Introduction
Non-small cell lung cancer (NSCLC) is the most
common malignant neoplasm worldwide (1), with metastasis and recurrence
primarily responsible for mortality. The early identification of
metastasis and recurrence provides patients with time to undergo
salvage therapy in order to prolong their survival. Radical surgery
has been the standard treatment for a number of decades. However, a
large number of patients experience disease progression over a
short time. Over the past decades, various studies have attempted
to identify molecular biomarkers to predict the metastasis or
recurrence of NSCLC (2,3). Numerous promising biomarkers have
been evaluated as potential prognosis predictors, however, none of
these have been demonstrated to be sufficiently effective for
clinical use. The majority of these markers are somewhat
controversial and inconclusive.
More recently, significant attention has been given
to the association between malignancies and coagulation (4,5). A
hypercoagulability state is one of the signs of a more aggressive
disease, while a thromboembolism is one of the major causes of
mortality in cancer patients (6).
Several studies have demonstrated that an elevated platelet count
correlates with a poor prognosis in numerous types of solid cancer,
including colorectal cancer (7,8),
esophageal carcinoma (9) and
gastric cancer (10,11). A prognostic significance between
the platelet count and lung cancer has also been identified
(12–17). However, the majority of these
studies included small cell lung cancer and the samples were
relatively small.
In the present study, a retrospective clinical
analysis was designed for a total of 510 operable NSCLC patients to
investigate the correlation between platelet count, patients’
characteristics and prognosis.
Patients and methods
Patients and treatment
The present study enrolled 510 patients who had been
diagnosed with NSCLC between 2006 and 2009 at Zhejiang Provincial
Corps Hospital, China. All patients had their diagnoses freshly
confirmed and had not had any previous treatment. The patients with
the following characteristics were excluded from the present study:
patients who had any coexisting or previous cancers other than
NSCLC; patients with concomitant diseases suspected of increasing
the serum platelet concentration, including severe hypertension,
splenic disease and blood coagulation disorders; and patients who
had taken aspirin or other acetylsalicylic acid drugs one month
prior to the treatment. The study population had a median age of 60
years (range, 37–82 years). The patients comprised of 388 (76.1%)
males and 122 (23.9%) females. Approval for the present study was
obtained from the institutional review board of the Zhejiang
Provincial Corps Hospital. All patients provided informed consent
prior to undergoing surgery.
A lobectomy, bilobectomy or pneumonectomy was
performed according to the location or size of the lung neoplasm in
each patient. Systematic mediastinum lymph node dissection was also
performed in each patient. In total, 203 patients were treated with
adjuvant platinum-based chemotherapy, adjuvant radiotherapy or a
combination of the two. Detailed information about the patient’s
characteristics and tumor histopathology were collected
retrospectively from the medical records.
Platelet measurement
A blood sample was obtained by peripheral venous
puncture, before breakfast 3 days prior to the surgery. A complete
blood count was regularly taken and elevated platelet counts were
defined as >300×109/l.
Follow-up
All patients received a standardized follow-up,
occurring at 3-month intervals for two years, at 6-month intervals
in the third year and yearly thereafter. An evaluation comprised a
physical examination, complete blood count, chest computed
tomography (CT), brain magnetic resonance imaging (MRI) and
abdominal ultrasound. Local recurrence and distance metastasis were
histologically confirmed whenever possible.
Statistical analysis
The Chi-square test was performed to evaluate the
association between the clinicopathological variables and the
platelet count. Disease-free survival (DFS) was defined from the
date of the definitive surgery to the date of local or distant
progression, mortality by any cause or the date of the last
follow-up. Overall survival (OS) was calculated as the time from
the pulmonary surgery to the time of mortality or censoring.
Kaplan-Meier curves were used to estimate the distribution of DFS
and OS and a two-sided log-rank test was performed to compare the
difference between the survival curves. The Cox proportional
hazards model was used with a backward selection method for the
univariate and multivariate analyses. All factors with an effect on
DFS and OS in the univariate analysis (P≤0.10) were included in the
multivariate analysis. All statistical calculations were performed
with SPSS 13.0 for Windows (SPSS, Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patients’ characteristics
A total of 510 NSCLC patients were enrolled in the
present study. The characteristics of the patients are summarized
in Table I. According to the
criteria of the World Health Organization/International Association
for the Study of Lung Cancer (WHO/IASLC) classification of lung
tumors, 253 tumors were squamous cell carcinomas, 25 were large
cell lung carcinomas and 232 were adenocarcinomas; of these, 29
were well differentiated, 263 were moderately differentiated and
218 were poorly differentiated. There were 354 patients who had
smoked at a some time in their lives. In terms of the new IASLC
staging system, 234 cases were categorized as stage I, 128 as stage
II and 148 as stage III.
 | Table IAssociation of platelet count with the
parameters of patients with NSCLC. |
Table I
Association of platelet count with the
parameters of patients with NSCLC.
| Parameters | Patients, n (%) | Platelet count
|
|---|
| Median (5th–95th
percentile) | P-value | ≤300, nb (%) | >300, nb (%) | P-value |
|---|
| Gender | | | 0.312 | | | 0.074 |
| Male | 388 (76.1) | 204.5
(112.8–373.5) | | 336 (86.6) | 52 (13.4) | |
| Female | 122 (23.9) | 198.5
(117.6–352.5) | | 113 (92.6) | 9 (7.4) | |
| Age (years) | | | 0.263 | | | 0.158 |
| <65 | 353 (69.2) | 206.0
(117.0–373.6) | | 306 (86.7) | 47 (13.3) | |
| ≥65 | 157 (30.8) | 193.0
(103.6–357.1) | | 143 (91.1) | 14 (8.9) | |
| Smoking | | | 0.338 | | | 0.094 |
| Never | 156 (30.6) | 199.0
(115.6–345.5) | | 143 (91.7) | 13 (8.3) | |
| Smoker | 354 (69.4) | 205.5
(114.0–378.3) | | 306 (86.4) | 48 (13.6) | |
| Histological
type | | | 0.058 | | | 0.127 |
| Squamous cell
carcinoma | 253 (49.6) | 215.0
(117.7–361.0) | | 216 (85.4) | 37 (14.6) | |
| Adenocarcinoma | 232 (45.5) | 196.0
(107.0–376.1) | | 209 (90.1) | 23 (9.9) | |
| Other | 25 (4.9) | 192.0
(121.0–299.5) | | 24 (96.0) | 1 (4.0) | |
| Differentiation | | | 0.122 | | | 0.959 |
| Well | 29 (5.7) | 187.0
(78.0–314.5) | | 26 (89.7) | 3 (10.3) | |
| Moderately | 263 (51.6) | 201.0
(118.4–333.6) | | 231 (87.8) | 32 (12.2) | |
| Poorly | 218 (42.7) | 210.5
(114.8–380.1) | | 192 (88.1) | 26 (11.9) | |
| T stage | | | <0.001a | | | <0.001a |
| T1 | 52 (10.2) | 196.5
(96.3–325.8) | | 47 (90.4) | 5 (9.6) | |
| T2 | 362 (71.0) | 199.5
(117.0–331.4) | | 329 (90.9) | 33 (9.1) | |
| T3 | 60 (11.8) | 253.5
(127.4–423.5) | | 43 (71.7) | 17 (28.3) | |
| T4 | 36 (7.1) | 263.0
(148.1–406.1) | | 30 (83.3) | 6 (16.7) | |
| N stage | | | 0.001a | | | 0.001a |
| N0 | 270 (52.9) | 192.0
(107.1–312.5) | | 251 (93.0) | 19 (7.0) | |
| N1 | 135 (26.5) | 217.0
(110.6–379.2) | | 114 (84.4) | 21 (15.6) | |
| N2 | 105 (20.6) | 221.0
(117.3–395.3) | | 84 (80.0) | 21 (20.0) | |
| Clinical stage | | | <0.001a | | | <0.001a |
| I | 234 (45.9) | 190.5
(107.5–305.3) | | 221 (94.4) | 13 (5.6) | |
| II | 128 (25.1) | 220.5
(113.7–383.8) | | 107 (83.6) | 21 (16.4) | |
| III | 148 (29.0) | 225.0
(115.9–387.2) | | 121 (81.8) | 27 (18.2) | |
Platelet count and patients’
characteristics
The median platelet count in the NSCLC patients was
203×109/l (95% CI, 115–358×109/l). A total of
449 (88.0%) patients had a platelet count of ≤300×109/l,
which was defined as within the normal range. The correlation
between the characteristics of the patients and their platelet
count is shown in Table I. There
was no significant correlation between the platelet count and
gender, age, smoking status, tumor histological type or cancer cell
differentiation. The median platelet count in the N2 patients was
significantly higher than that in the N1 or N0 patients (median,
221×109/l vs. 217×109/l or
192×109/l, respectively; P= 0.001). There was a
statistically significant correlation between the platelet count
and the T and clinical stages. The median platelet count in the T3
and T4 patients was significantly higher than that in the T1 and T2
patients (median, 263 and 253.5×109/l vs. 199.5 and
196.5×109/l, respectively; P<0.001). When the value
of the platelet count was analyzed as a dichotomous variable
(elevated and normal platelet count groups), the frequency of the
patients with T3 and T4 was higher in the group that had an
elevated platelet count. The increased platelet count was also
associated with the N and clinical stages (P<0.05).
Association of platelet count with DFS
and OS
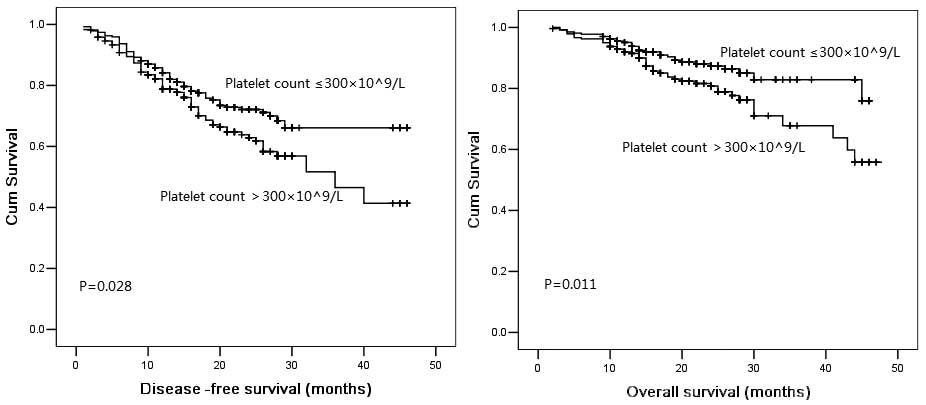
Overall, the 3-year DFS and OS probabilities were
57.3 and 75.7%, respectively. The median DFS period was 34.0 months
in the patients with a normal platelet count and 27.4 months in the
patients with an elevated platelet count. The 3-year cumulative OS
probability was 75.3% for patients with a normal platelet count and
59.2% for patients with an elevated platelet count. The
Kaplan-Meier DFS and OS curves of the normal versus elevated
platelet counts showed a highly significant separation, as shown in
Fig. 1. When stratified by gender,
age, smoking status, tumor histological type, tumor differentiation
and the T, N and clinical stages in each subgroup, the patients
with a normal platelet count had a longer mean DFS time than those
with an elevated platelet count. Within the subgroups of age <65
years, female, poor differentiation, T2 stage, N0 stage and
clinical stage I in particular, the Kaplan-Meier DFS curves of the
normal versus elevated platelet counts showed a significant
separation.
In the multivariate survival analysis, the platelet
count and patient age, but not the smoking status, tumor
differentiation or the T, N and clinical stages, were associated
with DFS and OS (Table II).
 | Table IIMultivariate analysis of DFS and OS
rate. |
Table II
Multivariate analysis of DFS and OS
rate.
| Parameter | HR | 95% CI | P-value |
|---|
| DFS | | | |
| Age: <65 vs.
≥65 years | 1.416 | 1.009–1.987 | 0.044a |
| Smoking: ever vs.
never | 1.029 | 0.596–1.777 | 0.919 |
| T stage: T1 and 2
vs. T3 and 4 | 1.395 | 0.892–2.183 | 0.145 |
| N stage: N0 vs.
N1-2 | 1.113 | 0.733–1.692 | 0.615 |
| Clinical stage:
I, II vs. III | 1.325 | 0.817–2.147 | 0.254 |
| Platelet count:
≤300 × 109 vs. >300 × 109 cells/l | 1.568 | 1.015–2.453 | 0.030a |
| OS | | | |
| Age: <65 vs.
≥65 years | 1.795 | 1.170–2.755 | 0.007a |
| Smoking: ever vs.
never | 1.367 | 0.868–2.154 | 0.178 |
| T stage: T1-2 vs.
T3-4 | 1.157 | 0.668–2.004 | 0.602 |
| N stage: N0 vs.
N1-2 | 1.436 | 0.843–2.444 | 0.183 |
| Clinical stage:
I, II vs. III | 1.496 | 0.834–2.683 | 0.176 |
| Platelet count:
≤300 × 109 vs. >300 × 109 cells/l | 1.689 | 1.005–2.380 | 0.017a |
Discussion
The results of the present study indicated that the
pre-operative platelet count may be used as a biomarker for
predicting the outcome in NSCLC. An elevated platelet count was
correlated with a worse prognosis in the NSCLC patients. To the
best of our knowledge, this is the largest sample study to reveal a
correlation between platelet count and the prognosis of operable
NSCLC patients.
In the present study, the platelet count was
significantly associated with tumor clinical stage and patient
outcome. An elevated platelet count was associated with a worse
patient outcome. Moreover, multivariate analysis using a Cox
proportional hazards model showed that the pre-operative platelet
count was an independent prognostic factor in operable NSCLC
patients. The patients with an elevated platelet count had a
1.57-fold greater risk of disease progression than those with a
normal fibrinogen level. These findings were consistent with a
previous study (18), which were
based on relatively small sample sizes. Tomita et
al(18) reported that the
pre-operative platelet count was a prognostic factor for resectable
NSCLC patients. The 5-year survival probabilities of patients with
normal or elevated platelet counts were reported as 28.87 and
63.73%, respectively. The major strengths of the present study are
the inclusion of a large population of NSCLC patients, which was
used to investigate the prognostic value of the platelet count. The
large size of the study may avoid bias and heterogeneity. However,
the present study was also a retrospective study and there was
insufficient information on post-recurrence treatment, which may
have lead to differences in the survival rates. A prospective study
is required to determine the prognostic and treatment value of
serum fibrinogen.
Platelets play various significant roles in
physiological pathways, including homeostasis and inflammation.
Also, platelets correlate with the progression of malignancies. The
precise reason for the association between an elevated platelet
count and a worse outcome for NSCLC remains unknown. Firstly, the
increase in platelet count may promote tumor cell growth and
angiogenesis. Platelets release various cytokines, including
vascular endothelial growth factor (VEGF) and platelet-derived
growth factor (PDGF), during blood clotting. The VEGF and PDGF
family of proteins has a significant role in regulating
angiogenesis. The invasiveness of the cancer cells may be enhanced
by the plasma components in stored platelets (19). Additionally, bevacizumab, an
inhibitor of VEGF, is able to reduce this promotive effect.
Moreover, platelets promote the formation of capillary-like
structures by endothelial cells, via integrins mediating cell-cell
adhesion (20). Secondly,
platelets enhance tumor metastasis by protecting the tumor cells
from the host’s immune system. Platelets expressing
immunoregulatory proteins, including glucocorticoid-induced
TNFR-related (GITR) protein, may protect the cancer cells (21). The inhibition of platelet
activation significantly decreases the metastatic potential of
tumor cells (22).
In summary, there is evolving evidence that platelet
counts are an independent new prognostic biomarker for DFS and OS
in operable NSCLC. An assessment of the platelet count should be
included in the work up of patients with NSCLC in future
prospective trials to confirm its prognostic significance.
References
|
1.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2.
|
O’Byrne KJ, Gatzemeier U, Bondarenko I, et
al: Molecular biomarkers in non-small-cell lung cancer: a
retrospective analysis of data from the phase 3 FLEX study. Lancet
Oncol. 12:795–805. 2011.PubMed/NCBI
|
|
3.
|
Douillard JY, Shepherd FA, Hirsh V, et al:
Molecular predictors of outcome with gefitinib and docetaxel in
previously treated non-small-cell lung cancer: data from the
randomized phase III INTEREST trial. J Clin Oncol. 28:744–752.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Komurcuoglu B, Ulusoy S, Gayaf M, et al:
Prognostic value of plasma D-dimer levels in lung carcinoma.
Tumori. 97:743–748. 2011.PubMed/NCBI
|
|
5.
|
Unsal E, Atalay F, Atikcan S and Yilmaz A:
Prognostic significance of hemostatic parameters in patients with
lung cancer. Respir Med. 98:93–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
van Doormaal FF, Raskob GE, Davidson BL,
et al: Treatment of venous thromboembolism in patients with cancer:
subgroup analysis of the Matisse clinical trials. Thromb Haemost.
101:762–769. 2009.PubMed/NCBI
|
|
7.
|
Monreal M, Fernandez-Llamazares J, Piñol
M, et al: Platelet count and survival in patients with colorectal
cancer - a preliminary study. Thromb Haemost. 79:916–918.
1998.PubMed/NCBI
|
|
8.
|
Costantini V, Zacharski LR, Moritz TE and
Edwards RL: The platelet count in carcinoma of the lung and colon.
Thromb Haemost. 64:501–505. 1990.PubMed/NCBI
|
|
9.
|
Shimada H, Oohira G, Okazumi S, et al:
Thrombocytosis associated with poor prognosis in patients with
esophageal carcinoma. J Am Coll Surg. 198:737–741. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ikeda M, Furukawa H, Imamura H, et al:
Poor prognosis associated with thrombocytosis in patients with
gastric cancer. Ann Surg Oncol. 9:287–291. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Hwang SG, Kim KM, Cheong JH, et al: Impact
of pretreatment thrombocytosis on blood-borne metastasis and
prognosis of gastric cancer. Eur J Surg Oncol. 38:562–567. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Gonzalez Barcala FJ, Garcia Prim JM,
Moldes Rodriguez M, et al: Platelet count: association with
prognosis in lung cancer. Med Oncol. 27:357–362. 2010.PubMed/NCBI
|
|
13.
|
Gislason T and Nõu E: Sedimentation rate,
leucocytes, platelet count and haemoglobin in bronchial carcinoma:
an epidemiological study. Eur J Respir Dis. 66:141–146.
1985.PubMed/NCBI
|
|
14.
|
Engan T and Hannisdal E: Blood analyses as
prognostic factors in primary lung cancer. Acta Oncol. 29:151–154.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Pedersen LM and Milman N: Prognostic
significance of thrombocytosis in patients with primary lung
cancer. Eur Respir J. 9:1826–1830. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Aoe K, Hiraki A, Ueoka H, et al:
Thrombocytosis as a useful prognostic indicator in patients with
lung cancer. Respiration. 71:170–173. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Cox G, Walker RA, Andi A, et al:
Prognostic significance of platelet and microvessel counts in
operable non-small cell lung cancer. Lung Cancer. 29:169–177. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Tomita M, Shimizu T, Hara M, et al:
Prognostic impact of thrombocytosis in resectable non-small cell
lung cancer. Interact Cardiovasc Thorac Surg. 7:613–615. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Dineen SP, Roland CL, Toombs JE, et al:
The acellular fraction of stored platelets promotes tumor cell
invasion. J Surg Res. 153:132–137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Pipili-Synetos E, Papadimitriou E and
Maragoudakis ME: Evidence that platelets promote tube formation by
endothelial cells on matrigel. Br J Pharmacol. 125:1252–1257. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Placke T, Kopp HG and Salih HR: Modulation
of natural killer cell anti-tumor reactivity by platelets. J Innate
Immun. 3:374–382. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Nieswandt B, Hafner M, Echtenacher B and
Männel DN: Lysis of tumor cells by natural killer cells in mice is
impeded by platelets. Cancer Res. 59:1295–1300. 1999.PubMed/NCBI
|