with ketamine (40
mg/kg) and xylazine (5 mg/kg) by intramuscular injection. Access to
the right common femoral artery was obtained via surgical cutdown,
where a 2.7/2.9 Fr microcatheter (Terumo, Tokyo, Japan) was
introduced. Arteriography of the common hepatic artery was
performed to demonstrate the hepatic arterial anatomy and the
location, size and vascularity of the tumor. The catheter was
selectively advanced into the tumor feeding artery in which,
depending on the subject’s group, Rg3 (6.0 mg/kg), lipiodol (0.1
ml/kg) or a mixture of both was injected through the catheter until
a reduction of blood flow to the tumor was observed. In the case of
Group 4, 1 ml saline was injected through the catheter in each
subject. The catheter was then removed and the common femoral
artery was ligated with absorbable sutures.
Two weeks later, all rabbits were sacrificed with
high-dose ketamine. The tumor tissue was carefully dissected and
divided into two sections. One of the sections was placed in 4%
paraformaldehyde and 0.1 mol/l phosphate-buffered saline at pH 7.0
and 4°C for 24 h and then embedded in paraffin, and the other was
frozen in liquid nitrogen and stored at −80°C until further
analysis. Paraffin sections (5 μm thick) were mounted on
poly-L-lysine-coated slides and stained with hematoxylin and eosin
for observation under a microscope.
Immunohistochemistry
To investigate the angiogenesis and cell apoptosis
in the tumor, we detected the expression of CD31, VEGF and
caspase-3. The sections were deparaffinized in xylene and immersed
in graded ethanol and distilled water. Immunohistochemical staining
was performed using the avidin-biotin peroxidase complex (ABC)
method according to the manufacturer’s instructions (Dako,
Carpinteria, CA, USA). The primary antibodies for CD31 (Abcam,
Boston, MA, USA), VEGF (Abcam) and caspase-3 (Santa Cruz
Biotechnology, Inc. Santa Cruz, CA, USA) were diluted to 1:500. The
primary antibody was omitted as a negative control for the
immunostaining. An image of each section was captured using a light
microscope (Olympus, Tokyo, Japan) at ×400 magnification and the
integrated optical density (IOD) of the positively stained tissue
in each image was determined using Image Pro Plus software, version
6.0 (Media Cybernetics, Silver Spring, MD, USA).
Cell culture
The human hepatocellular carcinoma cell line HepG2
was cultured in Dulbecco’s modifed Eagle’s medium (DMEM) routinely
supplemented with 10% fetal bovine serum plus ampicillin and
streptomycin, and incubated in 5% CO2 at 37°C.
MTT assay
The MTT assay was performed to examine cell
proliferation. Cells in the logarithmic phase were collected, and
were seeded into 96-well plates, 5×103 cells per well
with 100 μl medium, and the plates were incubated at 37°C in
a humidified incubator with 5% CO2 for 24 h. The medium
was removed and 100 μl medium with different concentrations
of Rg3 was added to each well. A control group and four Rg3 groups
were created, the final concentrations of Rg3 were 0, 25, 50, 75
and 100 mg/l. Each group had six replicates. MTT reagent (20
μl; Sigma, St. Louis, MO, USA) was added to each well at 12
h, 24 h, 36 h and 48 h. After culturing the cells for 4 h, the
medium was removed and 150 μl DMSO was added to each well
and incubated for 15 min. The absorbance of the plates at 490 nm
were read using a microplate reader (Model 680, Bio-Rad, Hercules,
CA, USA). Experiments were performed in triplicate in three
independent experiments.
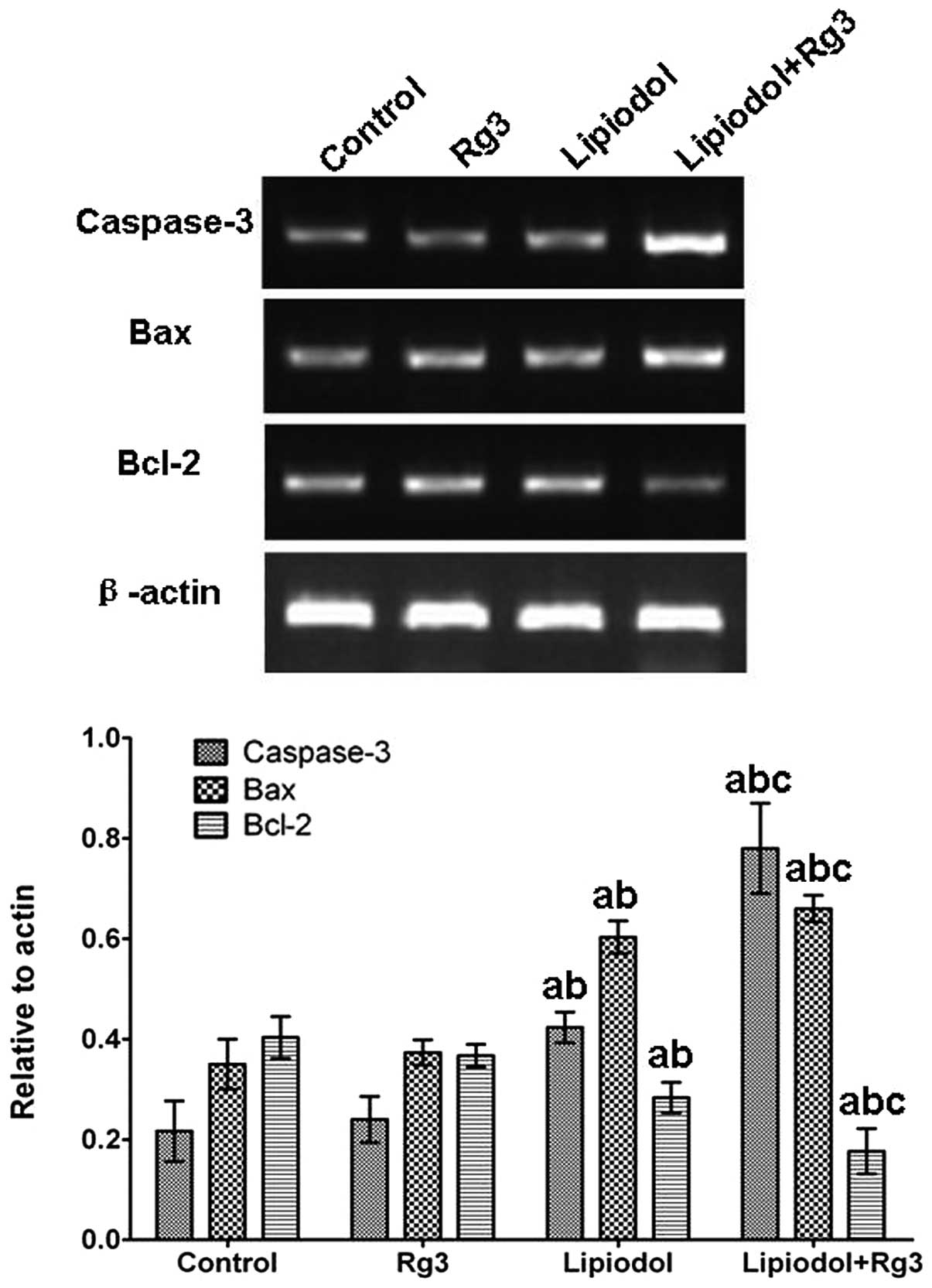
RNA isolation and semi-quantitative
RT-PCR
Tumor tissues were rapidly excised and powdered in
liquid nitrogen. Total RNA was extracted using TRIzol (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
For semi-quantitative RT-PCR, cDNA synthesis and PCR analysis were
performed using the RevertAid™ First Strand cDNA Synthesis kit
(Fermentas, Thermo Fisher Scientific Inc., Waltham, MA, USA). PCR
was performed using an Eppendorf Mastercycler gradient thermal
cycler (Eppendorf, Hamburg, Germany). The reaction cycle consisted
of a hot start at 95°C for 10 min, then 30 cycles of denaturation
at 95°C for 30 sec, annealing at 60°C for 30 sec and extension at
72°C for 30 sec. The primer sequences used for PCR were as follows:
β-actin: 5′- C T T C CAG C C C T C C T T C C T - 3 ′ and
5′-GCCCGACTCGTCATACTCC-3′ (product size: 316 bp); caspase-3: 5′-
CAATGGACTCTGGGAAAT-3′ and 5′-GCAAGCCTGAATAATGA-3′ (product size:
489 bp); Bax: 5′-CCAAGAAGCTGAGCGAGTG-3′ and 5′-TTCCAG
ATGGTGAGTGAGG-3′ (product size: 400 bp); and Bcl-2:
5′-GTGGGATACTGGAGATGAAGA-3′ and 5′-GACGGT AGCGACGAG AGA-3′ (product
size: 233 bp). Experiments were performed in triplicate in three
independent experiments.
Following PCR amplification, 6 μl PCR
products plus 1 μl loading buffer were run on 1.5% agarose
gel containing 1 μg/ml ethidium bromide. The PCR products
were visualized and scanned using a Gel imaging system (Bio-Rad)
and the intensity of each band present in each lane was determined
using Quantity One software (Bio-Rad).
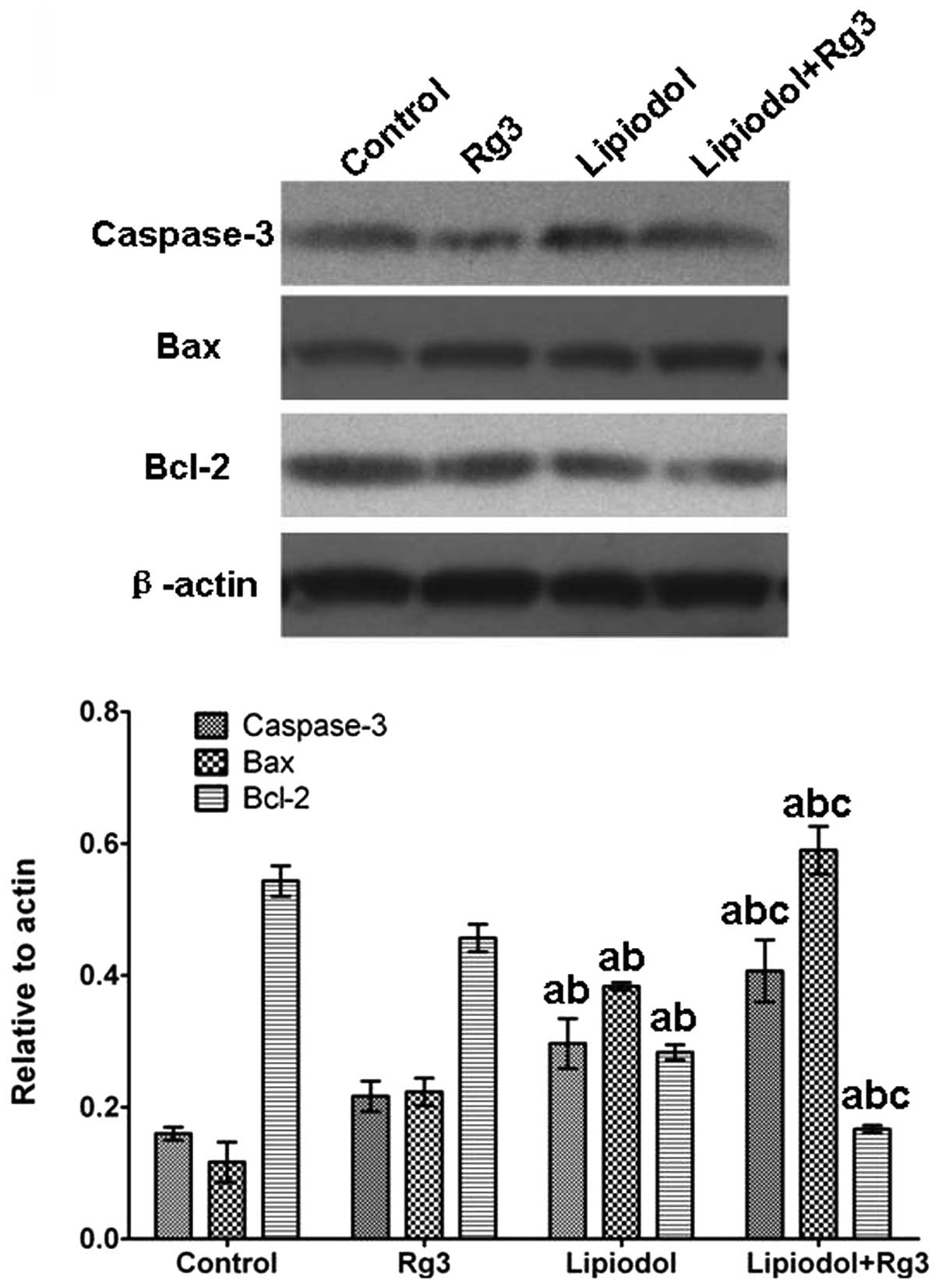
Western blot analysis
To confirm the effect of each treatment on tumor
cell apoptosis, the expression of the apoptosis-related genes,
caspase-3, Bax and Bcl-2, were detected by semi-quantitative RT-PCR
and the respective proteins were detected by western blot analysis.
For protein extraction, tumor tissue was ground into powder in
liquid nitrogen with a pre-cooled mortar and pestle. The powdered
tissue was homogenized in 500 μl lysis buffer (50 mmol/l
Tris-HCl, pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mmol/l
NaCl, 1 mmol/l EDTA, 1 mmol/l phenylmethylsulfonyl fluoride, 1
mmol/l Na3VO4, and 1 mmol/l NaF) and
incubated at 4°C for 30 min, followed by centrifuging at 10,000 × g
at 4°C for 20 min. The lysates were collected and the protein
concentration was determined using the BCA Protein Assay kit. Equal
amounts of proteins were separated by SDS-PAGE and transferred to
polyvinylidene difluoride membranes (GE Healthcare Biosciences).
The membranes were incubated with primary antibodies against
caspase-3 (1:1000; Santa Cruz Biotechnology), Bax (1:1000; Sigma),
Bcl-2 (1:1000; Sigma) or VEGF (1:1000; Santa Cruz Biotechnology).
Antibody binding was revealed by incubation with horse-radish
peroxidase-conjugated secondary antibodies (Santa Cruz
Biotechnology) and an ECL Plus immunoblotting detection system (GE
Healthcare Biosciences, Fairfield, CT, USA). Signals were
quantified using NIH ImageJ 1.63 software (National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
Data were analyzed and calculated with one-way ANOVA
using SPSS version 11.0 software (SPSS, Inc., Chicago, IL, USA) and
were expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant result.
Results
Rg3 inhibits the proliferation and VEGF
expression of hepatocellular carcinoma cells in vitro
To determine whether Rg3 functions as a novel
chemotherapeutic agent for human hepatocellular carcinoma, we first
examined the effect of Rg3 on the proliferative activity of human
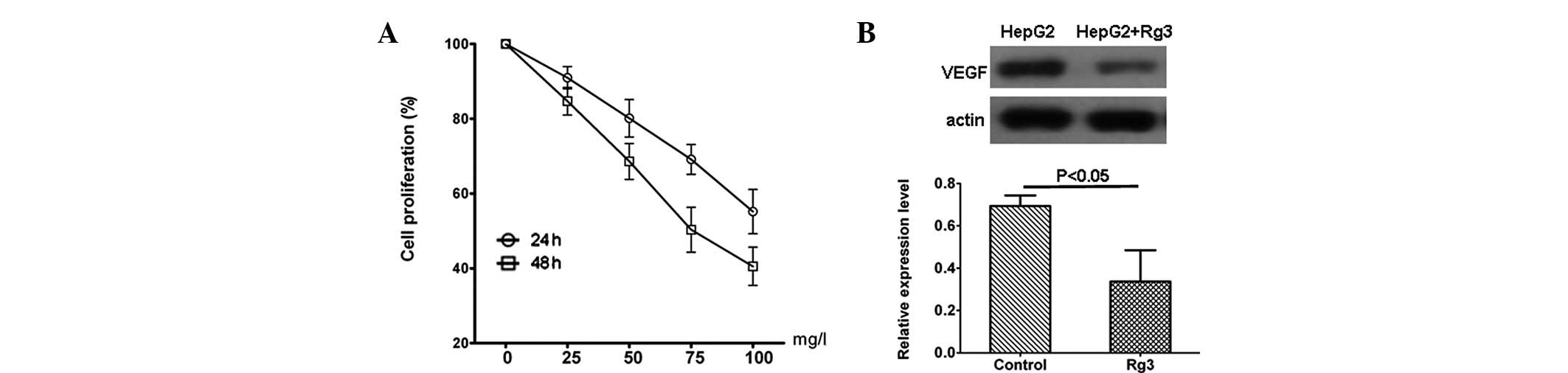
hepatocellular carcinoma cells. As shown in Fig. 1A, the proliferation of human
hepatocellular carcinoma cell line HepG2 was inhibited by Rg3 in a
concentration-dependent manner in vitro. In addition,
western blotting results indicated that Rg3 inhibited the VEGF
expression of HepG2 cells significantly (P<0.05; Fig. 1B).
TAE combined with Rg3 inhibits tumor
growth in vivo
A total of 48 rabbits with VX2 liver tumors were
included in this experiment. There was no significant difference in
the mean volume of the tumor between each group prior to
intervention. Following treatment, the tumors continued to increase
in volume in each group. At 2 weeks, the mean tumor volume and
growth rate were significantly lower in Groups 2 (TAE) and 3 (Rg3
and TAE) than in the control (P<0.05). In addition, the mean
tumor growth rate in the rabbits treated with Rg3 and TAE was
significantly lower than that in the TAE group (P<0.05). There
was no significant difference in the mean tumor growth rate between
Group 1 (Rg3) and the control (P>0.05), and notably, no
adverse-effects were observed in this experiment (Table I).
 | Table I.Pre- and post-treatment tumor volume
and post-treatment tumor growth rate in each group at 2 weeks. |
Table I.
Pre- and post-treatment tumor volume
and post-treatment tumor growth rate in each group at 2 weeks.
| Group | Tumor volume
(cm3) pre-treatment | Tumor volume
(cm3) post-treatment | Tumor growth rate (%)
post-treatment |
|---|
| Rg3 | 1.89±0.57 | 3.72±1.09 | 196.80±21 |
| Lipiodol | 2.04±0.98 | 2.98±1.29a | 146.07±14a,b |
| Lipiodol+Rg3 | 2.07±0.85 |
2.54±1.07a–c |
117.05±12a–c |
| Control | 1.96±0.68 | 3.95±1.91 | 201.50±15 |
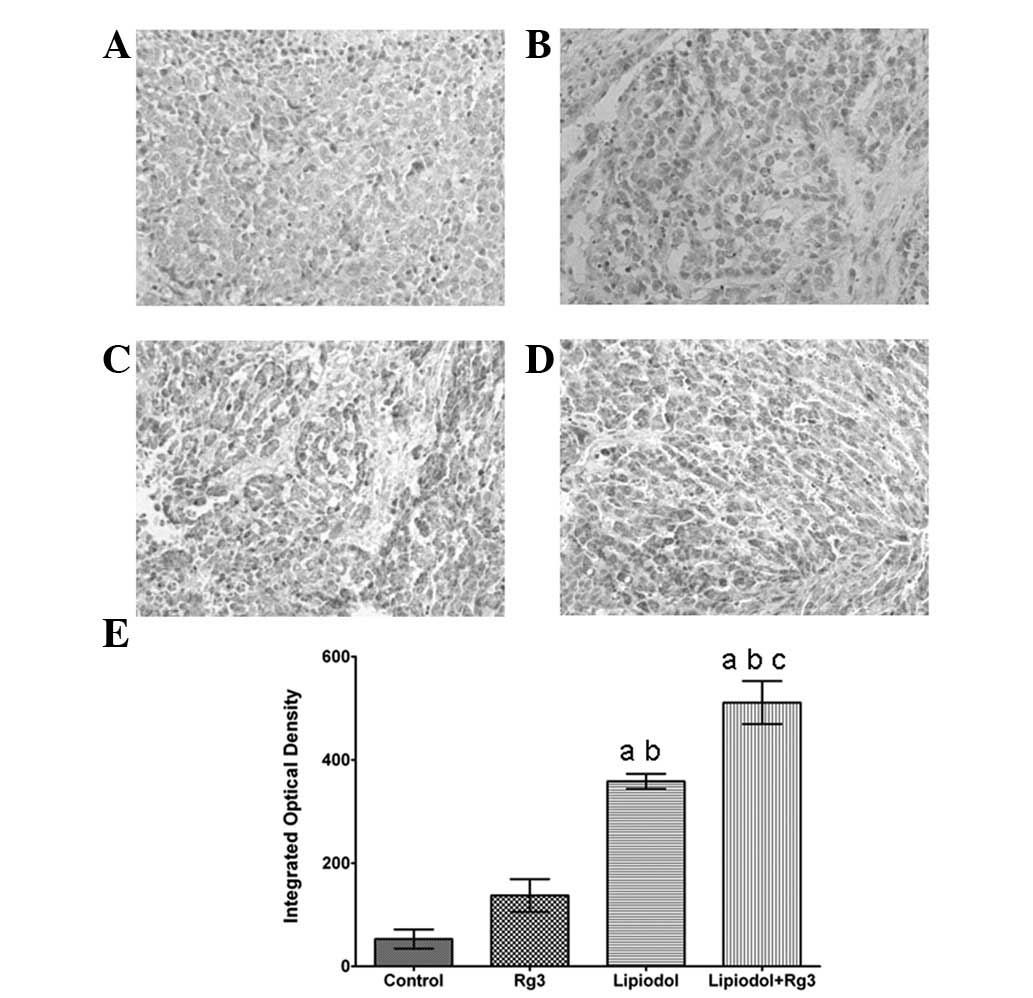
TAE combined with Rg3 inhibits expression
of CD31 and VEGF in vivo
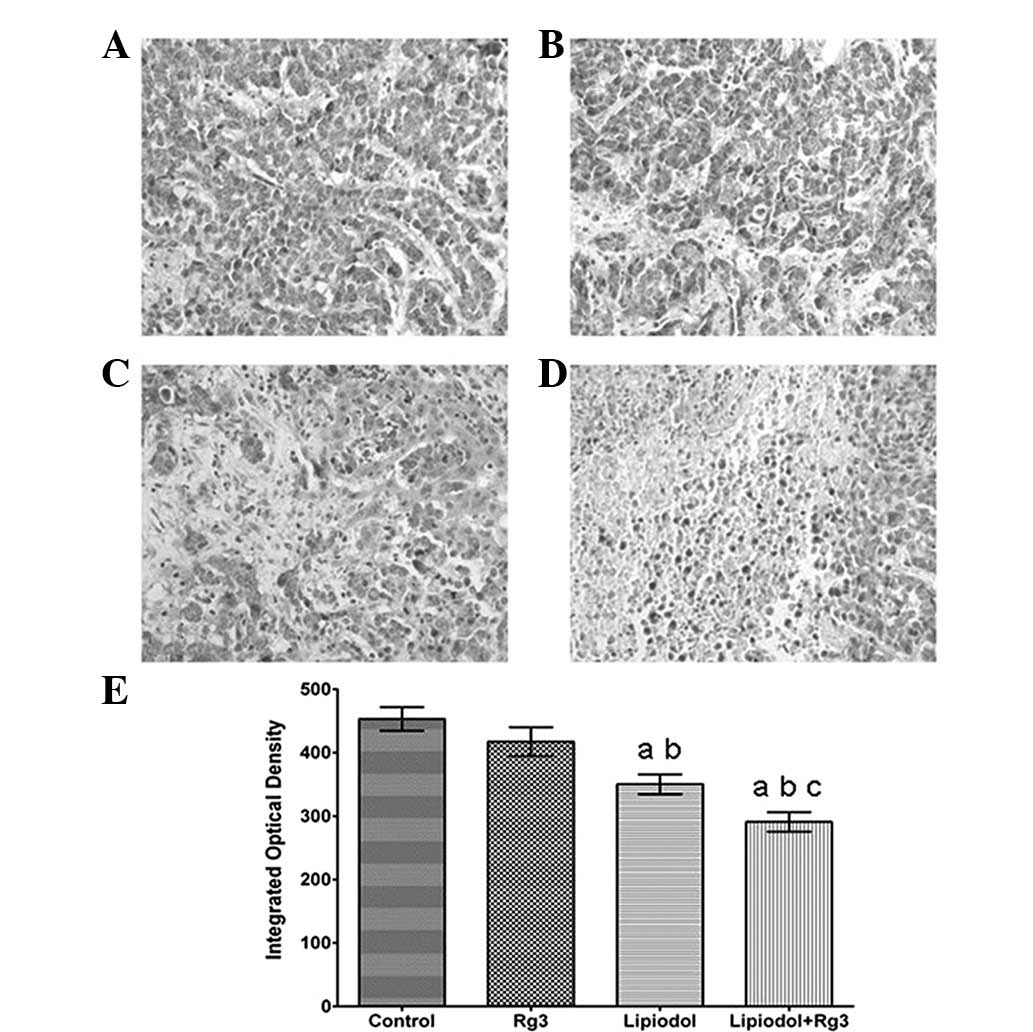
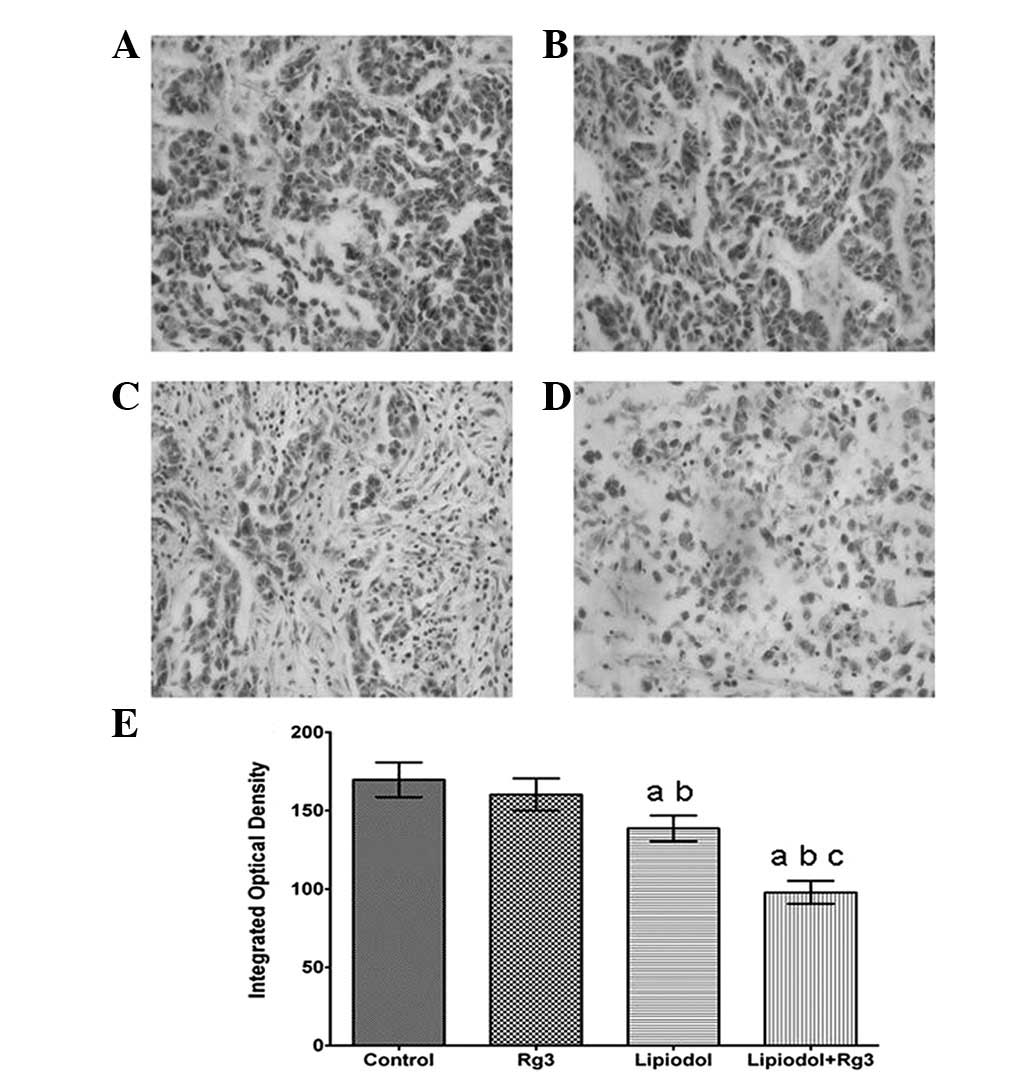
Immunohistochemical analysis demonstrated that the
tumor tissues were weakly stained for VEGF (Fig. 2) and CD31 (Fig. 3) antibody in Group 2 (TAE) and
particularly weakly in Group 3 (Rg3 and TAE) compared with Group 1
(Rg3) and the control. Groups 2 (TAE) and 3 (Rg3 and TAE) each had
a significantly lower integrated optical density (Fig. 2E and Fig. 3E) than the other groups. Group 1
(Rg3) also had a lower integrated optical density than the control
group, however, there was no significant difference between the two
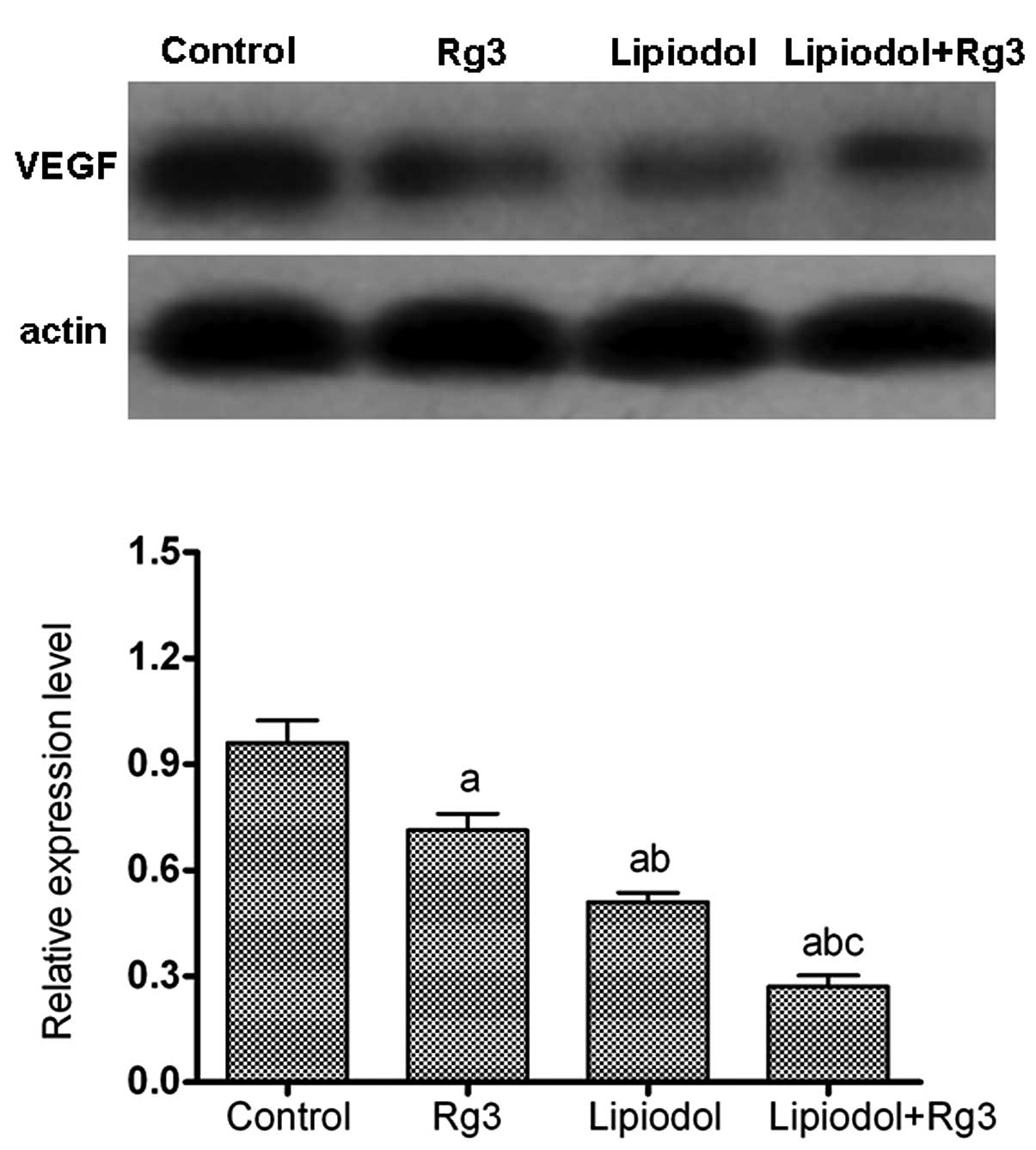
groups. A western blot analysis of VEGF (Fig. 4) showed similar results to those of
the immunohistochemical analysis. The mean relative protein
expression of VEGF was 0.95±0.10, 0.71±0.07, 0.50±0.08 and
0.26±0.04 in the control group, Rg3 group, lipiodol group and
lipiodol + Rg3 group, respectively (Fig. 4). The expression level of VEGF in
the group treated with TAE and Rg3 was significantly decreased
compared with that in the other groups (P<0.05; Fig. 4).
TAE combined with Rg3 upregulates the
expression of apoptosis-related genes in vivo
Caspase-3 and Bax expression levels in tumor tissues
from Group 3 (Rg3 and TAE) were significantly increased compared
with those from the other groups at the mRNA (Fig. 5) and protein (Fig. 6) levels. Group 2 (TAE) also had
significantly increased expression levels compared with the control
group, but the increase was inferior compared with that in Group 3
(Rg3 and TAE). No significant difference was observed between Group
1 (Rg3) and the control. With respect to Bcl-2, the expression
level in Group 3 (Rg3 and TAE) was significantly lower than in the
other groups. By contrast, the control group and Group 1 (Rg3) had
significantly higher Bcl-2 levels and there was no significant
difference in Bcl-2 level between Group 1 (Rg3) and the control.
The immunohistochemical staining of caspase-3 also confirmed these
results (Fig. 7).
Discussion
The most prominent constituents of ginseng, a
traditional Chinese medicinal herb, are ginsenosides. Thus far,
over 40 types of ginsenosides have been isolated and have been
demonstrated to affect the central nervous system and immune
system, thereby producing anti-stress, anti-ageing, anti-fatigue,
anti-hyperlipemic and anti-angiogenic effects (9–12).
Of all ginsenosides isolated from ginseng, ginsenoside Rg3 has
gained much attention for its antitumor properties (13). Previous studies have shown that Rg3
may inhibit cancer cell proliferation, inhibit metastasis, promote
cancer cell apoptosis and enhance immunity (14–17).
Kim et al demonstrated that chemotherapeutics combined with
Rg3 may have enhanced therapeutic efficacy and reduced side-effects
(18). At present, the oral
administration of Rg3 is employed by physicians worldwide. However,
the therapeutic effect of Rg3 when administered selectively via an
artery has not been investigated.
Numerous studies in recent years have revealed that
angiogenesis is essential for the growth of cancer in solid tumors
(4,5). Angiogenesis plays an important role
in tumor progression; it is responsible for accelerated cancer cell
replication and may also promote tumor metastasis (4). For these reasons, the inhibition of
angiogenesis in solid tumors is an attractive target for cancer
therapy. Arterial embolization is an effective method for the
treatment of liver tumors, but its long-term efficacy remains
limited (3). It has been suggested
that ischemia and hypoxia induced by TAE stimulates tumor
angiogenic activities (5,19), which may partially explain the poor
long-term efficacy and the frequent requirement for TAE to be
repeated (20).
In the present study, TAE therapy was combined with
selective arterial administration of Rg3 for the treatment of VX2
liver tumors in rabbits. Compared with either Rg3 or TAE
monotherapy, the combination of the two modalities may achieve a
greater therapeutic outcome. While TAE congests the microvessels
feeding the tumor, Rg3 may inhibit angiogenic activities caused by
tumor ischemia and hypoxia. In addition, the hepatic arterial
administration of Rg3 combined with TAE increases the local
concentration of Rg3, which may also facilitate therapeutic
efficacy. The results of the present study were in agreement with
this hypothesis and demonstrated that the hepatic arterial
administration of Rg3 combined with TAE has a better therapeutic
effect than either therapy alone.
Immunohistochemistry and western blot analysis
demonstrated that VEGF and CD31 expression in Groups 2 (TAE) and 3
(Rg3 and TAE) was significantly lower than in the control group.
Group 3 (Rg3 and TAE) displayed a lower VEGF and CD31 expression
than Group 2 (TAE). These results indicate that Rg3 may inhibit
angiogenesis following TAE. Previous studies have indicated that
TAE enhances tumor hypoxia, which upregulates the expression of
VEGF (1–3). Notably, in the present study, the
VEGF expression was significantly downregulated in the group
treated with TAE compared with the control group. This may be due
to the TAE therapy causing the necrosis and shrinkage of tumors.
The results of histological and morphological examination were also
confirmed by the necrosis or apoptosis of tumor cells observed in
the group treated with TAE. A previous study has shown that the
rate of cancer cell proliferation and apoptosis is closely
correlated with the progression of cancer (21), therefore, pro-apoptotic and
anti-apoptotic genes were monitored in order to evaluate the
antitumor and anti-angiogenic activity of Rg3 and TAE. The results
of the present study showed that TAE alone and hepatic arterial
administration of Rg3 in combination with TAE may downregulate the
Bcl-2 expression and upregulate the caspase-3 and Bax expression
significantly. Compared with TAE monotherapy, there was a
significantly lower Bcl-2 expression and higher caspase-3 and Bax
expression in Group 3 (Rg3 and TAE). These results suggest that
selective hepatic arterial administration of Rg3 in combination
with TAE may significantly inhibit tumor cell proliferation and
promote tumor cell apoptosis through a caspase-dependent
mechanism.
This study investigated the therapeutic efficacy of
hepatic arterial administration of Rg3 combined with TAE for the
treatment of VX2 liver cancer in rabbits. The results demonstrated
that Rg3 combined with TAE may induce VX2 liver tumor cell
apoptosis and inhibit angiogenesis, and showed that it is an
effective and safe method for the treatment of VX2 liver tumors in
rabbits. However, the application of Rg3 combined with TAE in the
clinic requires further investigation.
Acknowledgements
This study was supported by The
National Natural Science Foundation of China (Grant No.
30770984,81171444).
References
|
1.
|
Acunaş B and Rozanes I: Hepatocellular
carcinoma: treatment with transcatheter arterial chemoembolization.
Eur J Radiol. 32:86–89. 1999.PubMed/NCBI
|
|
2.
|
Lo CM, Ngan H, Tso WK, et al: Randomized
controlled trial of transarterial lipiodol chemoembolization for
unresectable hepatocellular carcinoma. Hepatology. 35:1164–1171.
2002. View Article : Google Scholar
|
|
3.
|
Sergio A, Cristofori C, Cardin R, et al:
Transcatheter arterial chemoembolization (TACE) in hepatocellular
carcinoma (HCC): the role of angiogenesis and invasiveness. Am J
Gastroenterol. 103:914–921. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kim KR, Moon HE and Kim KW:
Hypoxia-induced angiogenesis in human hepatocellular carcinoma. J
Mol Med (Berl). 80:703–714. 2008. View Article : Google Scholar
|
|
5.
|
von Marschall Z, Cramer T, Höcker M, et
al: Dual mechanism of vascular endothelial growth factor
upregulation by hypoxia in human hepatocellular carcinoma. Gut.
48:87–96. 2001.PubMed/NCBI
|
|
6.
|
Kaku T and Kawashima Y: Isolation and
characterization of ginsenoside-Rg2, 20R-prosapogenin,
20S-prosapogenin and delta 20-prosapogenin. Chemical studies on
saponins of Panax ginseng C. A. Meyer, Third report.
Arzneimittelforschung. 30:936–943. 1980.PubMed/NCBI
|
|
7.
|
Kim HS, Lee EH, Ko SR, et al: Effects of
ginsenosides Rg3 and Rh2 on the proliferation of prostate cancer
cells. Arch Pharm Res. 27:429–435. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Wang CZ, Aung HH, Ni M, et al: Red
American ginseng: ginsenoside constituents and antiproliferative
activities of heat-processed Panax quinquefolius roots.
Planta Med. 73:669–674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Yue PY, Wong DY, Wu PK, et al: The
angiosuppressive effects of 20(R)-ginsenoside Rg3. Biochem
Pharmacol. 72:437–445. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Rous P, Kidd JG and Smith WE: Experiments
on the cause of the rabbit carcinomas derived from virus-induced
papillomas. II. Loss by the Vx2 carcinoma of the power to immunize
hosts against the papilloma virus. J Exp Med. 96:159–174. 1952.
View Article : Google Scholar
|
|
11.
|
Khalil WK, Ahmed KA, Park MH, et al: The
inhibitory effects of garlic and Panax ginseng extract
standardized with ginsenoside Rg3 on the genotoxicity, biochemical,
and histological changes induced by ethylenediaminetetraacetic acid
in male rats. Arch Toxicol. 82:183–195. 2008.PubMed/NCBI
|
|
12.
|
Chen CF, Chiou WF and Zhang JT: Comparison
of the pharmacological effects of Panax ginseng and Panax
quinquefolium. Acta Pharmacol Sin. 29:1103–1108. 2008.
View Article : Google Scholar
|
|
13.
|
Jia L, Zhao Y and Liang XJ: Current
evaluation of the millennium phytomedicine - ginseng (II):
Collected chemical entities, modern pharmacology, and clinical
applications emanated from traditional Chinese medicine. Curr Med
Chem. 16:2924–2942. 2009. View Article : Google Scholar
|
|
14.
|
Keum YS, Han SS, Chun KS, et al:
Inhibitory effects of the ginsenoside Rg3 on phorbol ester-induced
cyclooxygenase-2 expression, NF-kappaB activation and tumor
promotion. Mutat Res. 523–524:75–85. 2003.
|
|
15.
|
Popovich DG and Kitts DD:
Structure-function relationship exists for ginsenosides in reducing
cell proliferation and inducing apoptosis in the human leukemia
(THP-1) cell line. Arch Biochem Biophys. 406:1–8. 2002. View Article : Google Scholar
|
|
16.
|
Yoon SR, Lee GD, Park JH, et al:
Ginsenoside composition and antiproliferative activities of
explosively puffed ginseng (Panax ginseng C.A. Meyer). J
Food Sci. 75:378–382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Liu WK, Xu SX and Che CT:
Anti-proliferative effect of ginseng saponins on human prostate
cancer cell line. Life Sci. 67:1297–1306. 2010.PubMed/NCBI
|
|
18.
|
Kim SM, Lee SY, Yuk DY, et al: Inhibition
of NF-kappaB by ginsenoside Rg3 enhances the susceptibility of
colon cancer cells to docetaxel. Arch Pharm Res. 32:755–765. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Suzuki H, Mori M, Kawaguchi C, et al:
Serum vascular endothelial growth factor in the course of
transcatheter arterial embolization of hepatocellular carcinoma.
Int J Oncol. 14:1087–1090. 1999.
|
|
20.
|
Wu XZ, Xie GR and Chen D: Hypoxia and
hepatocellular carcinoma: The therapeutic target for hepatocellular
carcinoma. J Gastroenterol Hepatol. 22:1178–1182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Burz C, Berindan-Neagoe I, Balacescu O and
Irimie A: Apoptosis in cancer: key molecular signaling pathways and
therapy targets. Acta Oncol. 48:811–821. 2009. View Article : Google Scholar : PubMed/NCBI
|